Molecular Characterization of Equine Staphylococcus aureus Isolates Exhibiting Reduced Oxacillin Susceptibility
Abstract
:1. Introduction
2. Results
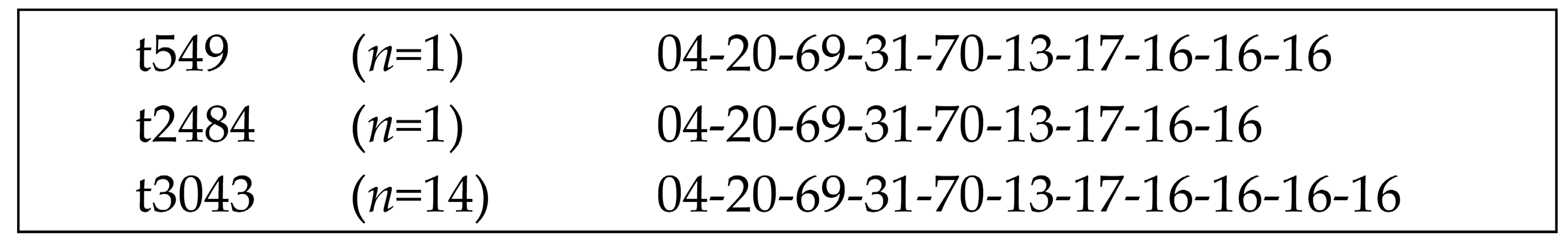
2.1. Molecular Typing of Equine BORSA Isolates
2.2. Phylogenetic Analysis
2.3. Virulence Factors of the 19 Equine BORSA Isolates
2.4. Antimicrobial Resistance Properties
2.5. Investigation of the Reduced Susceptibility to Oxacillin
2.6. Overexpression and Induction of blaZ
2.7. Susceptibility to Biocides
3. Discussion
4. Materials and Methods
4.1. Background Information and Bacterial Isolates
4.2. Characterization of the Isolates
4.3. Phylogenetic Analysis
4.4. Antimicrobial Susceptibility Testing
4.5. Investigation of β-Lactamase-Production
4.6. Biocide Susceptibility Testing
4.7. Statistical Analysis
4.8. WGS Submitted to GenBank
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schwarz, S.; Feßler, A.T.; Loncaric, I.; Wu, C.; Kadlec, K.; Wang, Y.; Shen, J. Antimicrobial resistance among staphylococci of animal origin. In Antimicrobial Resistance in Bacteria from Livestock and Companion Animals, 1st ed.; Schwarz, S., Cavaco, L., Shen, J., Eds.; American Society for Microbiology: Washington, DC, USA, 2018; pp. 127–157. ISBN 9781555819798. [Google Scholar]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.; Borges, A.; Simões, M. Staphylococcus aureus toxins and their molecular activity in infectious diseases. Toxins 2018, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Gudiol, C.; Cuervo, G.; Shaw, E.; Pujol, M.; Carratalà, J. Pharmacotherapeutic options for treating Staphylococcus aureus bacteremia. Expert Opin. Pharmacother. 2017, 18, 1947–1963. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Wilson, B.; Gould, I.M. Current and future treatment options for community-associated MRSA infection. Expert Opin. Pharmacother. 2018, 19, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, U.; von Lützau, K.; Schlattmann, A.; Rösler, U.; Köck, R.; Becker, K. Zoonotic multidrug-resistant microorganisms among non-hospitalized horses from Germany. One Health 2019, 7, 100091. [Google Scholar] [CrossRef]
- Bino, E.; Lauková, A.; Ščerbová, J.; Kubašová, I.; Kandričáková, A.; Strompfová, V.; Miltko, R.; Belzecki, G. Fecal coagulase-negative staphylococci from horses, their species variability, and biofilm formation. Folia Microbiol. 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fouché, N.; Gerber, V.; Thomann, A.; Perreten, V. Antimicrobial susceptibility patterns of blood culture isolates from foals in Switzerland. Schweiz. Arch. Tierheilkd. 2018, 160, 665–671. [Google Scholar] [CrossRef]
- Schnellmann, C.; Gerber, V.; Rossano, A.; Jaquier, V.; Panchaud, Y.; Doherr, M.G.; Thomann, A.; Straub, R.; Perreten, V. Presence of new mecA and mph(C) variants conferring antibiotic resistance in Staphylococcus spp. isolated from the skin of horses before and after clinic admission. J. Clin. Microbiol. 2006, 44, 4444–4454. [Google Scholar] [CrossRef]
- Schwarz, S.; Roberts, M.C.; Werckenthin, C.; Pang, Y.; Lange, C. Tetracycline resistance in Staphylococcus spp. from domestic animals. Vet. Microbiol. 1998, 63, 217–227. [Google Scholar] [CrossRef]
- Cuny, C.; Witte, W. MRSA in equine hospitals and its significance for infections in humans. Vet. Microbiol. 2017, 200, 59–64. [Google Scholar] [CrossRef]
- Waqar, N.; Amin, Q.; Munir, T.; Ikram, M.S.; Shahzad, N.; Mirza, A.; Ali, A.; Arshad, M.I. A cross-sectional study of methicillin-resistant Staphylococcus aureus at the equine-human interface. Trop. Anim. Health Prod. 2019, 51, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Walther, B.; Klein, K.S.; Barton, A.K.; Semmler, T.; Huber, C.; Merle, R.; Tedin, K.; Mitrach, F.; Lübke-Becker, A.; Gehlen, H. Equine methicillin-resistant sequence type 398 Staphylococcus aureus (MRSA) harbor mobile genetic elements promoting host adaptation. Front. Microbiol. 2018, 9, 2516. [Google Scholar] [CrossRef] [PubMed]
- Köck, R.; Cuny, C. Multidrug-resistant bacteria in animals and humans. Med. Klin. Intensivmed. Notfmed. 2018. (Epub ahead of print). [Google Scholar] [CrossRef]
- Aires-de-Sousa, M. Methicillin-resistant Staphylococcus aureus among animals: Current overview. Clin. Microbiol. Infect. 2017, 23, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Hryniewicz, M.M.; Garbacz, K. Borderline oxacillin-resistant Staphylococcus aureus (BORSA)—A more common problem than expected? J. Med. Microbiol. 2017, 66, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; van Alen, S.; Idelevich, E.A.; Schleimer, N.; Seggewiß, J.; Mellmann, A.; Kaspar, U.; Peters, G. Plasmid-encoded transferable mecB-mediated methicillin resistance in Staphylococcus aureus. Emerg. Infect. Dis. 2018, 24, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Pantosti, A. Methicillin-resistant Staphylococcus aureus associated with animals and its relevance to human health. Front. Microbiol. 2012, 3, 127. [Google Scholar] [CrossRef]
- Köck, R.; Schaumburg, F.; Mellmann, A.; Köksal, M.; Jurke, A.; Becker, K.; Friedrich, A.W. Livestock-associated methicillin-resistant Staphylococcus aureus (MRSA) as causes of human infection and colonization in Germany. PLoS ONE 2013, 8, e55040. [Google Scholar] [CrossRef]
- Van Duijkeren, E.; Moleman, M.; Sloet van Oldruitenborgh-Oosterbaan, M.M.; Multem, J.; Troelstra, A.; Fluit, A.C.; van Wamel, W.J.B.; Houwers, D.J.; de Neeling, A.J.; Wagenaar, J.A. Methicillin-resistant Staphylococcus aureus in horses and horse personnel: An investigation of several outbreaks. Vet. Microbiol. 2010, 141, 96–102. [Google Scholar] [CrossRef]
- Sieber, S.; Gerber, V.; Jandova, V.; Rossano, A.; Evison, J.M.; Perreten, V. Evolution of multidrug-resistant Staphylococcus aureus infections in horses and colonized personnel in an equine clinic between 2005 and 2010. Microb. Drug Resist. 2011, 17, 471–478. [Google Scholar] [CrossRef]
- Abdelbary, M.M.H.; Wittenberg, A.; Cuny, C.; Layer, F.; Kurt, K.; Wieler, L.H.; Walther, B.; Skov, R.; Larsen, J.; Hasman, H.; et al. Phylogenetic analysis of Staphylococcus aureus CC398 reveals a sub-lineage epidemiologically associated with infections in horses. PLoS ONE 2014, 9, e88083. [Google Scholar] [CrossRef] [PubMed]
- Vincze, S.; Stamm, I.; Kopp, P.A.; Hermes, J.; Adlhoch, C.; Semmler, T.; Wieler, L.H.; Lübke-Becker, A.; Walther, B. Alarming proportions of methicillin-resistant Staphylococcus aureus (MRSA) in wound samples from companion animals, Germany 2010–2012. PLoS ONE 2014, 9, e85656. [Google Scholar] [CrossRef] [PubMed]
- Maddox, T.W.; Clegg, P.D.; Williams, N.J.; Pinchbeck, G.L. Antimicrobial resistance in bacteria from horses: Epidemiology of antimicrobial resistance. Equine Vet. J. 2015, 47, 756–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuny, C.; Abdelbary, M.M.H.; Köck, R.; Layer, F.; Scheidemann, W.; Werner, G.; Witte, W. Methicillin-resistant Staphylococcus aureus from infections in horses in Germany are frequent colonizers of veterinarians but rare among MRSA from infections in humans. One Health 2015, 2, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.Z.; Espinosa-Gongora, C.; Damborg, P.; Sieber, R.N.; Munk, R.; Husted, L.; Moodley, A.; Skov, R.; Larsen, J.; Guardabassi, L. Horses in Denmark are a reservoir of diverse clones of methicillin-resistant and -susceptible Staphylococcus aureus. Front. Microbiol. 2017, 8, 543. [Google Scholar] [CrossRef] [PubMed]
- Loncaric, I.; Lepuschitz, S.; Ruppitsch, W.; Trstan, A.; Andreadis, T.; Bouchlis, N.; Marbach, H.; Schauer, B.; Szostak, M.P.; Feßler, A.T.; et al. Increased genetic diversity of methicillin-resistant Staphylococcus aureus (MRSA) isolated from companion animals. Vet. Microbiol. 2019, 235, 118–126. [Google Scholar] [CrossRef]
- DANMAP. Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark; DANMAP, Statens Serum Institute: Copenhagen, Denmark, 2017; ISSN 1600-2032. [Google Scholar]
- Toleman, M.S.; Reuter, S.; Jamrozy, D.; Wilson, H.J.; Blane, B.; Harrison, E.M.; Coll, F.; Hope, R.J.; Kearns, A.; Parkhill, J.; et al. Prospective genomic surveillance of methicillin-resistant Staphylococcus aureus (MRSA) associated with bloodstream infection, England, 1 October 2012 to 30 September 2013. Euro Surveill. 2019, 24. [Google Scholar] [CrossRef]
- Pérez-Montarelo, D.; Viedma, E.; Larrosa, N.; Gómez-González, C.; Ruiz de Gopegui, E.; Muñoz-Gallego, I.; San Juan, R.; Fernández-Hidalgo, N.; Almirante, B.; Chaves, F. Molecular epidemiology of Staphylococcus aureus bacteremia: Association of molecular factors with the source of infection. Front. Microbiol. 2018, 9, 2210. [Google Scholar] [CrossRef]
- Elstrøm, P.; Grøntvedt, C.A.; Gabrielsen, C.; Stegger, M.; Angen, Ø.; Åmdal, S.; Enger, H.; Urdahl, A.M.; Jore, S.; Steinbakk, M.; et al. Livestock-associated MRSA CC1 in Norway; Introduction to pig farms, zoonotic transmission, and eradication. Front. Microbiol. 2019, 10, 139. [Google Scholar] [CrossRef]
- Güven Gökmen, T.; Kalayci, Y.; Yaman, A.; Köksal, F. Molecular characterization of methicillin-resistant Staphylococcus aureus strains by spa typing and pulsed field gel electrophoresis methods. BMC Microbiol. 2018, 18, 155. [Google Scholar] [CrossRef]
- Manara, S.; Pasolli, E.; Dolce, D.; Ravenni, N.; Campana, S.; Armanini, F.; Asnicar, F.; Mengoni, A.; Galli, L.; Montagnani, C.; et al. Whole-genome epidemiology, characterisation, and phylogenetic reconstruction of Staphylococcus aureus strains in a paediatric hospital. Genome Med. 2018, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; de Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Šiširak, M.; Zvizdić, A.; Hukić, M. Methicillin-resistant Staphylococcus aureus (MRSA) as a cause of nosocomial wound infections. Bosn. J. Basic Med. Sci. 2010, 10, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Hardy, K.; Sunnucks, K.; Gil, H.; Shabir, S.; Trampari, E.; Hawkey, P.; Webber, M. Increased usage of antiseptics is associated with reduced susceptibility in clinical isolates of Staphylococcus aureus. MBio 2018, 9, e00894–e00918. [Google Scholar] [CrossRef] [PubMed]
- Feßler, A.T.; Schug, A.R.; Geber, F.; Scholtzek, A.D.; Brombach, J.; Hensel, V.; Meurer, M.; Michael, G.B.; Reinhardt, M.; Speck, S.; et al. Development and evaluation of a broth macrodilution method to determine the biocide susceptibility of bacteria. Vet. Microbiol. 2018, 223, 59–64. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 4th ed.; CLSI supplement VET08; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; ISBN1 978-1-68440-010-2. (Print); ISBN2 978-1-68440-011-9. (Electronic). [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 29th ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019; ISBN1 978-1-68440-032-4. [Print]; ISBN2 978-1-68440-033-1. [Electronic]. [Google Scholar]
- Watanabe, S.; Ito, T.; Hiramatsu, K. Susceptibilities of healthcare- and community-associated methicillin-resistant staphylococci to the novel des-F(6)-quinolone DX-619. J. Antimicrob. Chemother. 2007, 60, 1384–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauschild, T.; Feßler, A.T.; Billerbeck, C.; Wendlandt, S.; Kaspar, H.; Mankertz, J.; Schwarz, S.; Kadlec, K. Target gene mutations among methicillin-resistant Staphylococcus aureus and methicillin-susceptible S. aureus with elevated MICs of enrofloxacin obtained from diseased food-producing animals or food of animal origin. Antimicrob. Chemother. 2012, 67, 1791–1793. [Google Scholar] [CrossRef]
- Berger-Bächi, B.; Tschierske, M. Role of fem factors in methicillin resistance. Drug Resist. Updat. 1998, 1, 325–335. [Google Scholar] [CrossRef]
- Seidl, K.; Stucki, M.; Ruegg, M.; Goerke, C.; Wolz, C.; Harris, L.; Berger-Bächi, B.; Bischoff, M. Staphylococcus aureus CcpA affects virulence determinant production and antibiotic resistance. Antimicrob. Agents Chemother. 2006, 50, 1183–1194. [Google Scholar] [CrossRef]
- Sadykov, M.R.; Hartmann, T.; Mattes, T.A.; Hiatt, M.; Jann, N.J.; Zhu, Y.; Ledala, N.; Landmann, R.; Herrmann, M.; Rohde, H.; et al. CcpA coordinates central metabolism and biofilm formation in Staphylococcus epidermidis. Microbiology 2011, 157 Pt 12, 3458–3468. [Google Scholar] [CrossRef]
- Griffiths, J.M.; O’Neill, A.J. Loss of function of the GdpP protein leads to joint β-lactam/glycopeptide tolerance in Staphylococcus aureus. Antimicrob. Agents Chemother. 2012, 56, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Argudín, M.A.; Roisin, S.; Nienhaus, L.; Dodémont, M.; de Mendonça, R.; Nonhoff, C.; Deplano, A.; Denis, O. Genetic diversity among Staphylococcus aureus isolates showing oxacillin and/or cefoxitin resistance not linked to the presence of mec genes. Antimicrob. Agents Chemother. 2018, 62, e00091–e00118. [Google Scholar] [CrossRef] [PubMed]
- Commichau, F.M.; Heidemann, J.L.; Ficner, R.; Stülke, J. Making and breaking of an essential poison: The cyclases and phosphodiesterases that produce and degrade the essential second messenger cyclic di-AMP in bacteria. J. Bacteriol. 2018, 201, e00462–e00518. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.N.; Woodward, J.J. Too much of a good thing: Regulated depletion of c-di-AMP in the bacterial cytoplasm. Curr. Opin. Microbiol. 2016, 30, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Ahn, J. Associations between resistance phenotype and gene expression in response to serial exposure to oxacillin and ciprofloxacin in Staphylococcus aureus. Lett. Appl. Microbiol. 2017, 65, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.J.; Lauderdale, T.L.; Lee, C.H.; Hsu, Y.C.; Huang, I.W.; Hsu, P.C.; Yang, C.S. Effect of a point mutation in mprF on susceptibility to daptomycin, vancomycin, and oxacillin in an MRSA clinical strain. Front. Microbiol. 2018, 9, 1086. [Google Scholar] [CrossRef] [PubMed]
- Rowland, S.J.; Dyke, K.G. Tn552, a novel transposable element from Staphylococcus aureus. Mol. Microbiol. 1990, 4, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Vincze, S. Staphylococcus aureus in Companion Animals: An Infection Source for the Community? Ph.D. Thesis, Freie Universität Berlin, Berlin, Germany, 2015. [Google Scholar]
- Gharsa, H.; Ben Sallem, R.; Ben Slama, K.; Gómez-Sanz, E.; Lozano, C.; Jouini, A.; Klibi, N.; Zarazaga, M.; Boudabous, A.; Torres, C. High diversity of genetic lineages and virulence genes in nasal Staphylococcus aureus isolates from donkeys destined to food consumption in Tunisia with predominance of the ruminant associated CC133 lineage. BMC Vet. Res. 2012, 8, 203. [Google Scholar] [CrossRef]
- Battisti, A.; Franco, A.; Merialdi, G.; Hasman, H.; Iurescia, M.; Lorenzetti, R.; Feltrin, F.; Zini, M.; Aarestrup, F.M. Heterogeneity among methicillin-resistant Staphylococcus aureus from Italian pig finishing holdings. Vet. Microbiol. 2010, 142, 361–366. [Google Scholar] [CrossRef]
- Franco, A.; Hasman, H.; Iurescia, M.; Lorenzetti, R.; Stegger, M.; Pantosti, A.; Feltrin, F.; Ianzano, A.; Porrero, M.C.; Liapi, M.; et al. Molecular characterization of spa type t127, sequence type 1 methicillin-resistant Staphylococcus aureus from pigs. J. Antimicrob. Chemother. 2011, 66, 1231–1235. [Google Scholar] [CrossRef]
- Alba, P.; Feltrin, F.; Cordaro, G.; Porrero, M.C.; Kraushaar, B.; Argudín, M.A.; Nykäsenoja, S.; Monaco, M.; Stegger, M.; Butaye, P.; et al. Livestock-associated methicillin resistant and methicillin susceptible Staphylococcus aureus sequence type (CC)1 in European farmed animals: High genetic relatedness of isolates from Italian cattle herds and humans. PLoS ONE 2015, 10, e0137143. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lin, X.; Jiang, T.; Peng, Z.; Xu, J.; Yi, L.; Li, F.; Fanning, S.; Baloch, Z. Prevalence and characterization of Staphylococcus aureus cultured from raw milk taken from dairy cows with mastitis in Beijing, China. Front. Microbiol. 2018, 9, 1123. [Google Scholar] [CrossRef] [PubMed]
- Seinige, D.; von Altrock, A.; Kehrenberg, C. Genetic diversity and antibiotic susceptibility of Staphylococcus aureus isolates from wild boars. Comp. Immunol. Microbiol. Infect. Dis. 2017, 54, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, S.A.; Chia, N.; Jeraldo, P.R.; Quest, D.J.; Johnson, J.A.; Boxrud, D.J.; Taylor, A.J.; Chen, J.; Jenkins, G.D.; Drucker, T.M.; et al. Comparison of whole-genome sequencing methods for analysis of three methicillin-resistant Staphylococcus aureus outbreaks. J. Clin. Microbiol. 2017, 55, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.X.; McCormick, J.K. Staphylococcal superantigens in colonization and disease. Front. Cell. Infect. Microbiol. 2012, 2, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettersson, H.; Forsberg, G. Staphylococcal enterotoxin H contrasts closely related enterotoxins in species reactivity. Immunology 2002, 106, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koop, G.; Vrieling, M.; Storisteanu, D.M.; Lok, L.S.; Monie, T.; van Wigcheren, G.; Raisen, C.; Ba, X.; Gleadall, N.; Hadjirin, N.; et al. Identification of LukPQ, a novel, equid-adapted leukocidin of Staphylococcus aureus. Sci. Rep. 2017, 7, 40660. [Google Scholar] [CrossRef]
- De Jong, N.W.M.; Vrieling, M.; Garcia, B.L.; Koop, G.; Brettmann, M.; Aerts, P.C.; Ruyken, M.; van Strijp., J.A.G.; Holmes, M.; Harrison, E.M.; et al. Identification of a staphylococcal complement inhibitor with broad host specificity in equid Staphylococcus aureus strains. J. Biol. Chem. 2018, 293, 4468–4477. [Google Scholar] [CrossRef]
- Monecke, S.; Coombs, G.; Shore, A.C.; Coleman, D.C.; Akpaka, P.; Borg, M.; Chow, H.; Ip, M.; Jatzwauk, L.; Jonas, D.; et al. A field guide to pandemic, epidemic and sporadic clones of methicillin-resistant Staphylococcus aureus. PLoS ONE 2011, 6, e17936. [Google Scholar] [CrossRef]
- Schwarz, S.; Silley, P.; Simjee, S.; Woodford, N.; van Duijkeren, E.; Johnson, A.P.; Gaastra, W. Assessing the antimicrobial susceptibility of bacteria obtained from animals. Vet. Microbiol. 2010, 141, 1–4. [Google Scholar] [CrossRef]
- Ghuysen, J.M. Serine beta-lactamases and penicillin-binding proteins. Annu. Rev. Microbiol. 1991, 45, 37–67. [Google Scholar] [CrossRef] [PubMed]
- Sauvage, E.; Kerff, F.; Terrak, M.; Ayala, J.A.; Charlier, P. The penicillin-binding proteins: Structure and role in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 2008, 32, 234–258. [Google Scholar] [CrossRef] [PubMed]
- Sauvage, E.; Terrak, M. Glycosyltransferases and transpeptidases/penicillin-binding proteins: Valuable targets for new antibacterials. Antibiotics 2016, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Morroni, G.; Brenciani, A.; Brescini, L.; Fioriti, S.; Simoni, S.; Pocognoli, A.; Mingoia, M.; Giovanetti, E.; Barchiesi, F.; Giacometti, A.; et al. High rate of ceftobiprole resistance among clinical methicillin-resistant Staphylococcus aureus isolates from a hospital in central Italy. Antimicrob. Agents Chemother. 2018, 62, e01663–e01718. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Milheiriço, C.; de Lencastre, H.; Tomasz, A. Antibiotic resistance as a stress response: Recovery of high-level oxacillin resistance in methicillin-resistant Staphylococcus aureus “auxiliary” (fem) mutants by induction of the stringent stress response. Antimicrob. Agents Chemother. 2017, 61, e00313–e00317. [Google Scholar] [CrossRef] [PubMed]
- De Lencastre, H.; de Jonge, B.L.; Matthews, P.R.; Tomasz, A. Molecular aspects of methicillin resistance in Staphylococcus aureus. J. Antimicrob. Chemother. 1994, 33, 7–24. [Google Scholar] [CrossRef]
- Giannouli, S.; Labrou, M.; Kyritsis, A.; Ikonomidis, A.; Pournaras, S.; Stathopoulos, C.; Tsakris, A. Detection of mutations in the FemXAB protein family in oxacillin-susceptible mecA-positive Staphylococcus aureus clinical isolates. J. Antimicrob. Chemother. 2010, 65, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Bizzini, A.; Entenza, J.M.; Moreillon, P. Loss of penicillin tolerance by inactivating the carbon catabolite repression determinant CcpA in Streptococcus gordonii. J. Antimicrob. Chemother. 2007, 59, 607–615. [Google Scholar] [CrossRef]
- Cabral, D.J.; Wurster, J.I.; Belenky, P. Antibiotic persistence as a metabolic adaptation: Stress, metabolism, the host, and new directions. Pharmaceuticals 2018, 11, 14. [Google Scholar] [CrossRef]
- Maalej, S.M.; Rhimi, F.M.; Fines, M.; Mnif, B.; Leclercq, R.; Hammami, A. Analysis of borderline oxacillin-resistant Staphylococcus aureus (BORSA) strains isolated in Tunisia. J. Clin. Microbiol. 2012, 50, 3345–3348. [Google Scholar] [CrossRef]
- Staude, M.W.; Frederick, T.E.; Natarajan, S.V.; Wilson, B.D.; Tanner, C.E.; Ruggiero, S.T.; Mobashery, S.; Peng, J.W. Investigation of signal transduction routes within the sensor/transducer protein BlaR1 of Staphylococcus aureus. Biochemistry 2015, 54, 1600–1610. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.E.; Christensen, H.; Aarestrup, F.M. Diversity and evolution of blaZ from Staphylococcus aureus and coagulase-negative staphylococci. J. Antimicrob. Chemother. 2006, 57, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xue, H.; Wu, Z.; Ma, J.; Zhao, X. Effect of bla regulators on the susceptible phenotype and phenotypic conversion for oxacillin-susceptible mecA-positive staphylococcal isolates. J. Antimicrob. Chemother. 2016, 71, 2105–2112. [Google Scholar] [CrossRef] [PubMed]
- Schug, A.R.; Feßler, A.T.; Scholtzek, A.D.; Meurer, M.; Brombach, J.; Hensel, V.; Schwarz, S. Determination of biocide susceptibility: Development of a broth microdilution method. In National Symposium on Zoonoses Research 2018, Program and Abstracts, National Symposium on Zoonoses Research 2018, Berlin, Germany, 17–19 October 2018; German Research Platform for Zoonoses: Berlin, Germany, 2018; Volume A22, pp. 158–159. [Google Scholar]
- Nurk, S.; Bankevich, A.; Antipov, D.; Gurevich, A.; Korobeynikov, A.; Lapidus, A.; Prjibelski, A.D.; Pyshkin, A.; Sirotkin, A.; Sirotkin, Y.; et al. Assembling genomes and mini-metagenomes from highly chimeric reads. J. Comput. Biol. 2013, 20, 714–737. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST server: Rapid annotations using subsystems technology. BMC Genomics. 2008, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef]
- Jünemann, S.; Sedlazeck, F.J.; Prior, K.; Albersmeier, A.; John, U.; Kalinowski, J.; Mellmann, A.; Goesmann, A.; von Haeseler, A.; Stoye, J. Updating benchtop sequencing performance comparison. Nat. Biotechnol. 2013, 31, 294–296. [Google Scholar] [CrossRef] [Green Version]
- Leopold, S.R.; Goering, R.V.; Witten, A.; Harmsen, D.; Mellmann, A. Bacterial whole-genome sequencing revisited: Portable, scalable, and standardized analysis for typing and detection of virulence and antibiotic resistance genes. J. Clin. Microbiol. 2014, 52, 2365–2370. [Google Scholar] [CrossRef]
- DVG. Richtlinien für die Prüfung von Desinfektionsverfahren und Chemischen Desinfektionsmitteln, 4th ed.; Verlag der DVG Service GmbH: Gießen, Germany, 2007; ISBN 978-3-939902-44-7. [Google Scholar]



| ST | Isolate | hlb | fnbA | fnbB | ica | sei | selm | seln | selo | ϕent2 | selq | seh | lukD/E | lukP/Q |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ST1 | IMT39129 | - | + | + | + | - | - | - | - | - | - | + | + | + |
| ST1 | IMT39173 | - | + | + | + | - | - | - | - | - | - | + | + | + |
| ST1 | IMT39701 | - | + | + | + | - | - | - | - | - | - | + | + | + |
| ST1660 | IMT39637 | + | + | + | + | + | + | + | + | + | + | - | + | + |
| ST1660 | IMT37083 | + | + | + | + | + | + | + | + | + | + | - | + | + |
| ST1660 | IMT37341 | + | - | + | + | + | + | + | + | + | + | - | + | + |
| ST1660 | IMT37410 | + | + | + | + | + | + | + | + | + | + | - | + | + |
| ST1660 | IMT37728 | + | + | + | + | - | + | + | + | + | + | - | + | + |
| ST1660 | IMT39233 | + | + | + | + | + | + | + | - | + | + | - | + | + |
| ST1660 | IMT39841 | + | - | + | + | - | + | + | + | - | + | - | + | + |
| ST1660 | IMT40768 | + | + | + | + | + | + | + | + | + | + | - | + | + |
| ST1660 | IMT40820 | + | + | + | + | + | + | + | + | + | + | - | + | + |
| ST1660 | IMT40952 | + | + | + | + | + | + | + | + | + | + | - | + | + |
| ST1660 | IMT41452 | + | + | + | + | + | + | + | + | + | + | - | + | + |
| ST1660 | IMT41468 | + | + | + | + | - | + | + | + | + | + | - | + | + |
| ST1660 | IMT43228 | + | + | + | + | + | + | + | + | + | + | - | + | + |
| ST1660 | IMT43231 | + | + | + | + | + | + | + | + | + | + | - | + | + |
| ST1660 | IMT43240 | + | + | + | + | - | + | + | + | + | + | - | + | + |
| ST1660 | IMT41899 | + | - | - | + | - | + | + | + | + | + | - | + | + |
| No. of Isolates with MIC (mg/L) | Susceptible | Intermediate | Resistant | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial Agent(s) | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | no. | % | no. | % | no. | % |
| Oxacillin | - | - | - | - | 2 | 1 | 9 | 7 | - | - | - | 19 | 100 | - | - | - | - | |||||
| Penicillin | - | - | - | - | - | - | - | 3 | - | - | - | - | 16 | - | - | - | - | 19 | 100 | |||
| Ampicillin | - | - | - | - | - | 1 | 2 | - | - | - | - | 1 | 15 | |||||||||
| Amoxicillin/clavulanic acid a | - | - | - | 3 | - | 9 | 7 | - | - | - | - | - | - | |||||||||
| Imipenem | 12 | 7 | - | - | - | - | - | - | - | - | - | - | - | |||||||||
| Ceftiofur | - | - | - | - | 1 | 11 | 6 | 1 | - | - | - | - | - | |||||||||
| Cefquinome | - | - | - | - | - | 8 | 11 | - | - | - | - | - | - | |||||||||
| Cefalothin | - | - | 3 | 16 | - | - | - | - | - | - | - | - | - | |||||||||
| Cefotaxime | - | - | - | - | - | - | - | 11 | 8 | - | - | - | - | |||||||||
| Cefoperazone | - | - | - | - | - | 3 | 8 | 8 | - | - | - | - | - | |||||||||
| Erythromycin | - | - | - | - | 10 | 8 | 1 | - | - | - | - | - | - | 18 | 94.7 | 1 | 5.3 | - | - | |||
| Tylosin tartrate | 1 | - | - | - | 11 | 7 | - | - | - | - | - | - | - | |||||||||
| Tulathromycin | - | - | - | - | - | - | 3 | 12 | 4 | - | - | |||||||||||
| Tilmicosin | - | - | - | - | 12 | 7 | - | - | - | - | - | - | - | |||||||||
| Clindamycin | - | - | 15 | 4 | - | - | - | - | - | - | - | - | - | 19 | 100 | - | - | - | - | |||
| Pirlimycin | - | - | - | - | 13 | 6 | - | - | - | - | - | - | - | |||||||||
| Tiamulin | - | - | - | - | 4 | 15 | - | - | - | - | - | - | - | |||||||||
| Ciprofloxacin | - | - | - | 9 | 6 | 1 | 2 | 1 | - | - | - | - | 18 | 94.7 | 1 | 5.3 | - | - | ||||
| Enrofloxacin | - | 1 | 4 | 10 | 1 | 2 | 1 | - | - | - | - | - | 15 | 78.9 | 1 | 5.3 | 3 | 15.8 | ||||
| Marbofloxacin | - | - | - | - | 14 | 2 | 3 | - | - | - | - | - | ||||||||||
| Nalidixic acid | - | - | - | - | - | - | - | 1 | 15 | - | 3 | - | ||||||||||
| Gentamicin | - | - | - | - | - | - | - | - | 7 | 11 | 1 | - | - | - | - | - | - | 19 | 100 | |||
| Kanamycin | - | - | - | - | - | - | - | 5 | 14 | |||||||||||||
| Streptomycin | - | - | - | - | - | 14 | 5 | - | - | - | - | - | ||||||||||
| Neomycin | - | - | 11 | 5 | - | - | 2 | 1 | - | - | - | |||||||||||
| Tetracycline | - | 16 | - | - | - | - | - | - | 1 | 2 | - | - | - | 16 | 84.2 | - | - | 3 | 15.8 | |||
| Doxycycline | - | 10 | 6 | - | - | - | 1 | 2 | - | - | - | - | - | 10 | 52.6 | 6 | 31.6 | 3 | 15.8 | |||
| Sulfamethoxazole/trimethoprima | - | - | - | 1 | - | 7 | 8 | - | 3 | - | - | - | - | 16 | 84.2 | - | - | 3 | 15.8 | |||
| Florfenicol | - | - | - | - | - | 18 | 1 | - | - | - | - | - | - | |||||||||
| Linezolid | - | - | - | - | - | 2 | 14 | 3 | - | - | - | - | - | 19 | 100 | - | - | - | - | |||
| Vancomycin | - | - | - | - | - | 8 | 11 | - | - | - | - | - | - | 19 | 100 | - | - | - | - | |||
| Quinupristin/dalfopristin | - | - | - | - | 10 | 9 | - | - | - | - | - | - | - | 19 | 100 | - | - | - | - | |||
| ST a | Isolate | Zone Diameter (mm) b,c | |||||
|---|---|---|---|---|---|---|---|
| PEN (10 U) | OXA (1 µg) | AMP (10 µg) | SAM (10/10 µg) | AMX (20/10 µg) | AMC (10 µg) | ||
| ST1 | IMT39129 | 18 | 20 | 18 | 26 | 7 | 30 |
| ST1 | IMT39173 | 18 | 19 | 18 | 22 | 8 | 30 |
| ST1 | IMT39701 | 16 | 19 | 17 | 26 | 8 | 30 |
| ST1660 | IMT39637 | 10 | 8 | 9 | 18 | no zone | 22 |
| ST1660 | IMT37083 | 12 | 14 | 12 | 19 | 7 | 22 |
| ST1660 | IMT37341 | 10 | 12 | 10 | 16 | no zone | 20 |
| ST1660 | IMT37410 | 10 | 12 | 10 | 18 | no zone | 20 |
| ST1660 | IMT37728 | 10 | 12 | 10 | 18 | no zone | 20 |
| ST1660 | IMT39233 | 10 | 14 | 10 | 18 | no zone | 22 |
| ST1660 | IMT39841 | 8 | 14 | 10 | 16 | no zone | 20 |
| ST1660 | IMT40768 | 9 | 14 | 9 | 16 | no zone | 21 |
| ST1660 | IMT40820 | 10 | 14 | 10 | 17 | no zone | 20 |
| ST1660 | IMT40952 | 11 | 14 | 10 | 18 | no zone | 21 |
| ST1660 | IMT41452 | 9 | 14 | 10 | 18 | no zone | 20 |
| ST1660 | IMT41468 | 10 | 15 | 10 | 17 | 7 | 20 |
| ST1660 | IMT43228 | 10 | 14 | 11 | 18 | no zone | 21 |
| ST1660 | IMT43231 | 10 | 14 | 8 | 17 | 7 | 22 |
| ST1660 | IMT43240 | 12 | 15 | 12 | 20 | 7 | 24 |
| ST1660 | IMT41899 | 11 | 14 | 11 | 19 | 7 | 22 |
| Method a and ST | BAC b (MIC in %) | GLU (MIC in %) | CHX (MIC in %) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.00006 | 0.000125 | 0.00025 | 0.0005 | 0.125 | 0.25 | 0.5 | 0.00006 | 0.000125 | 0.00025 | |
| Micro ST1 | 3 | 1 | 2 | 3 | ||||||
| Macro ST1 | 3 | 2 | 1 | 3 | ||||||
| Micro ST1660 | 1 | 11 | 4 | 13 | 3 | 9 | 7 | |||
| Macro ST1660 | 1 | 1 | 5 | 9 | 3 | 12 | 1 | 4 | 12 | |
| Isolate | Date | History | Surgery | Sample Material | Age | Sex |
|---|---|---|---|---|---|---|
| IMT39129 a | 9 April 2016 | arthritis | yes | synovial fluid | 5 years | gelding |
| IMT39173 a | 11 April 2016 | arthritis | yes | tissue sample | 5 years | gelding |
| IMT39701 | 12 July 2016 | fracture | yes | wound sample | <1 year | stallion |
| IMT39637 | 22 June 2016 | trauma | no | wound sample | n.k. | gelding |
| IMT37083 | 13 July 2015 | fracture | yes | wound sample | 14 years | mare |
| IMT37341 | 24 August 2015 | colic surgery | yes | abscess | 8 years | stallion |
| IMT37410 | 11 September 2015 | castration | yes | wound sample | 1 year | gelding |
| IMT37728 | 5 October 2015 | colic surgery | yes | wound sample | 11 years | gelding |
| IMT39233 | 25 April 2016 | rupture of the urinary bladder | yes | wound sample | 2.5 weeks | stallion |
| IMT39841 | 11 August 2016 | wound healing disorder | yes | wound sample | 14 years | gelding |
| IMT40768 | 7 December 2016 | fracture | yes | wound sample | 4 years | mare |
| IMT40820 | 28 December 2016 | colic surgery | yes | wound sample | 1 year | mare |
| IMT40952 | 17 January 2017 | colic surgery | yes | TBS | 8 years | gelding |
| IMT41452 | 27 January 2017 | wound healing disorder | yes | wound sample | 15 years | gelding |
| IMT41468 | 2 February 2017 | sinusitis | yes | wound sample | 5 years | gelding |
| IMT43228 b | 16 June 2017 | fracture | yes | wound sample | 7 years | mare |
| IMT43231 b | 19 June 2017 | fracture | yes | wound sample | 7 years | mare |
| IMT43240 | 22 June 2017 | colic surgery | yes | wound sample | 6 years | gelding |
| IMT41899 | 15 February 2017 | fracture | yes | wound sample | 14 years | mare |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scholtzek, A.D.; Hanke, D.; Walther, B.; Eichhorn, I.; Stöckle, S.D.; Klein, K.-S.; Gehlen, H.; Lübke-Becker, A.; Schwarz, S.; Feßler, A.T. Molecular Characterization of Equine Staphylococcus aureus Isolates Exhibiting Reduced Oxacillin Susceptibility. Toxins 2019, 11, 535. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11090535
Scholtzek AD, Hanke D, Walther B, Eichhorn I, Stöckle SD, Klein K-S, Gehlen H, Lübke-Becker A, Schwarz S, Feßler AT. Molecular Characterization of Equine Staphylococcus aureus Isolates Exhibiting Reduced Oxacillin Susceptibility. Toxins. 2019; 11(9):535. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11090535
Chicago/Turabian StyleScholtzek, Anissa D., Dennis Hanke, Birgit Walther, Inga Eichhorn, Sabita D. Stöckle, Katja-Sophia Klein, Heidrun Gehlen, Antina Lübke-Becker, Stefan Schwarz, and Andrea T. Feßler. 2019. "Molecular Characterization of Equine Staphylococcus aureus Isolates Exhibiting Reduced Oxacillin Susceptibility" Toxins 11, no. 9: 535. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11090535







