Paralytic Shellfish Toxin Uptake, Assimilation, Depuration, and Transformation in the Southeast Asian Green-Lipped Mussel (Perna viridis)
Abstract
:1. Introduction
2. Results
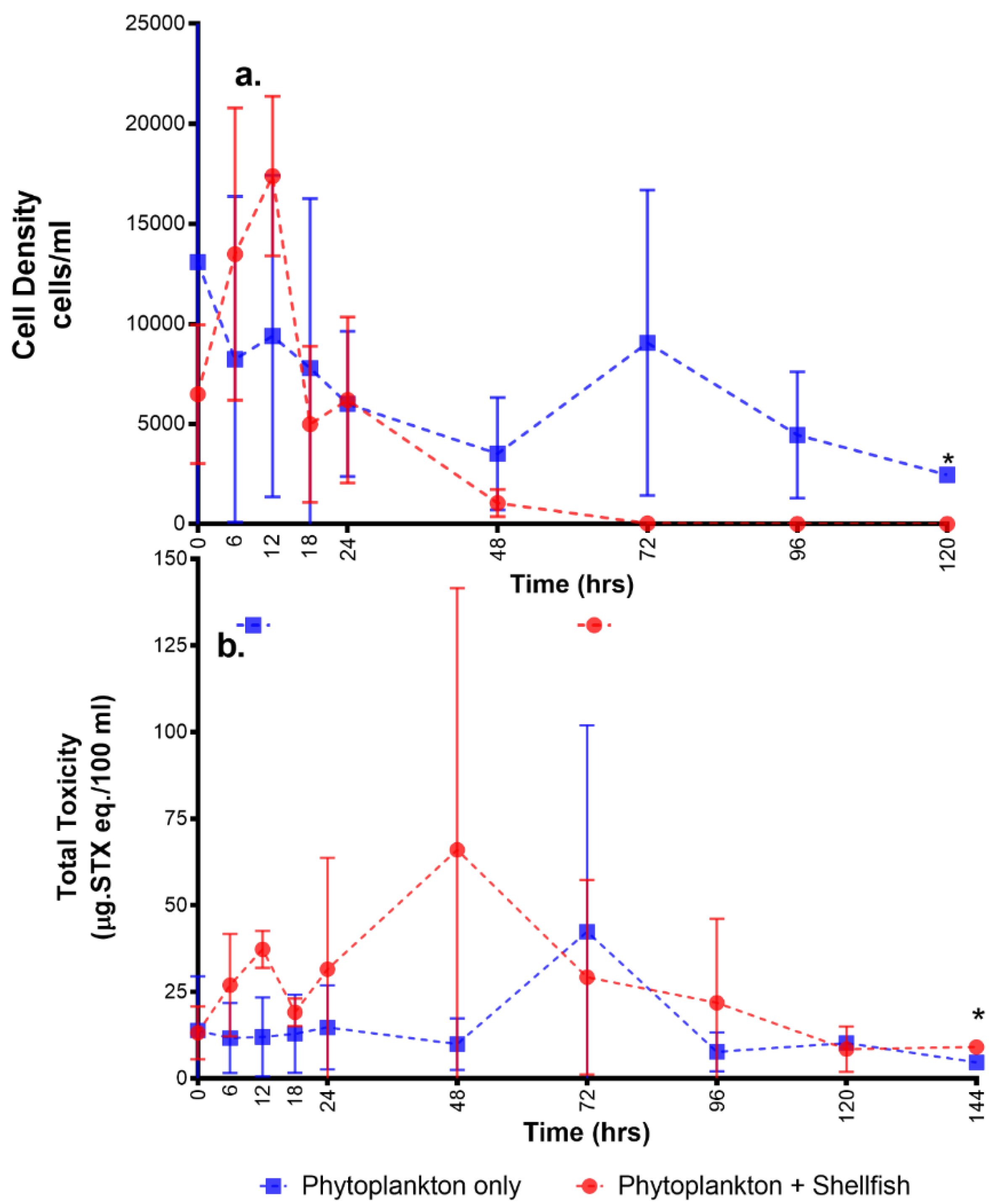
2.1. Overall Toxicity, Uptake, and Depuration
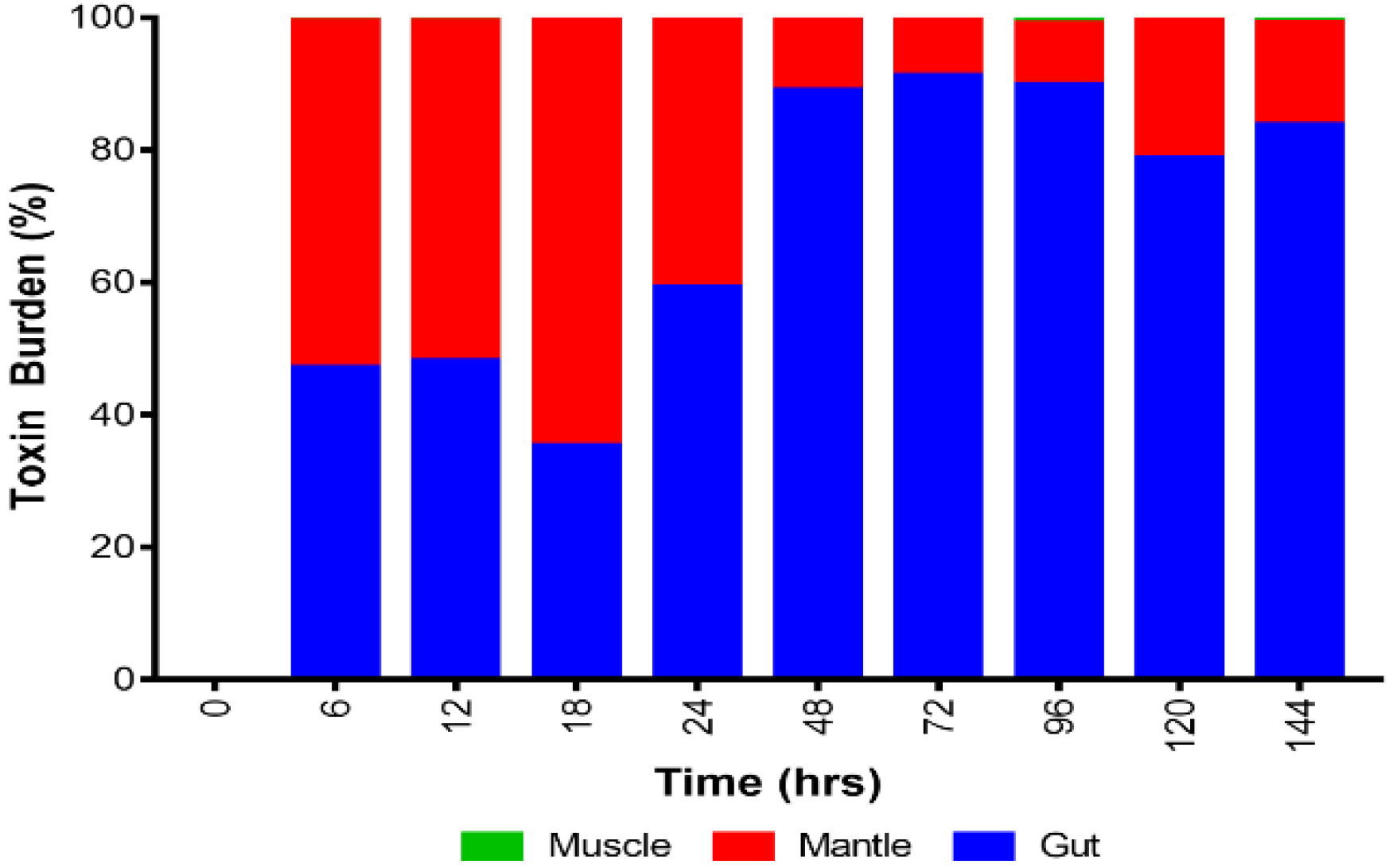
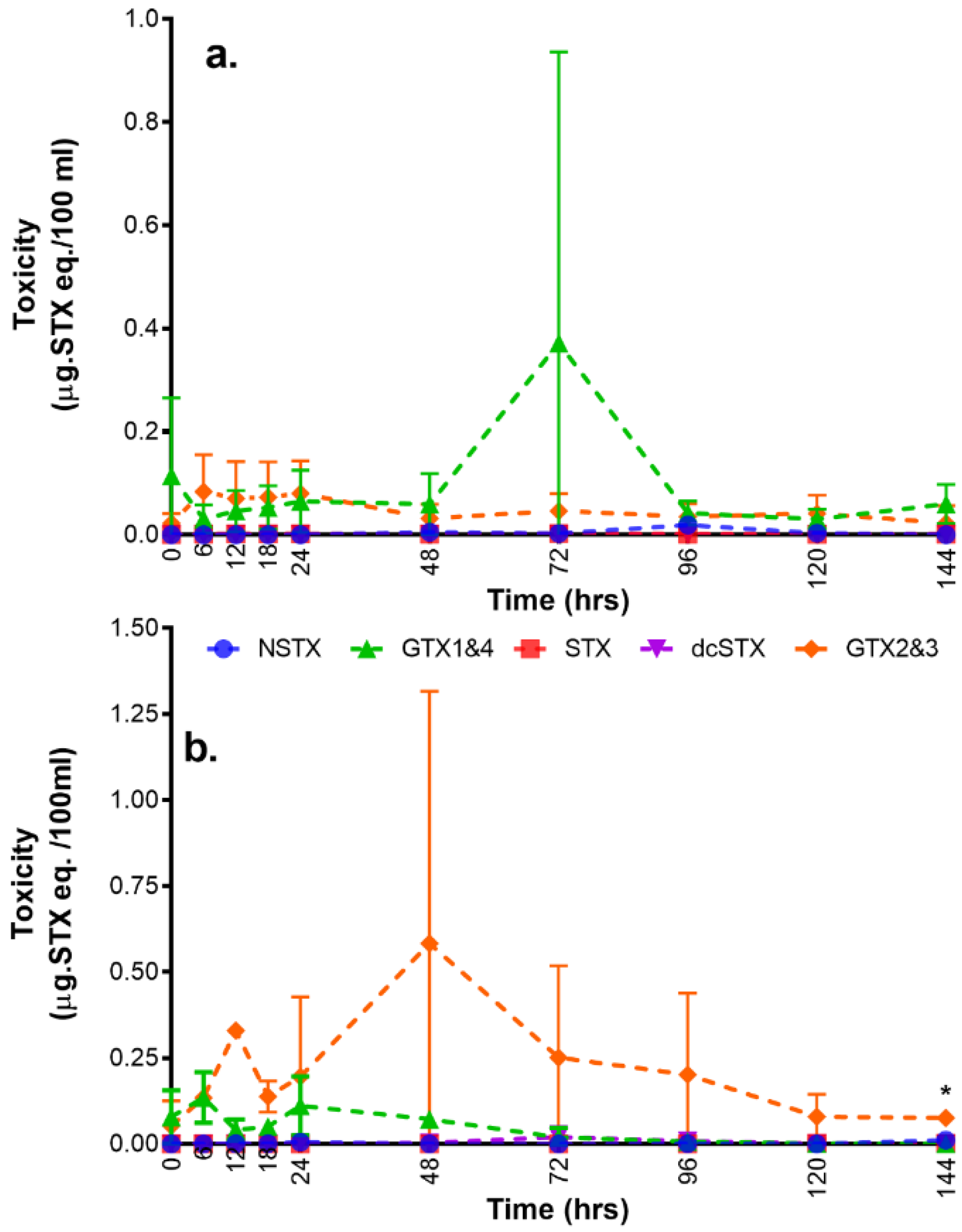
2.2. Toxin Compartmentalisation
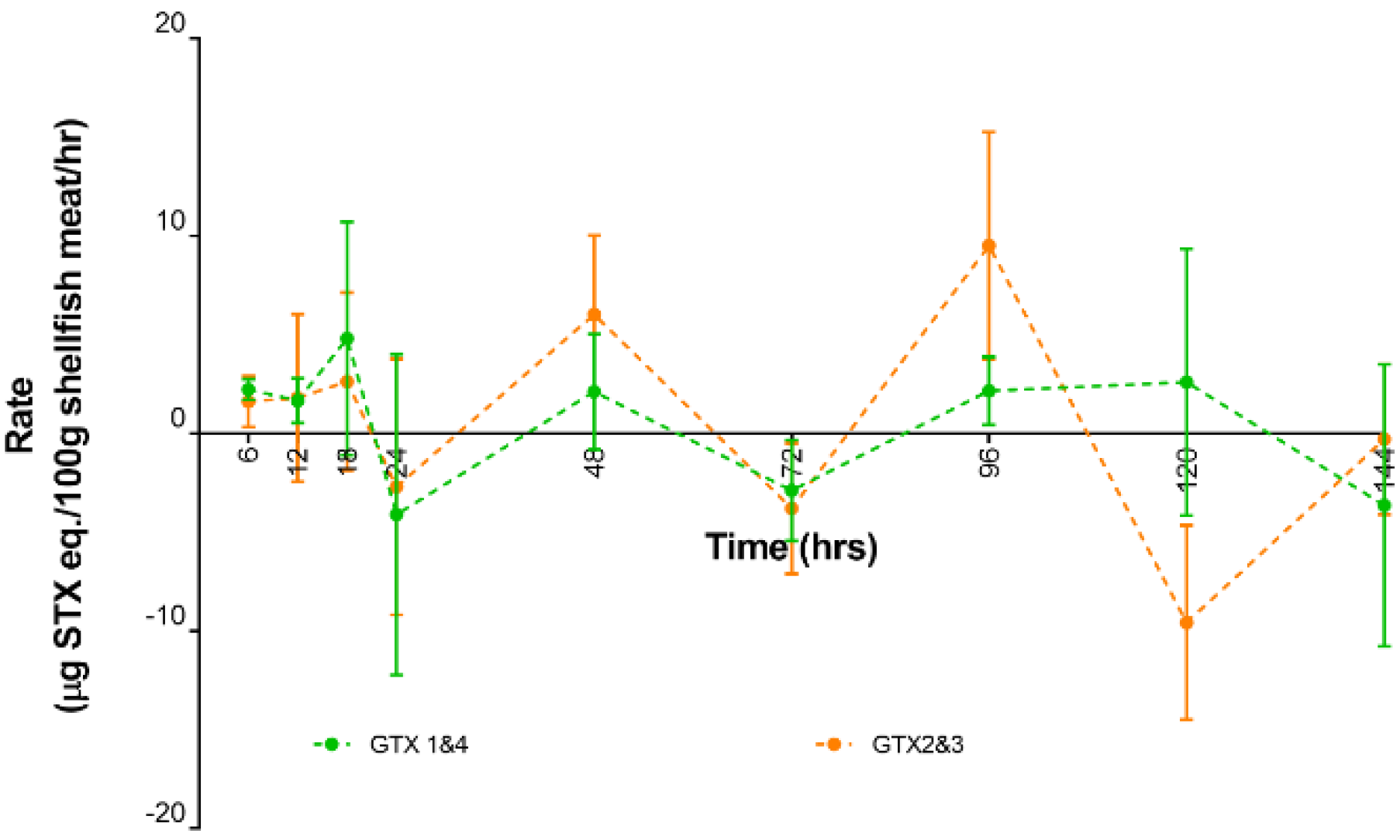
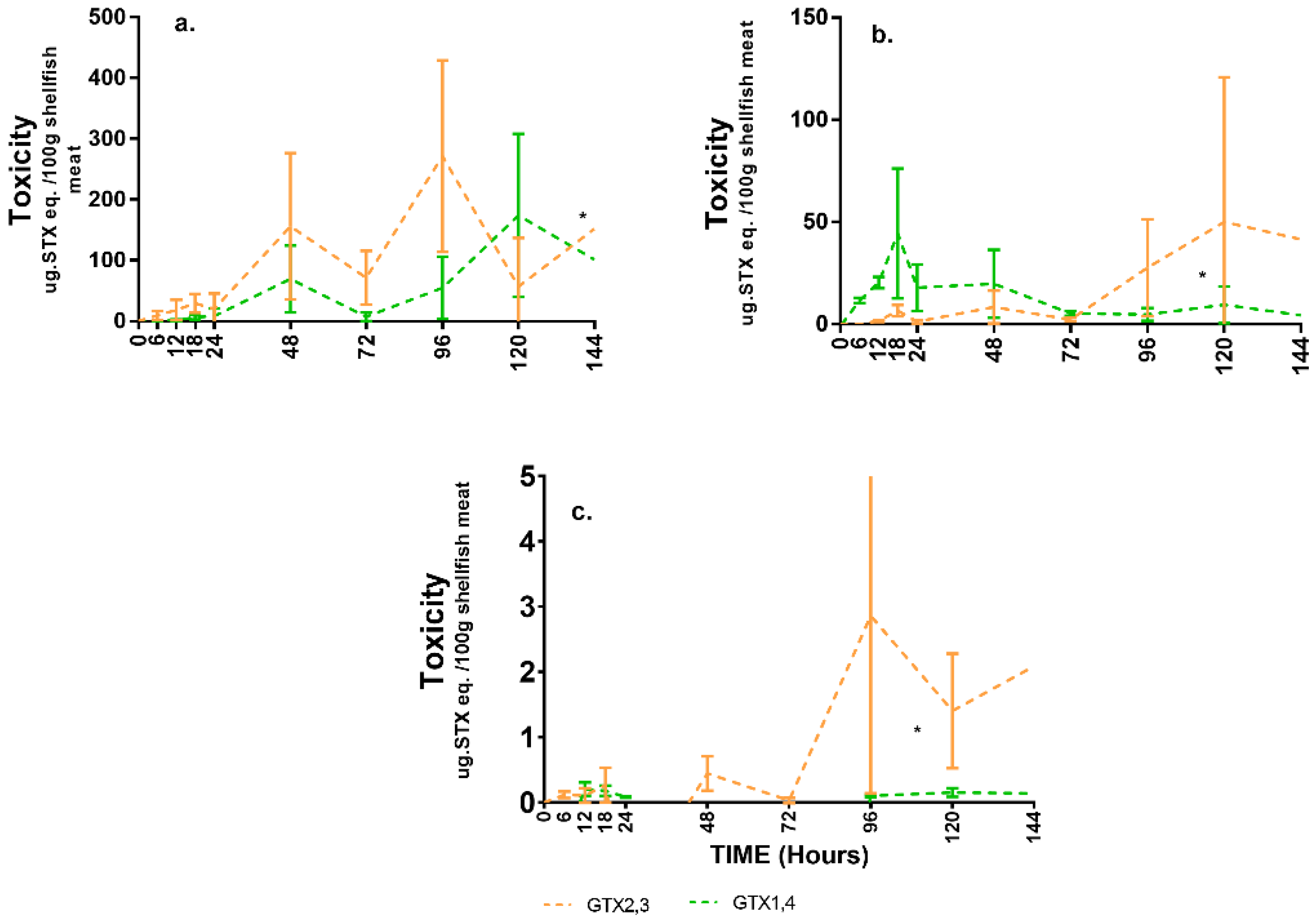
2.3. Toxin Types and Their Distributions
3. Discussion
3.1. Uptake and Depuration Patterns
3.2. Toxin Distribution within Shellfish
3.3. Toxin Transformation
4. Conclusions
5. Materials and Methods
5.1. Cell Cultures
5.2. Acclimation
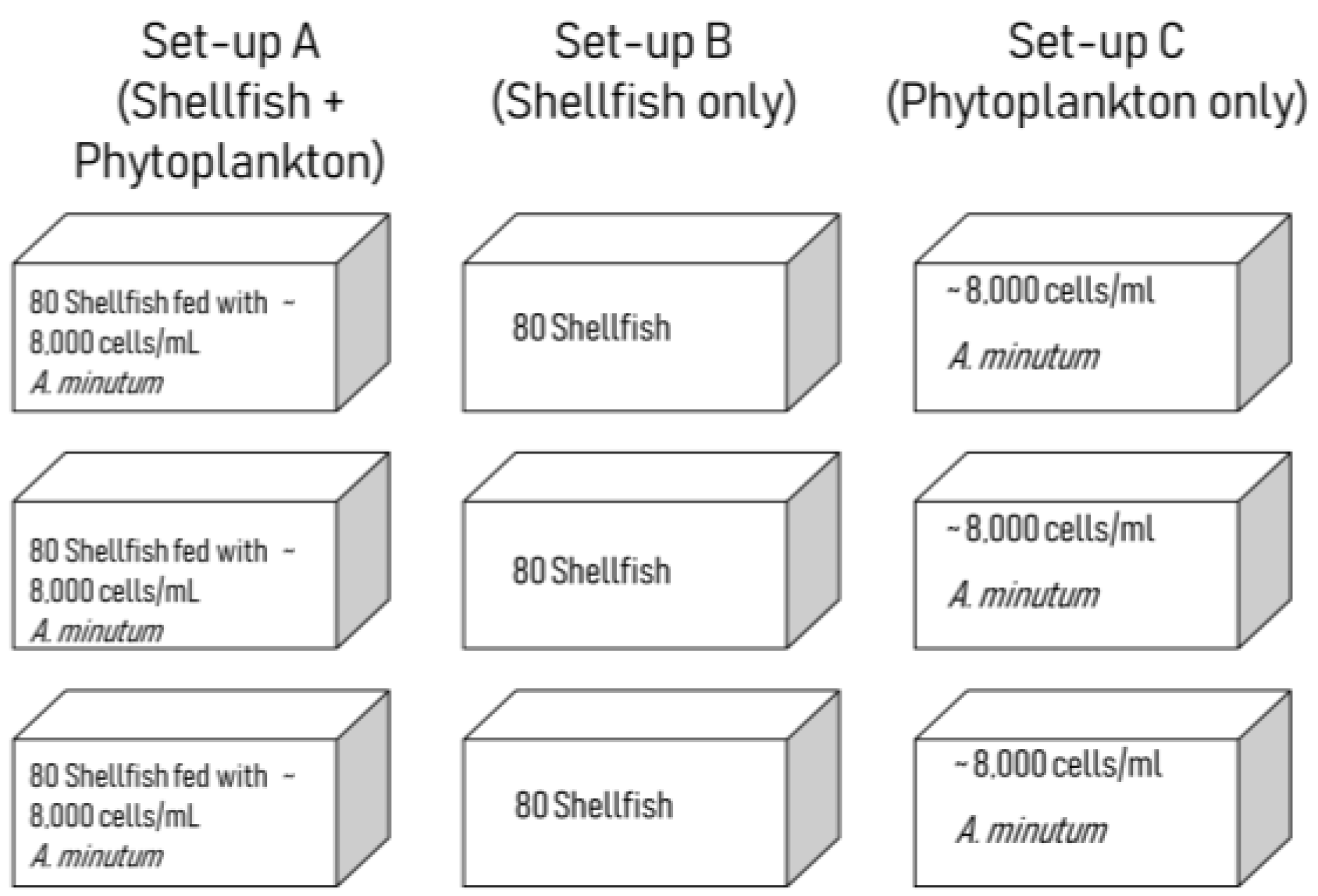
5.3. Grazing Experiment
5.4. Toxin Analysis and Cell Counts
5.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mak, K.C.; Li, A.M.; Hsieh, D.P.; Wong, P.S.; Lam, M.H.; Wu, R.S.; Richardson, B.J.; Lam, P.K. Paralytic shellfish toxins in green-lipped mussels, Perna viridis, in Hong Kong. Mar. Pollut. Bull. 2003, 46, 258–268. [Google Scholar] [CrossRef]
- Ding, L.; Qiu, J.; Li, A. Proposed biotransformation pathways of new metabolites of paralytic shellfish toxins based in field and experimental mussel samples. J. Agric. Food Chem. 2017, 65, 5494–5502. [Google Scholar] [CrossRef] [PubMed]
- Kwong, R.W.; Wang, W.X.; Lam, P.K.; Peter, K.N. The uptake, distribution and elimination of paralytic shellfish toxins in mussels and fish exposed to toxic dinoflagellates. Aquat. Toxicol. 2006, 80, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.N.; Choi, M.C.; Shen, X.; Wu, R.; Wang, W.; Lam, P. Modeling of depuration of paralytic shellfish toxins in Chlamys nobilis and Perna viridis. Mar. Pollut. Bull. 2005, 50, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Bricelj, V.M.; Shumway, S. Paralytic shellfish toxins in bivalve molluscs: Occurrence, transfer kinetics, and biotransformation. Rev. Fish. Sci. 1998, 6, 315–383. [Google Scholar] [CrossRef]
- Montojo, U.; Sakamoto, S.; Cayme, M.; Gatdula, N.; Furio, E.; Relox, J.; Sato, S.; Fukuyo, Y.; Kodama, M. Remarkable difference in accumulation of paralytic shellfish toxins among bivalve toxins exposed to Pyrodinium bahamense var. compressum bloom in Masinloc Bay, Philippines. Toxicon 2006, 48, 85–92. [Google Scholar] [CrossRef]
- Li, A.M.; Yu, P.K.; Hsieh, D.P.; Wang, W.-X.; Wu, R.S.; Lam, P.K. Uptake and depuration of paralytic shellfish toxins in green-lipped mussel, Perna viridis: A dynamic model. Environ. Toxicol. Chem. 2005, 24, 129–135. [Google Scholar] [CrossRef]
- Choi, M.-C.; Hsieh, D.P.H.; Lam, P.K.S.; Wang, W.-X.; Lam, K.S.P.; Wang, W. Field depuration and biotransformation of paralytic shellfish toxins in scallop Chlamys nobilis and green-lipped mussel Perna viridis. Mar. Biol. 2003, 143, 927–934. [Google Scholar] [CrossRef]
- Guéguen, M.; Baron, R.; Bardouil, M.; Truquet, P.; Haberkorn, H.; Lassus, P.; Barillé, L.; Amzil, Z. Modelling of paralytic shellfish toxin biotransformation in the course of Crassostrea gigas detoxification kinetics. Ecol. Model. 2011, 222, 3394–3402. [Google Scholar] [CrossRef]
- Contreras, A.; Marsden, I.; Munro, M. Effects of short-term exposure to paralytic shellfish toxins on clearance rates and toxin uptake in five species of New Zealand bivalve. Mar. Freshw. Res. 2011, 63, 166–174. [Google Scholar] [CrossRef]
- Wong, W.H.; Cheung, S.G. Feeding behaviour of the green mussel, Perna viridis (L.): Responses to variation in seston quantity and quality. J. Exp. Mar. Biol. Ecol. 1999, 236, 191–207. [Google Scholar] [CrossRef]
- Costa, P.R.; Baugh, K.A.; Wright, B.; Ralonde, R.; Nance, S.L.; Tatarenkova, N.; Etheridge, S.M.; Lefebvre, K.A. Comparative determination of paralytic shellfish toxins (PSTs) using five different toxin detection methods in shellfish species collected in the Aleutian Islands, Alaska. Toxicon 2009, 54, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Van De Riet, J.; Gibbs, R.S.; Muggah, P.M.; Rourke, W.A.; MacNeil, J.D.; Quilliam, M.A. Liquid Chromatography Post-Column Oxidation (PCOX) Method for the Determination of Paralytic Shellfish Toxins in Mussels, Clams, Oysters, and Scallops: Collaborative Study. J. AOAC Int. 2011, 94, 1154–1176. [Google Scholar] [PubMed]
- Turner, A.D.; Hatfield, R.G.; Maskrey, B.H.; Algoet, M.; Lawrence, J.F. Evaluation of the New European Union Reference Method for Paralytic Shellfish Toxins in Shellfish: A Review of Twelve Years Regulatory Monitoring Using Pre-Column Oxidation LC-FLD. Trends Anal. Chem. 2019, 113, 124–139. [Google Scholar] [CrossRef]
- Turner, A.D.; McNabb, P.S.; Harwood, D.T.; Selwood, A.I.; Boundy, M.J. Single laboratory validation of a multitoxin LC-hydrophilic interaction LC-MS/MS method for quantitation of Paralytic Shellfish Toxins in bivalve shellfish. J. AOAC Int. 2015, 98, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, W.; Hsieh, D. Feeding and absorption of the toxic dinoflagellate Alexandrium tamarense by two marine bivalves from the South. China Sea. Mar. Biol. 2001, 139, 617–624. [Google Scholar] [CrossRef]
- Smith, E.A.; Grant, F.; Ferguson, C.M.J.; Gallacher, S. Biotransformations of paralytic shellfish toxins by bacteria isolated from bivalve molluscs. Appl. Environ. Microbiol. 2001, 67, 2345–2353. [Google Scholar] [CrossRef] [PubMed]
- Persson, A.; Smith, B.C.; Wikfors, G.H.; Quilliam, M. Grazing on toxic Alexandrium fundyense resting cysts and vegetative cells by the eastern oyster (Crassostrea virginica). Harmful Algae 2006, 5, 678–684. [Google Scholar] [CrossRef]
- Sombrito, E.; Honrado, M.; de Vera, A.; Tabbada, R.; Rañada, M.L.; Relox, J., Jr.; Tangonan, M.D. Use of Perna viridis as a Bioindicator of Paralytic Shellfish Toxins at Low Pyrodinium bahamense var compressum Density using a Radioreceptor Assay. Environ. Bioindic. 2007, 2, 264–272. [Google Scholar] [CrossRef]
- Ji, Y.; Qiu, J.; Xie, T.; McCarron, P.; Li, A. Accumulation and transformation of azaspiracids in scallops (Chlamys nobilis) and mussels (Mytilus galloprovincialis) fed with Azadinium popurum, and response of antioxidant enzymes. Toxicon 2018, 143, 20–28. [Google Scholar] [CrossRef]
- Asakawa, M.; Beppu, R.; Ito, K.; Tsubota, M.; Takayama, H.; Miyazawa, K. Accumulation of paralytic shellfish poison (PSP)and biotransformation of its components in oysters Crassostrea gigas fed with the toxic dinoflagellate Alexandrium tamarense. Shokuhin Eiseigaku Zasshi 2005, 47, 28–32. [Google Scholar] [CrossRef]
- Botelho, M.J.; Vale, C.; Mota, A.; Simoes-Goncalves, M. Depuration kinetics of paralytic shellfish toxins in Mytilus galloprovincialis exposed to Gymnodinium catenatum: Laboratory and field experiments. J. Environ. Monit. 2010, 12, 2269–2275. [Google Scholar] [CrossRef] [PubMed]
- Homan, N.; Hallegraeff, G.; Van Ruth, R.; Van Ginkel, P.; McNabb, P.; Kiermeier, A.; Deveney, M.; McLeod, C. Uptake, Distribution and Depuration of Paralytic Shellfish Toxins in Australian Greenlip Abalone, Haliotis laevigata; Australian Seafood Cooperative Research Centre: Deakin, Australia, 2010; pp. 1–26. [Google Scholar]
- Lewis, A.M.; Coates, L.N.; Turner, A.D.; Percy, L.; Lewis, J. A review of the global distribution of Alexandrium minutum (Dinophyceae) and comments on ecology and associated paralytic shellfish toxin profiles, with a focus on Northern Europe. J. Phycol. 2018, 54, 581–598. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.N.P.; Kwong, R.W.; Wang, W.-X.; Lam, P.K.; Lam, K.S.P. Biokinetics of paralytic shellfish toxins in the green-lipped mussel Perna viridis. Mar. Pollut. Bull. 2007, 54, 1031–1071. [Google Scholar] [CrossRef] [PubMed]
- Manfrin, C.; de Moro, G.; Torboli, V.; Venier, P.; Pallavacini, A.; Gerdol, M. Physiological and molecular responses of bivalves to toxic dinoflagellates. Invertebr. Surviv. J. 2012, 9, 184–199. [Google Scholar]
- Ichimi, K.; Suzuki, T.; Yamasaki, M. Non-selective retention of PSP toxins by the mussel Mytilus galloprovincialis fed with the toxic dinoflagellate Alexandrium tamarense. Toxicon Off. J. Int. Soc. Toxinol. 2002, 39, 1917–1921. [Google Scholar] [CrossRef]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic Alkaloids: Saxitoxin and Its Analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaime, E.; Gerdts, G.; Luckas, B. In vitro transformation of PSP toxins by different shellfish tissues. Harmful Algae 2007, 6, 308–316. [Google Scholar] [CrossRef]
- Yñiguez, A.T.; Maister, J.; Villanoy, C.L.; Deauna, J.D.; Peñaflor, E.; Almo, A.; David, L.T.; Benico, G.A.; Hibay, E.; Mora, I.; et al. Insights into the dynamics of harmful algal blooms in a tropical estuary through an integrated hydrodynamic-Pyrodinium-shellfish model. Harmful Algae 2018, 80, 1–14. [Google Scholar] [CrossRef]
- Kudela, R. Passive sampling for freshwater and marine algal toxins. Compr. Anal. Chem. 2017, 78, 379–409. [Google Scholar]
- Lawrence, J.; Menard, C. Determination of marine toxins by liquid chromatography. J. Anal. Chem. 1991, 339, 494–498. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G. Official Methods of Analysis, 18th ed.; Method 2005.06; AOAC INTERNATIONAL: Gaithersburg, MD, USA, 2005; Chapter 49. [Google Scholar]
- Andres, J.K. Saxitoxin Uptake, Assimilation, Depuration, and Analogue Changes in Perna viridis (Linnaeus). Unpublished Master’s Thesis, University of the Philippines Diliman, Quezon City, Philippines, 2019. [Google Scholar]

| Time (h) | NeoSTX | STX | dcSTX | GTX 1,4 | GTX 2,3 |
|---|---|---|---|---|---|
| 0 | n.d. | n.d. | n.d. | 0 ± 0.14 | 0 ± 0.04 |
| 6 | n.d. | n.d. | n.d. | 12.15 ± 3.05 | 9.71 ± 7.82 |
| 12 | n.d. | 0.60 ± 0.68 | n.d. | 22.14 ± 3.90 | 20.55 ± 17.57 |
| 18 | n.d. | 15.82 ± 14.45 | 0.10 ± 0.02 | 50.98 ± 31.95 | 36.30 ± 13.30 |
| 24 | n.d. | 0.78 ± 0.13 | 0.02 ± 0.03 | 26.37 ± 23.53 | 20.07 ± 28.44 |
| 48 | n.d. | 0.91 ± 0.66 | 0.01 ± 0.02 | 77.13 ± 70.38 | 164.9 ± 120.4 |
| 72 | n.d. | 0.50 ± 0.15 | 0.02 ± 0.02 | 8.05 ± 15.54 | 73.76 ± 44.09 |
| 96 | n.d. | 4.81 ± 1.81 | n.d. | 59.86 ± 54.36 | 302.1 ± 155.7 |
| 120 | n.d. | 2.82 ± 1.74 | n.d. | 183.6 ± 146.6 | 108.4 ± 62.92 |
| 144 | n.d. | 4.27 ± 2.47 | n.d. | 105.9 ± 61.12 | 195.5 ± 112.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andres, J.K.; Yñiguez, A.T.; Maister, J.M.; Turner, A.D.; Olano, D.E.B.; Mendoza, J.; Salvador-Reyes, L.; Azanza, R.V. Paralytic Shellfish Toxin Uptake, Assimilation, Depuration, and Transformation in the Southeast Asian Green-Lipped Mussel (Perna viridis). Toxins 2019, 11, 468. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11080468
Andres JK, Yñiguez AT, Maister JM, Turner AD, Olano DEB, Mendoza J, Salvador-Reyes L, Azanza RV. Paralytic Shellfish Toxin Uptake, Assimilation, Depuration, and Transformation in the Southeast Asian Green-Lipped Mussel (Perna viridis). Toxins. 2019; 11(8):468. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11080468
Chicago/Turabian StyleAndres, John Kristoffer, Aletta T. Yñiguez, Jennifer Mary Maister, Andrew D. Turner, Dave Eldon B. Olano, Jenelyn Mendoza, Lilibeth Salvador-Reyes, and Rhodora V. Azanza. 2019. "Paralytic Shellfish Toxin Uptake, Assimilation, Depuration, and Transformation in the Southeast Asian Green-Lipped Mussel (Perna viridis)" Toxins 11, no. 8: 468. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11080468





