TMA, A Forgotten Uremic Toxin, but Not TMAO, Is Involved in Cardiovascular Pathology
Abstract
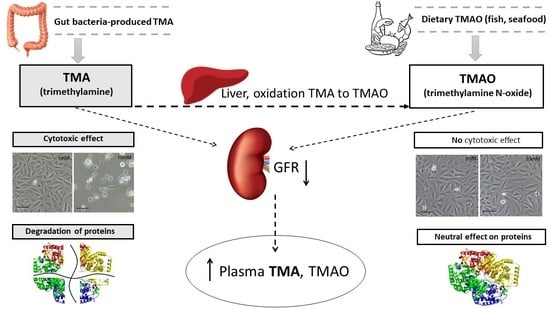
:1. Introduction
2. Results
2.1. Plasma TMA and TMAO in Healthy Humans and Cardiovascular Patients
2.2. The Effect of TMA and TMAO on Cardiomyocytes Viability (MTT Assay)
2.3. The Effect of TMA and TMAO on Protein Structure
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plasma TMA and TMAO Level in Healthy Humans and Cardiovascular Patients
5.2. The Effect of TMA and TMAO on Cardiomyocytes In Vitro
5.2.1. Cells and Treatment
5.2.2. Metabolic Activity Assay in Cardiomyocytes (MTT Assay)
5.3. The Effect of TMA and TMA on Protein Structure
5.4. Evaluation of TMA and TMAO Plasma Concentrations
5.5. Data Analysis and Statistics
Author Contributions
Funding
Conflicts of Interest
References
- Tang, W.H.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Troseid, M.; Ueland, T.; Hov, J.R.; Svardal, A.; Gregersen, I.; Dahl, C.P.; Aakhus, S.; Gude, E.; Bjorndal, B.; Halvorsen, B.; et al. Microbiota-Dependent metabolite trimethylamine-N-Oxide is associated with disease severity and survival of patients with chronic heart failure. J. Intern. Med. 2015, 277, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Li, X.S.; Obeid, S.; Klingenberg, R.; Gencer, B.; Mach, F.; Raber, L.; Windecker, S.; Rodondi, N.; Nanchen, D.; Muller, O.; et al. Gut microbiota-Dependent trimethylamine N-Oxide in acute coronary syndromes: A prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur. Heart J. 2017, 38, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Senthong, V.; Wang, Z.; Fan, Y.; Wu, Y.; Hazen, S.L.; Tang, W.H. Trimethylamine N-Oxide and Mortality Risk in Patients with Peripheral Artery Disease. J. Am. Heart Assoc. 2016, 5, 4237. [Google Scholar] [CrossRef]
- Qi, J.; You, T.; Li, J.; Pan, T.; Xiang, L.; Han, Y.; Zhu, L. Circulating trimethylamine N-Oxide and the risk of cardiovascular diseases: A systematic review and meta-Analysis of 11 prospective cohort studies. J. Cell. Mol. Med. 2018, 22, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Liao, S.X.; He, Y.; Wang, S.; Xia, G.H.; Liu, F.T.; Zhu, J.J.; You, C.; Chen, Q.; Zhou, L.; et al. Dysbiosis of Gut Microbiota with Reduced Trimethylamine-N-Oxide Level in Patients with Large-Artery Atherosclerotic Stroke or Transient Ischemic Attack. J. Am. Heart Assoc. 2015, 4, 2699. [Google Scholar] [CrossRef] [PubMed]
- Fukami, K.; Yamagishi, S.; Sakai, K.; Kaida, Y.; Yokoro, M.; Ueda, S.; Wada, Y.; Takeuchi, M.; Shimizu, M.; Yamazaki, H.; et al. Oral L-Carnitine supplementation increases trimethylamine-N-Oxide but reduces markers of vascular injury in hemodialysis patients. J. Cardiovasc. Pharmacol. 2015, 65, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.A.; Benton, T.Z.; Bennett, B.J.; Jacobs, D.R., Jr.; Lloyd-Jones, D.M.; Gross, M.D.; Carr, J.J.; Gordon-Larsen, P.; Zeisel, S.H. Microbiota-Dependent Metabolite Trimethylamine N-Oxide and Coronary Artery Calcium in the Coronary Artery Risk Development in Young Adults Study (CARDIA). J. Am. Heart Assoc. 2016, 5, 3970. [Google Scholar] [CrossRef]
- Shafi, T.; Powe, N.R.; Meyer, T.W.; Hwang, S.; Hai, X.; Melamed, M.L.; Banerjee, T.; Coresh, J.; Hostetter, T.H. Trimethylamine N-Oxide and Cardiovascular Events in Hemodialysis Patients. J. Am. Soc. Nephrol. 2017, 28, 321–331. [Google Scholar] [CrossRef]
- Kuhn, T.; Rohrmann, S.; Sookthai, D.; Johnson, T.; Katzke, V.; Kaaks, R.; von Eckardstein, A.; Muller, D. Intra-Individual variation of plasma trimethylamine-N-Oxide (TMAO), betaine and choline over 1 year. Clin. Chem. Lab. Med. 2017, 55, 261–268. [Google Scholar] [CrossRef]
- Cheung, W.; Keski-Rahkonen, P.; Assi, N.; Ferrari, P.; Freisling, H.; Rinaldi, S.; Slimani, N.; Zamora-Ros, R.; Rundle, M.; Frost, G.; et al. A metabolomic study of biomarkers of meat and fish intake. Am. J. Clin. Nutr. 2017, 105, 600–608. [Google Scholar] [CrossRef]
- Brown, J.M.; Hazen, S.L. Microbial modulation of cardiovascular disease. Nat. Revi. Microbiol. 2018, 16, 171. [Google Scholar] [CrossRef]
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-Oxide: The Good, the Bad and the Unknown. Toxins (Basel) 2016, 8, 326. [Google Scholar] [CrossRef]
- Cho, C.E.; Caudill, M.A. Trimethylamine-N-Oxide: Friend, Foe, or Simply Caught in the Cross-Fire? Trends Endocrinol. Metab. 2017, 28, 121–130. [Google Scholar] [CrossRef]
- Jethva, P.N.; Udgaonkar, J.B. The Osmolyte TMAO Modulates Protein Folding Cooperativity by Altering Global Protein Stability. Biochemistry 2018, 57, 5851–5863. [Google Scholar] [CrossRef]
- Chan, M.M.; Yang, X.; Wang, H.; Saaoud, F.; Sun, Y.; Fong, D. The microbial metabolite trimethylamine n-Oxide links vascular dysfunctions and the autoimmune disease rheumatoid arthritis. Nutrients 2019, 11, 1821. [Google Scholar] [CrossRef]
- Huc, T.; Drapala, A.; Gawrys, M.; Konop, M.; Bielinska, K.; Zaorska, E.; Samborowska, E.; Wyczalkowska-Tomasik, A.; Paczek, L.; Dadlez, M.; et al. Chronic, low-Dose TMAO treatment reduces diastolic dysfunction and heart fibrosis in hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, 1805–1820. [Google Scholar] [CrossRef]
- Jaworska, K.; Bielinska, K.; Gawrys-Kopczynska, M.; Ufnal, M. TMA (trimethylamine), but not its oxide TMAO (trimethylamine-oxide), exerts hemodynamic effects—Implications for interpretation of cardiovascular actions of gut microbiome. Cardiovasc. Res. 2019. [Google Scholar] [CrossRef]
- Jaworska, K.; Konop, M.; Hutsch, T.; Perlejewski, K.; Radkowski, M.; Grochowska, M.; Bielak-Zmijewska, A.; Mosieniak, G.; Sikora, E.; Ufnal, M. Tma but not tmao increases with age in rat plasma and affects smooth muscle cells viability. J. Gerontol. 2019. [Google Scholar] [CrossRef]
- Brieger, H.; Hodes, W.A. Toxic effects of exposure to vapors of aliphatic amines. Arch. Ind. Hyg. Occup. Med. 1951, 3, 287–291. [Google Scholar]
- Friemann, W.; Overhoff, W.; Wolter, J.R. Eye diseases in the fishing industry. Arch. Gewerbepathol. Gewerbehyg. 1959, 17, 1–56. [Google Scholar]
- Wills, M.R.; Savory, J. Biochemistry of renal failure. Ann. Clin. Lab. Sci. 1981, 11, 292–299. [Google Scholar]
- Ito, S.; Yoshida, M. Protein-Bound uremic toxins: New culprits of cardiovascular events in chronic kidney disease patients. Toxins (Basel) 2014, 6, 665–678. [Google Scholar] [CrossRef]
- Lekawanvijit, S. Cardiotoxicity of Uremic Toxins: A Driver of Cardiorenal Syndrome. Toxins (Basel) 2018, 10, 352. [Google Scholar] [CrossRef]
- Fujii, H.; Goto, S.; Fukagawa, M. Role of Uremic Toxins for Kidney, Cardiovascular, and Bone Dysfunction. Toxins (Basel) 2018, 10, 202. [Google Scholar] [CrossRef]
- Obeid, R.; Awwad, H.M.; Rabagny, Y.; Graeber, S.; Herrmann, W.; Geisel, J. Plasma trimethylamine N-Oxide concentration is associated with choline, phospholipids, and methyl metabolism. Am. J. Clin. Nutr. 2016, 103, 703–711. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Kelterer, D.; Fuchs, S.; Kaatz, M.; Grieshaber, R.; Kleesz, P.; Elsner, P. Additive impairment of the barrier function and irritation by biogenic amines and sodium lauryl sulphate: A controlled in vivo tandem irritation study. Skin Pharmacol. Physiol. 2005, 18, 88–97. [Google Scholar] [CrossRef]
- Guest, I.; Varma, D.R. Selective growth inhibition of the male progeny of mice treated with trimethylamine during pregnancy. Can. J. Physiol. Pharmacol. 1993, 71, 185–187. [Google Scholar] [CrossRef]
- Restini, C.B.; Fink, G.D.; Watts, S.W. Abstract p145: The bacterial metabolite trimethylamine (tma), but not trimethylamine n-Oxide (tmao), causes vascular contraction. Hypertension 2018, 72, 145. [Google Scholar] [CrossRef]
- Pospischil, E.; Johanson, G.; Nielsen, G.; Papameletiou, D.; Klein, C. SCOEL/REC/179 Trimethylamine. Publ. Sci. Comm. Occup. Expo. Lim. Eur. Union 2017. [Google Scholar] [CrossRef]
- Storino, G.; Moraes, C.; Saldanha, J.; Mafra, D. Cardiovascular mortality in chronic kidney patients: The role of uremic toxins. Int. J. Cardiovasc. Sci. 2015, 28, 327–334. [Google Scholar] [CrossRef]
- Kinney, L.; Burgess, B.; Chen, H.; Kennedy, G. Inhalation toxicology of trimethylamine. Inhal. Toxicol. 1990, 2, 41–51. [Google Scholar] [CrossRef]
- Zou, Q.; Bennion, B.J.; Daggett, V.; Murphy, K.P. The molecular mechanism of stabilization of proteins by TMAO and its ability to counteract the effects of urea. J. Am. Chem. Soc. 2002, 124, 1192–1202. [Google Scholar] [CrossRef]
- Yancey, P.H.; Speers-Roesch, B.; Atchinson, S.; Reist, J.D.; Majewski, A.R.; Treberg, J.R. Osmolyte Adjustments as a Pressure Adaptation in Deep-Sea Chondrichthyan Fishes: An Intraspecific Test in Arctic Skates (Amblyraja hyperborea) along a Depth Gradient. Physiol. Biochem. Zool. 2018, 91, 788–796. [Google Scholar] [CrossRef]
- Vigorita, M.; Cozzolino, S.; Oliva, R.; Graziano, G.; Del Vecchio, P. Counteraction ability of TMAO toward different denaturing agents. Biopolymers 2018, 109, 23104. [Google Scholar] [CrossRef]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Eng. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef]
- Manjunath, G.; Tighiouart, H.; Coresh, J.; Macleod, B.; Salem, D.N.; Griffith, J.L.; Levey, A.S.; Sarnak, M.J. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int. 2003, 63, 1121–1129. [Google Scholar] [CrossRef] [Green Version]
- Simenhoff, M.L.; Burke, J.F.; Saukkonen, J.J.; Ordinario, A.T.; Doty, R. Biochemical profile or uremic breath. N. Engl. J. Med. 1977, 297, 132–135. [Google Scholar] [CrossRef]
- Hao, X.; Liu, X.; Wang, W.; Ren, H.; Xie, J.; Shen, P.; Lin, D.; Chen, N. Distinct metabolic profile of primary focal segmental glomerulosclerosis revealed by NMR-Based metabolomics. PLoS ONE 2013, 8, 78531. [Google Scholar] [CrossRef]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Munoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; LeFevre, M.; Miller, F., Jr.; Otto, C.M. Recommendations on the echocardiographic assessment of aortic valve stenosis: A focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2016, 18, 254–275. [Google Scholar] [CrossRef]
- Jaworska, K.; Huc, T.; Samborowska, E.; Dobrowolski, L.; Bielinska, K.; Gawlak, M.; Ufnal, M. Hypertension in rats is associated with an increased permeability of the colon to TMA, A gut bacteria metabolite. PLoS ONE 2017, 12, 189310. [Google Scholar] [CrossRef]





| Characteristic | Healthy Group (n = 9) | AS Group (n = 19) | p-Value |
|---|---|---|---|
| Ethnicity (Caucasian/other) | 9/0 | 19/0 | - |
| Male/Female | 5/4 | 11/8 | - |
| Age (years) | 38.9 ± 4.8 | 74.5 ± 2.3 | <0.001 |
| eGFR (mL/min/1.73 m²) | 101.5 ± 6.3 | 57.4 ± 5.45 | <0.001 |
| Plasma TMA (µmol/L) | 23.2 ± 2.1 | 59.5 ± 3.9 | <0.001 |
| Plasma TMAO (µmol/L) | 3.6 ± 0.4 | 5.5 ± 0.6 | 0.07 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaworska, K.; Hering, D.; Mosieniak, G.; Bielak-Zmijewska, A.; Pilz, M.; Konwerski, M.; Gasecka, A.; Kapłon-Cieślicka, A.; Filipiak, K.; Sikora, E.; et al. TMA, A Forgotten Uremic Toxin, but Not TMAO, Is Involved in Cardiovascular Pathology. Toxins 2019, 11, 490. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11090490
Jaworska K, Hering D, Mosieniak G, Bielak-Zmijewska A, Pilz M, Konwerski M, Gasecka A, Kapłon-Cieślicka A, Filipiak K, Sikora E, et al. TMA, A Forgotten Uremic Toxin, but Not TMAO, Is Involved in Cardiovascular Pathology. Toxins. 2019; 11(9):490. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11090490
Chicago/Turabian StyleJaworska, Kinga, Dagmara Hering, Grażyna Mosieniak, Anna Bielak-Zmijewska, Marta Pilz, Michał Konwerski, Aleksandra Gasecka, Agnieszka Kapłon-Cieślicka, Krzysztof Filipiak, Ewa Sikora, and et al. 2019. "TMA, A Forgotten Uremic Toxin, but Not TMAO, Is Involved in Cardiovascular Pathology" Toxins 11, no. 9: 490. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11090490