Genetic Profiling of Aspergillus Isolates with Varying Aflatoxin Production Potential from Different Maize-Growing Regions of Kenya
Abstract
:1. Introduction
2. Results
2.1. Cultural Characterization of Aspergillus Isolates
2.2. Characterization of Aspergillus Isolates Based on Sequences of the ITS Region
2.3. Identification and Analysis of Polymorphism in Calmodulin Gene Sequences
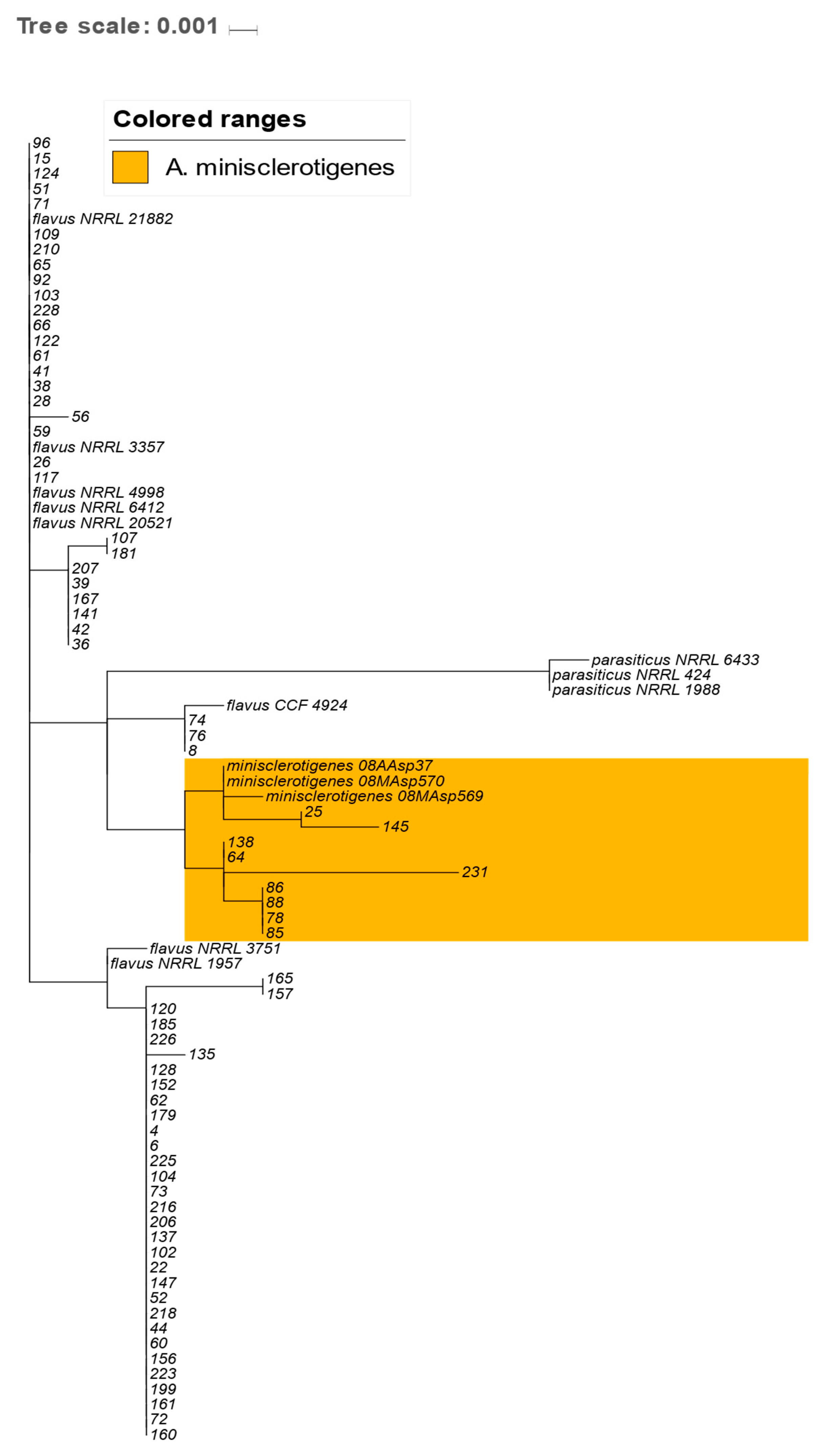
2.4. Analysis of the Genetic Relationships between A. flavus and A. minisclerotigenes Based on Concatenated Sequences of ITS Region and Partial Calmodulin Gene
2.5. Aflatoxin Production in Culture Media
2.6. Relationship between Aflatoxin Production in Culture Media and the Natural Contamination of Maize
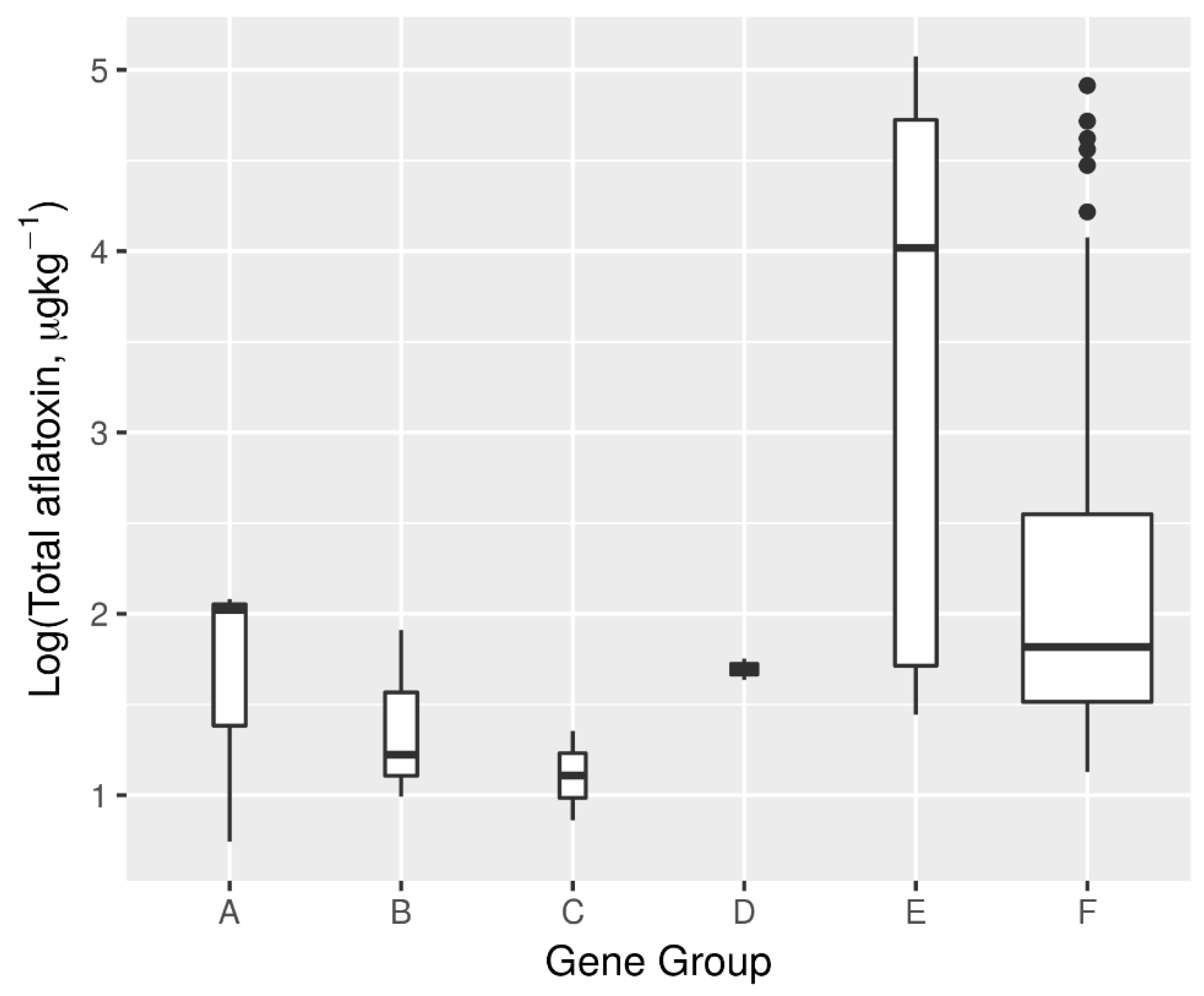
2.7. Aflatoxin Biosynthesis Gene Profile and Its Relationship with Aflatoxin Production Potential
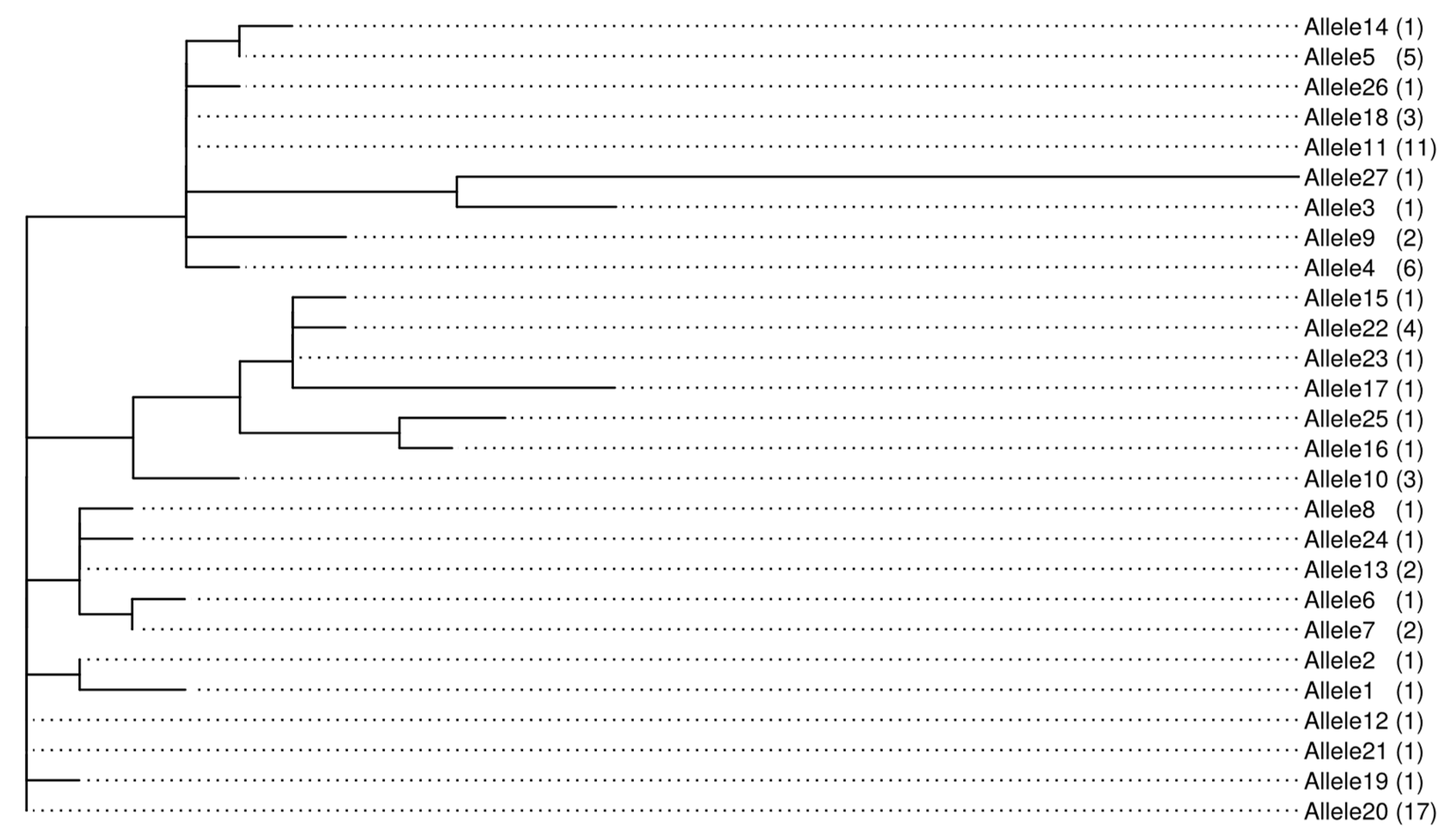
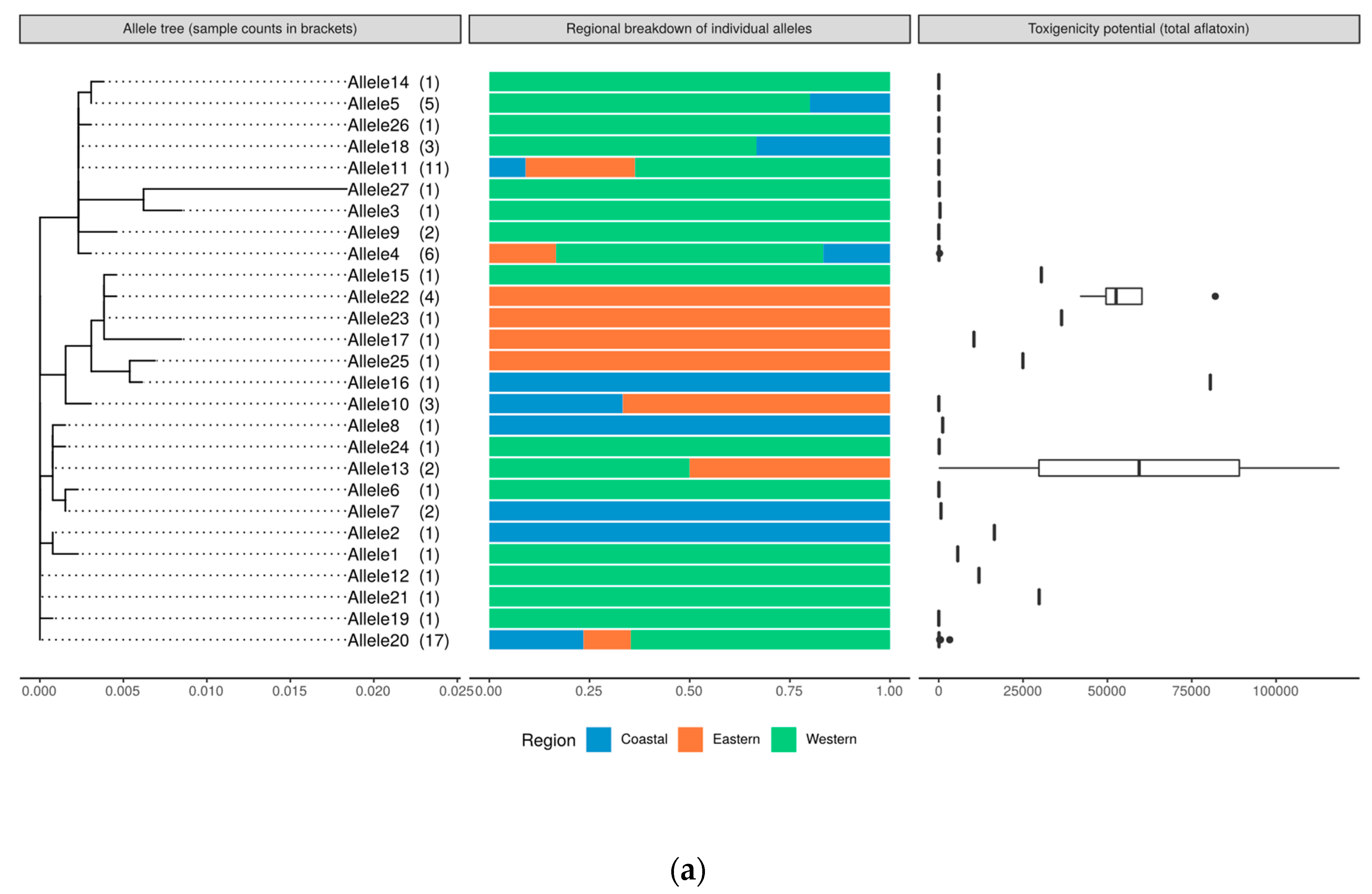
2.8. Genetic Relatedness and Aflatoxin Production Potential of Isolates
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Sites and Survey Design
5.2. Sample Collection
5.3. Isolation and Identification of Aspergillus flavus
5.4. DNA Extraction
5.5. PCR Amplification and Sequencing
5.6. Screening of Aflatoxin Genes
5.7. Aflatoxin Quantification Analysis
5.7.1. Aflatoxigenicity Assay and Extraction of Aflatoxin from Culture Media
5.7.2. Extraction of Aflatoxin in Maize
5.7.3. Analysis Using Ultra High-Performance Liquid Chromatography
5.8. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Okoth, S.; Boevre, M.; de Vidal, A.; Diana Mavungu, J.; Landschoot, S.; Kyallo, M.; Njuguna, J.; Harvey, J.; Saeger, S. De genetic and toxigenic variability within Aspergillus flavus population isolated from maize in two diverse environments in Kenya. Front. Microbiol. 2018, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Ouma, J.O.; de Groote, H. Maize varieties and production constraints: Capturing farmers’ perceptions through participatory rural appraisals (PRAs) in Eastern Kenya. J. Dev. Agric. Econ. 2011, 3, 679–688. [Google Scholar]
- Mutiga, S.K.; Were, V.; Hoffmann, V.; Harvey, J.W.; Milgroom, M.G.; Nelson, R.J. Extent and drivers of mycotoxin contamination: Inferences from a survey of Kenyan maize mills. Phytopathology 2014, 104, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Muthomi, J.W.; Nijega, L.N.; Gathumbi, J.; Chemining’wa, G. The occurrence of aflatoxins in maize and distribution of mycotoxin-producing fungi in Eastern Kenya. Plant Pathol. J. 2009, 8, 113–119. [Google Scholar] [CrossRef]
- Probst, C.; Njapau, H.; Cotty, P.J. Outbreak of an acute aflatoxicosis in Kenya in 2004: Identification of the causal agent. Appl. Env. Microbiol. 2007, 73, 2762–2764. [Google Scholar] [CrossRef] [PubMed]
- Okoth, S. Improving the Evidence Base on Aflatoxin Contamination and Exposure in Africa; CTA: Wageningen, The Netherlands, 2016. [Google Scholar]
- Wagacha, J.M.; Muthomi, J.W. Author’s personal copy mycotoxin problem in Africa: Current status, implications to food safety and health and possible management strategies. Int. J. Food Microbio. 2008, 124, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.; Callicott, K.A.; Cotty, P.J. Deadly strains of Kenyan Aspergillus are distinct from other aflatoxin producers. Eur. J. Plant. Pathol. 2012, 132, 419–429. [Google Scholar] [CrossRef]
- Cotty, P.J.; Cardwell, K.F. Divergence of West African and North American communities of Aspergillus section Flavi. Appl. Env. Microbiol. 1999, 65, 2264–2266. [Google Scholar]
- Maina, A.W.; Wagacha, J.M.; Mwaura, F.B.; Muthomi, J.W.; Woloshuk, C.P. Postharvest practices of maize farmers in Kaiti district, Kenya and the impact of hermetic storage on populations of Aspergillus spp. and aflatoxin contamination. J. Food Res. 2016, 5, 53. [Google Scholar] [CrossRef]
- Pildain, M.B.; Frisvad, J.C.; Vaamonde, G.; Cabral, D.; Varga, J.; Samson, R.A. Two novel aflatoxin-producing Aspergillus species from Argentinean peanuts. Int. J. Syst. Evol. Microbiol. 2008, 58, 725–735. [Google Scholar] [CrossRef]
- Geiser, D.M.; Pitt, J.I.; Taylor, J.W. Cryptic speciation and recombination in the aflatoxin-producing fungus Aspergillus flavus. Proc. Natl. Acad. Sci. USA 1998, 95, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Geiser, D.M.; Dorner, J.W.; Horn, B.W.; Taylor, J.W. The phylogenetics of mycotoxin and sclerotium production in Aspergillus flavus and Aspergillus oryzae. Fungal Genet. Biol. 2000, 31, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Klich, M.A. Aspergillus flavus: The major producer of aflatoxin. Mol. Plant. Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.; Venâncio, A.; Kozakiewicz, Z.; Lima, N. A polyphasic approach to the identification of aflatoxigenic and non-aflatoxigenic strains of Aspergillus section Flavi isolated from Portuguese almonds. Int. J. Food Microbiol. 2009, 129, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Horn, B.W. Biodiversity of Aspergillus section Flavi in the United States: A review. Food Addit. Contam. 2007, 24, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.A.; Varga, J.; Witiak, S.M.; Geiser, D.M. The species concept in Aspergillus: Recommendations of an international panel. Stud. Mycol. 2007, 59, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Balajee, S.A.; Gribskov, J.L.; Hanley, E.; Nickle, D.; Marr, K.A. Aspergillus lentulus sp. nov., a new sibling species of A. fumigatus. Eukaryot. Cell 2005, 4, 625–632. [Google Scholar] [CrossRef]
- Raquel, S.; Cristina, V.; Helena, P.; João, B.; Carla, V.; Elisabete, C.; Karl, V.C.; David, A.S. Molecular screening of 246 Portuguese Aspergillus isolates among different clinical and environmental sources. Med. Mycol. 2014, 52, 519–529. [Google Scholar]
- Yabe, K.; Chihaya, N.; Hamamatsu, S.; Sakuno, E.; Hamasaki, T.; Nakajima, H.; Bennett, J.W. Enzymatic conversion of averufin to hydroxyversicolorone and elucidation of a novel metabolic grid involved in aflatoxin biosynthesis. Appl. Env. Microbiol. 2003, 69, 66–73. [Google Scholar] [CrossRef]
- Yu, J.; Ehrlich, K. Aflatoxin biosynthetic pathway and pathway genes. In Aflatoxins—Biochemistry and Molecular Biology; USDA/ARS, Southern Regional Research Center: New Orlenas, LA, USA, 2011; ISBN 978-953-307-395-8. [Google Scholar]
- Yu, J.; Chang, P.K.; Ehrlich, K.C.; Cary, J.W.; Montalbano, B.; Dyer, J.M.; Bhatnagar, D.; Cleveland, T.E. Characterization of the critical amino acids of an Aspergillus parasiticus cytochrome P-450 monooxygenase encoded by ordA that is involved in the biosynthesis of aflatoxins B1, G1, B2, and G2. Appl. Env. Microbiol. 1998, 64, 4834–4841. [Google Scholar]
- Ehrlich, K.C.; Cary, J.W.; Montalbano, B.G. Characterization of the promoter for the gene encoding the aflatoxin biosynthetic pathway regulatory protein AFLR. Biochim. Biophys. Acta Gene Struct. Expr. 1999, 1444, 412–417. [Google Scholar] [CrossRef]
- Meyers, D.M.; Obrian, G.; Du, W.L.; Bhatnagar, D.; Payne, G.A. Characterization of aflJ, a gene required for conversion of pathway intermediates to aflatoxin. Appl. Env. Microbiol. 1998, 64, 3713–3717. [Google Scholar]
- Geisen, R. Molecular detection and monitoring of fungi in food. In Food Mycology: A Multifaceted Approach to Fungi and Food; Dijksterhuis, J., Samson, R.A., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 255–278. ISBN 9780849398186. [Google Scholar]
- Geisen, R. Multiplex polymerase chain reaction for the detection of potential aflatoxin and sterigmatocystin producing fungi. Syst. Appl. Microbiol. 1996, 19, 388–392. [Google Scholar] [CrossRef]
- Fakruddin, M.; Chowdhury, A.; Hossain, N.; Ahmed, M.M. Characterization of aflatoxin producing Aspergillus flavus from food and feed samples. SpringerPlus 2015, 4, 159. [Google Scholar] [CrossRef] [PubMed]
- Mutiga, S.K.; Hoffmann, V.; Harvey, J.W.; Milgroom, M.G.; Nelson, R.J. Assessment of aflatoxin and fumonisin contamination of maize in western Kenya. Phytopathology 2015, 105, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+ C-content biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar] [PubMed]
- Darnell, R.E.; Harvey, J.J.; Fox, G.P.; Fletcher, M.T.; Wainaina, J.; Wanjuki, I.; Turner, W.J. NIRS calibration of aflatoxin in maize. Aust. J. Chem. 2018. [Google Scholar] [CrossRef]
- Ahmed, N.; Ghose, A.; Agrawal, A.K.; Bhaumik, C.; Chandel, V.; Kumar, A.; Akaike, H.; Arnott, R.; de Palma, A.; Lindsey, R.; et al. Information theory and an extension of the maximum likelihood principle. In Selected Papers of Hirotugu Akaike; Springer Series in Statistics (Perspectives in Statistics); Parzen, E., Tanabe, K., Kitagawa, G., Eds.; Springer: New York, NY, USA, 1998. [Google Scholar]
- Okoth, S.; Nyongesa, B.; Ayugi, V.; Kang, E.; Korhonen, H.; Joutsjoki, V. Toxigenic potential of Aspergillus species occurring on maize kernels from two agro-ecological zones in Kenya. Toxins 2012, 4, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.W.; Geiser, D.M.; Burt, A.; Koufopanou, V. The evolutionary biology and population genetics underlying fungal strain typing. Clin. Microbiol. Rev. 1999, 12, 126–146. [Google Scholar] [CrossRef] [PubMed]
- Vaamonde, G.; Patriarca, A.; Fernández Pinto, V.; Comerio, R.; Degrossi, C. Variability of aflatoxin and cyclopiazonic acid production by Aspergillus section Flavi from different substrates in Argentina. Int. J. Food Microbiol. 2003, 88, 79–84. [Google Scholar] [CrossRef]
- Atehnkeng, J.; Ojiambo, P.S.; Donner, M.; Ikotun, T.; Sikora, R.A.; Cotty, P.J.; Bandyopadhyay, R. Distribution and toxigenicity of Aspergillus species isolated from maize kernels from three agro-ecological zones in Nigeria. Int. J. Food Microbiol. 2008, 122, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Giorni, P.; Magan, N.; Pietri, A.; Bertuzzi, T.; Battilani, P. Studies on Aspergillus section Flavi isolated from maize in northern Italy. Int. J. Food Microbiol. 2007, 113, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi-Abyaneh, M.; Shams-Ghahfarokhi, M.; Allameh, A.; Kazeroon-Shiri, A.; Ranjbar-Bahadori, S.; Mirzahoseini, H.; Rezaee, M.B. A survey on distribution of Aspergillus section Flavi in corn field soils in Iran: Population patterns based on aflatoxins, cyclopiazonic acid and sclerotia production. Mycopathologia 2006, 161, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.G.; Elliott, J.L.; Singh, R.; Horn, B.W.; Dorner, J.W.; Stone, E.A.; Chulze, S.N.; Barros, G.G.; Naik, M.K.; Wright, G.C.; et al. Sexuality generates diversity in the aflatoxin gene cluster: Evidence on a global scale. PLoS Pathog. 2013, 9, e1003574. [Google Scholar] [CrossRef] [PubMed]
- Fountain, J.C.; Bajaj, P.; Pandey, M.; Nayak, S.N.; Yang, L.; Kumar, V.; Jayale, A.S.; Chitikineni, A.; Zhuang, W.; Scully, B.T.; et al. Oxidative stress and carbon metabolism influence Aspergillus flavus transcriptome composition and secondary metabolite production. Sci. Rep. 2016, 6, 38747. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, J.D.; O’Keeffe, T.L.; Abbas, H.K. Microbial interactions with mycotoxigenic fungi and mycotoxins. Toxin Rev. 2008, 27, 261–285. [Google Scholar] [CrossRef]
- Mutiga, S.K.; Morales, L.; Angwenyi, S.; Wainaina, J.; Harvey, J.; Das, B.; Nelson, R.J. Field crops research association between agronomic traits and aflatoxin accumulation in diverse maize lines grown under two soil nitrogen levels in Eastern Kenya. Field Crop. Res. 2017, 205, 124–134. [Google Scholar] [CrossRef]
- Yin, Y.N.; Yan, L.Y.; Jiang, J.H.; Ma, Z.H. Biological control of aflatoxin contamination of crops. J. Zhejiang Univ. Sci. B 2008, 9, 787–792. [Google Scholar] [CrossRef]
- Levin, R.E. PCR detection of aflatoxin producing fungi and its limitations. Int. J. Food Microbiol. 2012, 156, 1–6. [Google Scholar] [CrossRef]
- Nyongesa, B.W.; Okoth, S.; Ayugi, V. Identification key for Aspergillus species isolated from maize and soil of Nandi. Adv. Microbiol. 2015, 5, 205–229. [Google Scholar] [CrossRef]
- Cochran, W.G. Sampling Techniques, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1999; Volume 3, ISBN 0-471-16240-X. [Google Scholar]
- Food and Agriculture Organization. Grain Storage Techniques—Evolution and Trends in Developing Countries; Proctor, D.L., Ed.; FAO Agricu Food and Agriculture Organization of the United Nations (FAO) Rome, 1994: Rome, Italy, 2011; ISBN 9251034567. [Google Scholar]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 2nd ed.; Blackie Academic & Professional: London, UK; New York, NY, USA, 1997; ISBN 0412554607. [Google Scholar]
- Klich, M.A. Identification of Common Aspergillus Species; Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2002. [Google Scholar]
- Jurjević, Ž.; Kubátová, A.; Kolařík, M.; Hubka, V. Taxonomy of Aspergillus section petersonii sect. nov. encompassing indoor and soil-borne species with predominant tropical distribution. Plant. Syst. Evol. 2015, 301, 2441–2462. [Google Scholar]
- Tam, E.W.T.; Chen, J.H.K.; Lau, E.C.L.; Ngan, A.H.Y.; Fung, K.S.C.; Lee, K.; Lam, C. Misidentification of Aspergillus nomius and Aspergillus tamarii as Aspergillus flavus: Characterization by internal transcribed spacer, β-tubulin, and calmodulin gene sequencing, metabolic of flight mass spectrometry. J. Clin. Microbiol. 2014, 52, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.J.; Pàmies, P.; Dobson, A.D. The use of reverse transcription-polymerase chain reaction (RT-PCR) for monitoring aflatoxin production in Aspergillus parasiticus 439. Int. J. Food Microbiol. 2000, 56, 97–103. [Google Scholar] [CrossRef]
- Davis, N.D.; Diener, U.L.; Eldridge, D.W. Production of Aflatoxins B1 and G1 by Aspergillus flavus in a semisynthetic medium. Appl. Env. Microbiol. 1966, 14, 378–380. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets brief communication. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview version 2-A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [PubMed]



| Nucleotide Position in Calmodulin Sequence | 39 | 144 | 424 | 466 | 691 | 701 |
|---|---|---|---|---|---|---|
| A. minisclerotigenes | A(3)/G(6) | G | G | G(7)/C(2) | A(4)/G(5) | A |
| A. flavus | G | A(60)/G(3) | A | C | A | T |
| Gene Effect | Magnitude of Gene Effect | Std Error of the Effect | t-Value | Significance p-Value |
|---|---|---|---|---|
| aflD’ | −0.8736 | 0.4439 | −1.968 | 0.053 |
| aflD” | 3.4093 | 1.162 | 2.934 | 0.005 |
| aflM | −0.239 | 0.6628 | −0.361 | 0.720 |
| aflR | 0.4392 | 0.7546 | 0.582 | 0.563 |
| aflS | 2.4061 | 0.7943 | 3.029 | 0.004 |
| aflD” and aflM | −2.109 | 1.4374 | −1.467 | 0.147 |
| aflD” and aflR | −2.2789 | 1.0133 | −2.249 | 0.028 |
| Intercept | 1.4965 | 0.423 | 3.538 | 0.001 |
| Target Gene | Primer Code | Primer DNA Sequence | Size (bp) | Reference |
|---|---|---|---|---|
| ITS | ITS 1F | 5′-CTTGGTCATTTAGAGGAAGTAA-3′ | 595 | [49] |
| ITS4 | 5′-TCCTCCGCTTATTGATATGC-3′ | |||
| CaM | CF1M | 5′-AGGCCGAYTCTYTGACYGA-3′ | 700 | [50] |
| CF4 | 5′-TTTYTGCATCATRAGYTGGAC-3′ | |||
| AflD | AflD-1 | 5′-CACTTAGCCATCACGGTCA-3′ | 852 | [27] |
| AflD-2rev | 5′-GAGTTGAGATCCATCCGTG-3′ | |||
| Nor1-F | 5′-ACCGCTACGCCGGCACTCTCGGCAC-3′ | 400 | [15] | |
| Nor1-R | 5′-GTTGGCCGCCAGCTTCGACACTCCG -3′ | |||
| AflM | AflM-1 | 5′-AAGTTAATGGCGGAGACG-3′ | 470 | [27] |
| AflM-2rev | 5′-TCTACCTGCTCATCGGTGA-3′ | |||
| AflO | AflO-1 | 5′-TCCAGAACAGACGATGTGG-3′ | 790 | [27] |
| AflO-2rev | 5′-CGTTGGCTAGAGTTTGAGG-3′ | |||
| AflP | AflP-1 | 5′-AGCCCCGAAGACCATAAAC-3′ | 870 | [27] |
| AflP-2rev | 5′-CCGAATGTCATGCTCCATC-3′ | |||
| AflQ | AflQ-1 | 5′-TCGTCCTTCCATCCTCTTG-3′ | 757 | [27] |
| AflQ-2rev | 5′-ATGTGAGTAGCATCGGCATTC-3′ | |||
| Ord1-gF | 5′-TTA AGG CAG CGG AAT ACA AG-3′ | 719 | [51] | |
| Ord1-gR | 5′-GAC GCC CAA AGC CGA ACA CAA A-3′ | |||
| AflR | AflR-1 | 5′-AAGCTCCGGGATAGCTGTA-3′ | 1079 | [27] |
| AflR-2rev | 5′-AGGCCACTAAACCCGAGTA-3′ | |||
| AflS | AflS-1 | 5′-TGAATCCGTACCCTTTGAGG-3′ | 684 | [27] |
| AflS-2rev | 5′-GGAATGGGATGGAGATGAGA-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dooso Oloo, R.; Okoth, S.; Wachira, P.; Mutiga, S.; Ochieng, P.; Kago, L.; Nganga, F.; Domelevo Entfellner, J.-B.; Ghimire, S. Genetic Profiling of Aspergillus Isolates with Varying Aflatoxin Production Potential from Different Maize-Growing Regions of Kenya. Toxins 2019, 11, 467. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11080467
Dooso Oloo R, Okoth S, Wachira P, Mutiga S, Ochieng P, Kago L, Nganga F, Domelevo Entfellner J-B, Ghimire S. Genetic Profiling of Aspergillus Isolates with Varying Aflatoxin Production Potential from Different Maize-Growing Regions of Kenya. Toxins. 2019; 11(8):467. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11080467
Chicago/Turabian StyleDooso Oloo, Richard, Sheila Okoth, Peter Wachira, Samuel Mutiga, Phillis Ochieng, Leah Kago, Fredrick Nganga, Jean-Baka Domelevo Entfellner, and Sita Ghimire. 2019. "Genetic Profiling of Aspergillus Isolates with Varying Aflatoxin Production Potential from Different Maize-Growing Regions of Kenya" Toxins 11, no. 8: 467. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11080467







