Uremic Toxins and Vascular Calcification–Missing the Forest for All the Trees
Abstract
:1. Relevance and Objective
2. Vascular Calcification
3. Uremic Toxins and Their Effects on VSMC
| Calcification -In Vitro | Calcification -In Vivo | Calcification -Clinical Studies | Osteogenesis | Oxidative Stress | Inflammation | Apoptosis | Senescence | Proliferation | Migration | Atherosclerosis | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| APN | ↓[31,32,33] —[34] | ↓[35,36] | ↑[37,38,39] ↓[40,41] —[42,43,44] | ↓[31,36] | ↓[45] | ↓[33,35] | ↓[46,47] | ↓[48,49] | ↓[49,50] | ||
| ADM | ↓[51,52] | ↓[51,53] | ↓[53] | ↓[54,55] | ↑[56,57] ↓[58,59] | ↓[60,61] | |||||
| ET | ↑[62] | ↑[62,63,64] | ↑[65,66,67] | ↑[68,69,70] | ↑[69,71] | ↑[72] ↓[73] | ↑[74,75] | ↑[76,77] | ↑[78,79] | ||
| IL-8 | ↑[80] | ↑[81] —[82] | ↓[83] | ↑[84,85] | ↑[84,85] | ||||||
| IL-18 | ↑[86,87] | ↑[88,89,90] | ↑[86,87] | ↑[91] | ↑[92,93] | ↑[94,95] | ↑[96,97] | ↑[98,99] | |||
| IL-1β | ↑[100,102] ~[103] | ↑[101,104,105] | —[101,102,106] | ↑[100,102] ~[107] | —[108] | ↑[109,110] | ↑[111,112] | ↑[102] | ↑[113,114] | ↑[114,115] | ↑[101,116] |
| IL-6 | ↑[117,118,119,120,121,122] | ↑[118,119,123] | ↑[41,106,124,125,126,127,128,129] | ↑[118,120,122,130] | ↑[131] | ↑↓[130] | ↑[132,133] | ↑[121] | ↑[134,135] | ↑[135,136] | ↑[118,120] |
| PTH | ↑[137,138] | ↑[137,139,140] ↓[141,142] | ↑[129,143] —[144] | ↑[137] | ↓[141] | —[145] | |||||
| TNF | ↑[107,146,147,148,149,150,151,152] | ↑[149] | ↑[126,129] —[44,128] | ↑[107,146,149,153] | ↑[154,155] | ↑[156,157] | ↑[158,159] | ↑[160,161] | ↑[160,162] | ↑[163,164] |
| Calcification -In Vitro | Calcification -In Vivo | Calcification -Clinical Studies | Osteogenesis | Oxidative Stress | Inflammation | Apoptosis | Senescence | Proliferation | Migration | Atherosclerosis | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hcy | ↑[165,166,167,168,169] | ↑[165,168,169,170,171] | ↑[170,172,173,174,175,176,177] —[106,128,178,179,180,181] | ↑[165,166,168,169] | ↑[182,183] | ↑[184,185] | ↑[186,187] —[188] | ↑[189,190] | ↑[191,192] | ↑[193,194] | |
| IS | ↑[29,80,195,196,197] | ↑[195,197,198] | ↑[199,200] —[201] | ↑[80,196,197,202] | ↑[202,203] | ↑[204,205] | ↑[29] | ↑[29] | ↑[206,207] | ↑[208,209] | ↑[29,210] |
| Leptin | ↑[211,212,213] | ↑[211,214,215] | ↑[40,216,217,218,219] —[41,44] | ↑[211,213,214,220] | ↑[221,222] | ↑[221,223] | ↑[222] ↓[224] | ↑[223,225] ↓[226] | ↑[227,228] | ↑[212,229] | |
| CML | ↑[230] | ↑[230] | ↑[231] | ↑[230] | ↑[232] | ↓[233] | ↑[233] | ↑[230] | |||
| pCS | ↑[198] | ↑[234] | ↑[235] | ↑[235,236] | ↑[198,235] | ↑[237] | ↑[237] | ↑[237,238] | |||
| SM | ↓[239] | ↑[240] | ↑[241,242] | ↑[243,244] ↓[245] |
| Calcification -In Vitro | Calcification -In Vivo | Calcification -Clinical Studies | Osteogenesis | Oxidative Stress | Inflammation | Apoptosis | Senescence | Proliferation | Migration | Atherosclerosis | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ADMA | ↓[246] | ↑[247,248,249,250,251] —[252] | ↑[253] | ↑[254] | ↑[253] | ↑[254,255] —[246] | ↓[256,257] | ||||
| G | —[246] | —[246] | |||||||||
| GAA | —[246] | —[246] | |||||||||
| GSA | ↓[246] | —[246] | |||||||||
| MG | —[246] | —[246] | |||||||||
| MMA | ↓[258] | ↓[258] | ↑[259] | ||||||||
| NA | ↑[260] | ↑[260] | ↑[260] | ↑[261,262] | ↑[263,264] | ||||||
| SDMA | ↓[246] | —[246] | |||||||||
| TMAO | ↑[265] | ↑[265] | —[266] | ↑[265] | ↑[265,267] | ↑[267] | |||||
| UA | ↑[268,269] | ↑[269] | ↑[270,271,272,273] —[274] | ↑[268,269] | ↑[275,276,277,278] | ↑[276,279] | ↑[280,281] | ↑[282] | ↑[276] | ||
| GPA | —[246] | —[246] | |||||||||
| GBA | —[246] | —[246] |
4. Challenges in Uremic Toxin–VC Research
5. Treatment Strategies
6. Future Perspectives
7. Summary
8. Search Strategy
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADM | Adrenomedullin |
| ADMA | Asymmetric Dimethylarginine |
| ALP | alkaline phosphatase |
| APN | Adiponectin |
| BMP2 | bone morphogenetic protein 2 |
| CAC | coronary artery calcification |
| CKD | Chronic kidney disease |
| CML | N(6)-Carboxymethyllysine |
| CRS | cardiorenal syndrome |
| CV | cardiovascular |
| CVD | cardiovascular disease |
| ESRD | End stage renal disease |
| ET | Endothelin |
| FBS | Foetal bovine serum |
| G | Guanidine |
| GAA | Guanidino acetic acid |
| GBA | γ-guanidinobutyric acid |
| Gla | gamma-carboxylated glutamate |
| GPA | β-Guanidinopropionic acid |
| GSA | Guanidino succinic acid |
| GSK | glycogen synthase kinase |
| HA | hydroxyapatite |
| Hcy | Homocystein |
| IL | Interleukin |
| IS | Indoxyl sulfate |
| kDa | kilodaltons |
| LDL | low density lipoprotein receptor |
| LMWS | low molecular weight solutes |
| MG | Methylguanidine |
| MGP | Matrix Gla-protein |
| MM | Middle molecules |
| MW | molecular weight |
| PBURM | protein bound uremic retention molecules |
| pCS | p cresyl sulfate |
| Pi | Inorganic phosphate |
| PTH | Parathyroid hormone |
| Runx2 | Runt-related transcription factor 2 |
| SDMA | Symmetric Dimethylarginine |
| SM | Spermine |
| TMAO | Trimethylamine-N-Oxide |
| TNF- α | Tumor necrosis factor alpha |
| VC | vascular calcification |
| VSMC | vascular smooth muscle cell |
References
- Kassebaum, N.J.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, J.; Carter, A.; Casey, D.C.; Charlson, F.J.; Coates, M.M.; Coggeshall, M.; et al. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1603–1658. [Google Scholar] [CrossRef] [Green Version]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Amedia, C.A. Economic burden of chronic kidney disease. J. Eval. Clin. Pract. 2008, 14, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.S.; Kazmi, W.H.; Abichandani, R.; Tighiouart, H.; Pereira, B.J.G.; Kausz, A.T. Health care utilization among patients with chronic kidney disease. Kidney Int. 2002, 62, 229–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unger, E.D.; Dubin, R.F.; Deo, R.; Daruwalla, V.; Friedman, J.L.; Medina, C.; Beussink, L.; Freed, B.H.; Shah, S.J. Association of chronic kidney disease with abnormal cardiac mechanics and adverse outcomes in patients with heart failure and preserved ejection fraction. Eur. J. Heart Fail. 2016, 18, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jager, D.J.; Grootendorst, D.C.; Jager, K.J.; Van Dijk, P.C.; Tomas, L.M.J.; Ansell, D.; Collart, F.; Finne, P.; Heaf, J.G.; Meester, J.D.; et al. Cardiovascular and Noncardiovascular Mortality Among Patients Starting Dialysis. JAMA 2009, 302, 1782–1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangaswami, J.; Bhalla, V.; Blair, J.E.A.; Chang, T.I.; Costa, S.; Lentine, K.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W.; et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement from the American Heart Association. Circulation 2019, 139, e840–e878. [Google Scholar] [CrossRef] [PubMed]
- Alani, H. Cardiovascular co-morbidity in chronic kidney disease: Current knowledge and future research needs. World J. Nephrol. 2014, 3, 156. [Google Scholar] [CrossRef]
- Vlagopoulos, P.T.; Sarnak, M.J. Traditional and Nontraditional Cardiovascular Risk Factors in Chronic Kidney Disease. Med. Clin. N. Am. 2005, 89, 587–611. [Google Scholar] [CrossRef]
- Di Lullo, L.; House, A.; Gorini, A.; Santoboni, A.; Russo, D.; Ronco, C. Chronic kidney disease and cardiovascular complications. Heart Fail. Rev. 2015, 20, 259–272. [Google Scholar] [CrossRef]
- Wang, X.-R.; Zhang, J.-J.; Xu, X.-X.; Wu, Y.-G. Prevalence of coronary artery calcification and its association with mortality, cardiovascular events in patients with chronic kidney disease: A systematic review and meta-analysis. Renal Fail. 2019, 41, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Rennenberg, R.J.M.W.; Kessels, A.G.H.; Schurgers, L.J.; Van Engelshoven, J.M.A.; De Leeuw, P.W.; Kroon, A.A. Vascular calcifications as a marker of increased cardiovascular risk: A meta-analysis. Vasc. Health Risk Manag. 2009, 5, 185–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha, P.G.; Boutouyrie, P.; Nilsson, P.M.; Laurent, S. Early Vascular Ageing (EVA): Definitions and Clinical Applicability. Curr. Hypertens. Rev. 2017, 13, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Luttropp, K.; McGuinness, D.; Witasp, A.; Qureshi, A.R.; Wernerson, A.; Nordfors, L.; Schalling, M.; Ripsweden, J.; Wennberg, L.; et al. CDKN2A/p16INK4a expression is associated with vascular progeria in chronic kidney disease. Aging (Albany NY) 2017, 9, 494–507. [Google Scholar] [CrossRef] [Green Version]
- Paloian, N.J.; Giachelli, C.M. A current understanding of vascular calcification in CKD. Am. J. Physiol. Ren. Physiol. 2014, 307, F891–F900. [Google Scholar] [CrossRef] [Green Version]
- Capusa, C.; Popescu, D. Mechanisms and Clinical Implications of Vascular Calcifications in Chronic Kidney Disease. Chronic Kidney Dis. Pathophysiol. Clin. Improv. 2017. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Rementer, C.; Giachelli, C.M. Vascular Calcification: An Update on Mechanisms and Challenges in Treatment. Calcif. Tissue Int. 2013, 93, 365–373. [Google Scholar] [CrossRef]
- Jaminon, A.; Reesink, K.; Kroon, A.; Schurgers, L. The Role of Vascular Smooth Muscle Cells in Arterial Remodeling: Focus on Calcification-Related Processes. Int. J. Mol. Sci. 2019, 20, 5694. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Gomez, D. Smooth Muscle Cell Phenotypic Diversity. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1715–1723. [Google Scholar] [CrossRef]
- Durham, A.L.; Speer, M.Y.; Scatena, M.; Giachelli, C.M.; Shanahan, C.M. Role of smooth muscle cells in vascular calcification: Implications in atherosclerosis and arterial stiffness. Cardiovasc. Res. 2018, 114, 590–600. [Google Scholar] [CrossRef]
- Leopold, J.A. Vascular calcification: Mechanisms of vascular smooth muscle cell calcification. Trends Cardiovasc. Med. 2015, 25, 267–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Solis, A.J.; González-Pacheco, F.R.; Deudero, J.J.P.; Neria, F.; Albalate, M.; Petkov, V.; Susanibar, L.; Fernandez-Sanchez, R.; Calabia, O.; Ortiz, A.; et al. Alkalinization potentiates vascular calcium deposition in an uremic milieu. J. Nephrol. 2009, 22, 647–653. [Google Scholar] [PubMed]
- Cazaña-Pérez, V.; Cidad, P.; Donate-Correa, J.; Martín-Núñez, E.; López-López, J.R.; Pérez-García, M.T.; Giraldez, T.; Navarro-González, J.F.; Alvarez de la Rosa, D. Phenotypic Modulation of Cultured Primary Human Aortic Vascular Smooth Muscle Cells by Uremic Serum. Front. Physiol. 2018, 9, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciceri, P.; Galassi, A.; Alfieri, C.; Messa, P.; Cozzolino, M. Uremic Patients with Increased Vascular Calcification Score Have Serum with High Calcific Potential: Role of Vascular Smooth Muscle Cell Osteoblastic Differentiation and Apoptosis. Blood Purif. 2019, 48, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zheng, L.; Xu, H.; Tang, D.; Lin, L.; Zhang, J.; Li, C.; Wang, W.; Yuan, Q.; Tao, L.; et al. Oxidative stress contributes to vascular calcification in patients with chronic kidney disease. J. Mol. Cell. Cardiol. 2020, 138, 256–268. [Google Scholar] [CrossRef] [Green Version]
- Vanholder, R.; Pletinck, A.; Schepers, E.; Glorieux, G. Biochemical and Clinical Impact of Organic Uremic Retention Solutes: A Comprehensive Update. Toxins 2018, 10, 33. [Google Scholar] [CrossRef] [Green Version]
- Evenepoel, P.; Poesen, R.; Meijers, B. The gut-kidney axis. Pediatr. Nephrol. 2017, 32, 2005–2014. [Google Scholar] [CrossRef]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef] [Green Version]
- Hénaut, L.; Mary, A.; Chillon, J.-M.; Kamel, S.; Massy, Z. The Impact of Uremic Toxins on Vascular Smooth Muscle Cell Function. Toxins 2018, 10, 218. [Google Scholar] [CrossRef] [Green Version]
- Madero, M.; Cano, K.B.; Campos, I.; Tao, X.; Maheshwari, V.; Brown, J.; Cornejo, B.; Handelman, G.; Thijssen, S.; Kotanko, P. Removal of Protein-Bound Uremic Toxins during Hemodialysis Using a Binding Competitor. CJASN 2019, 14, 394–402. [Google Scholar] [CrossRef]
- Zhan, J.-K.; Wang, Y.-J.; Wang, Y.; Tang, Z.-Y.; Tan, P.; Huang, W.; Liu, Y.-S. Adiponectin attenuates the osteoblastic differentiation of vascular smooth muscle cells through the AMPK/mTOR pathway. Exp. Cell Res. 2014, 323, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.-K.; Wang, Y.-J.; Wang, Y.; Wang, S.; Tan, P.; Huang, W.; Liu, Y.-S. The mammalian target of rapamycin signalling pathway is involved in osteoblastic differentiation of vascular smooth muscle cells. Can. J. Cardiol. 2014, 30, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Son, B.-K.; Akishita, M.; Iijima, K.; Kozaki, K.; Maemura, K.; Eto, M.; Ouchi, Y. Adiponectin antagonizes stimulatory effect of tumor necrosis factor-alpha on vascular smooth muscle cell calcification: Regulation of growth arrest-specific gene 6-mediated survival pathway by adenosine 5′-monophosphate-activated protein kinase. Endocrinology 2008, 149, 1646–1653. [Google Scholar] [CrossRef] [PubMed]
- Niknam, S.; Ghatreh-Samani, K.; Farrokhi, E. The effect of adiponectin on osteonectin gene expression by oxidized low density lipoprotein-treated vascular smooth muscle cells. Int. J. Mol. Cell. Med. 2015, 4, 60–66. [Google Scholar]
- Lu, Y.; Bian, Y.; Wang, Y.; Bai, R.; Wang, J.; Xiao, C. Globular adiponectin reduces vascular calcification via inhibition of ER-stress-mediated smooth muscle cell apoptosis. Int. J. Clin. Exp. Pathol. 2015, 8, 2545–2554. [Google Scholar]
- Luo, X.-H.; Zhao, L.-L.; Yuan, L.-Q.; Wang, M.; Xie, H.; Liao, E.-Y. Development of arterial calcification in adiponectin-deficient mice: Adiponectin regulates arterial calcification. J. Bone Miner. Res. 2009, 24, 1461–1468. [Google Scholar] [CrossRef]
- Maahs, D.M.; Ogden, L.G.; Kinney, G.L.; Wadwa, P.; Snell-Bergeon, J.K.; Dabelea, D.; Hokanson, J.E.; Ehrlich, J.; Eckel, R.H.; Rewers, M. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation 2005, 111, 747–753. [Google Scholar] [CrossRef] [Green Version]
- Steffes, M.W.; Gross, M.D.; Lee, D.-H.; Schreiner, P.J.; Jacobs, D.R. Adiponectin, visceral fat, oxidative stress, and early macrovascular disease: The Coronary Artery Risk Development in Young Adults Study. Obesity 2006, 14, 319–326. [Google Scholar] [CrossRef]
- Tanna, N.; Patel, K.; Moore, A.E.; Dulnoan, D.; Edwards, S.; Hampson, G. The relationship between circulating adiponectin, leptin and vaspin with bone mineral density (BMD), arterial calcification and stiffness: A cross-sectional study in post-menopausal women. J. Endocrinol. Investig. 2017, 40, 1345–1353. [Google Scholar] [CrossRef]
- Aoqui, C.; Cuppari, L.; Kamimura, M.A.; Canziani, M.E.F. Increased visceral adiposity is associated with coronary artery calcification in male patients with chronic kidney disease. Eur. J. Clin. Nutr. 2013, 67, 610–614. [Google Scholar] [CrossRef]
- Larsen, B.A.; Laughlin, G.A.; Cummins, K.; Barrett-Connor, E.; Wassel, C.L. Adipokines and severity and progression of coronary artery calcium: Findings from the Rancho Bernardo Study. Atherosclerosis 2017, 265, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Värri, M.; Niskanen, L.; Tuomainen, T.; Honkanen, R.; Kröger, H.; Tuppurainen, M.T. Association of adipokines and estradiol with bone and carotid calcifications in postmenopausal women. Climacteric 2016, 19, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Hyun, Y.Y.; Kim, H.; Oh, Y.K.; Oh, K.-H.; Ahn, C.; Sung, S.A.; Choi, K.H.; Kim, S.W.; Lee, K.-B. High fibroblast growth factor 23 is associated with coronary calcification in patients with high adiponectin: Analysis from the KoreaN cohort study for Outcome in patients with Chronic Kidney Disease (KNOW-CKD) study. Nephrol. Dial. Transplant. 2019, 34, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Patel, J.; Al Rifai, M.; Ayers, C.R.; Neeland, I.J.; Kanaya, A.M.; Kandula, N.; Blaha, M.J.; Nasir, K.; Blumenthal, R.S.; et al. Inflammation and coronary artery calcification in South Asians: The Mediators of Atherosclerosis in South Asians Living in America (MASALA) study. Atherosclerosis 2018, 270, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, T.; Haraguchi, G.; Konishi, M.; Ohigashi, H.; Saito, K.; Nakano, Y.; Isobe, M. Effect of adiponectin on cardiac allograft vasculopathy. Circ. J. 2011, 75, 2005–2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, L.; Zheng, W.; Lian, G.; Chen, H.; Li, L.; Xu, C.; Xie, L. Combination treatment of adipose-derived stem cells and adiponectin attenuates pulmonary arterial hypertension in rats by inhibiting pulmonary arterial smooth muscle cell proliferation and regulating the AMPK/BMP/Smad pathway. Int. J. Mol. Med. 2018, 41, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Lam, K.S.L.; Xu, J.Y.; Lu, G.; Xu, L.Y.; Cooper, G.J.S.; Xu, A. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J. Biol. Chem. 2005, 280, 18341–18347. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, M.; Shimomura, I.; Sata, M.; Arita, Y.; Nishida, M.; Maeda, N.; Kumada, M.; Okamoto, Y.; Nagaretani, H.; Nishizawa, H.; et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J. Biol. Chem. 2002, 277, 37487–37491. [Google Scholar] [CrossRef] [Green Version]
- Saneipour, M.; Ghatreh-Samani, K.; Heydarian, E.; Farrokhi, E.; Abdian, N. Adiponectin inhibits oxidized low density lipoprotein-induced increase in matrix metalloproteinase 9 expression in vascular smooth muscle cells. ARYA Atheroscler. 2015, 11, 191–195. [Google Scholar]
- Okamoto, Y.; Kihara, S.; Ouchi, N.; Nishida, M.; Arita, Y.; Kumada, M.; Ohashi, K.; Sakai, N.; Shimomura, I.; Kobayashi, H.; et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation 2002, 106, 2767–2770. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Teng, X.; Pan, C.; Duan, X.; Tang, C.; Qi, Y. Adrenomedullin up-regulates osteopontin and attenuates vascular calcification via the cAMP/PKA signaling pathway. Acta Pharmacol. Sin. 2010, 31, 1359–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Li, J.; Jiang, Z.; Qi, Y.; Tang, C.; Du, J. Effects of adrenomedullin, C-type natriuretic peptide, and parathyroid hormone-related peptide on calcification in cultured rat vascular smooth muscle cells. J. Cardiovasc. Pharmacol. 2003, 42, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-B.; Gao, Q.; Li, P.; Han, Y.; Zhang, F.; Qi, Y.-F.; Tang, C.-S.; Gao, X.-Y.; Zhu, G.-Q. Adrenomedullin attenuates vascular calcification in fructose-induced insulin resistance rats. Acta Physiol. 2013, 207, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shimosawa, T.; Matsui, H.; Meng, F.; Supowit, S.C.; DiPette, D.J.; Ando, K.; Fujita, T. Adrenomedullin inhibits angiotensin II-induced oxidative stress via Csk-mediated inhibition of Src activity. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1714–H1721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimoto, T.; Fukai, N.; Sato, R.; Sugiyama, T.; Ozawa, N.; Shichiri, M.; Hirata, Y. Antioxidant effect of adrenomedullin on angiotensin II-induced reactive oxygen species generation in vascular smooth muscle cells. Endocrinology 2004, 145, 3331–3337. [Google Scholar] [CrossRef] [PubMed]
- Shichiri, M.; Fukai, N.; Ozawa, N.; Iwasaki, H.; Hirata, Y. Adrenomedullin is an autocrine/paracrine growth factor for rat vascular smooth muscle cells. Regul. Pept. 2003, 112, 167–173. [Google Scholar] [CrossRef]
- Iwasaki, H.; Eguchi, S.; Shichiri, M.; Marumo, F.; Hirata, Y. Adrenomedullin as a novel growth-promoting factor for cultured vascular smooth muscle cells: Role of tyrosine kinase-mediated mitogen-activated protein kinase activation. Endocrinology 1998, 139, 3432–3441. [Google Scholar] [CrossRef]
- Kano, H.; Kohno, M.; Yasunari, K.; Yokokawa, K.; Horio, T.; Ikeda, M.; Minami, M.; Hanehira, T.; Takeda, T.; Yoshikawa, J. Adrenomedullin as a novel antiproliferative factor of vascular smooth muscle cells. J. Hypertens. 1996, 14, 209–213. [Google Scholar] [CrossRef]
- Ma, J.; Feng, Y.; Li, Z.; Tang, C. The effect of adrenomedullin and proadrenomedullin N-terminal 20 peptide on angiotensin II induced vascular smooth muscle cell proliferation. Iran. J. Basic Med. Sci. 2016, 19, 49–54. [Google Scholar]
- Kohno, M.; Yokokawa, K.; Kano, H.; Yasunari, K.; Minami, M.; Hanehira, T.; Yoshikawa, J. Adrenomedullin is a potent inhibitor of angiotensin II-induced migration of human coronary artery smooth muscle cells. Hypertension 1997, 29, 1309–1313. [Google Scholar] [CrossRef]
- Horio, T.; Kohno, M.; Kano, H.; Ikeda, M.; Yasunari, K.; Yokokawa, K.; Minami, M.; Takeda, T. Adrenomedullin as a novel antimigration factor of vascular smooth muscle cells. Circ. Res. 1995, 77, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Zhang, B.H.; Pan, C.S.; Jiang, H.F.; Pang, Y.Z.; Tang, C.S.; Qi, Y.F. Endothelin-1 is a potent regulator in vivo in vascular calcification and in vitro in calcification of vascular smooth muscle cells. Peptides 2003, 24, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Essalihi, R.; Ouellette, V.; Dao, H.H.; McKee, M.D.; Moreau, P. Phenotypic modulation of vascular smooth muscle cells during medial arterial calcification: A role for endothelin? J. Cardiovasc. Pharmacol. 2004, 44 (Suppl. 1), S147–S150. [Google Scholar] [CrossRef]
- Larivière, R.; Gauthier-Bastien, A.; Ung, R.-V.; St-Hilaire, J.; Mac-Way, F.; Richard, D.E.; Agharazii, M. Endothelin type A receptor blockade reduces vascular calcification and inflammation in rats with chronic kidney disease. J. Hypertens. 2017, 35, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, T.; Cong, X.; Hou, Z.; Lu, B.; Zhou, Z.; Chen, X. The Value of Big Endothelin-1 in the Assessment of the Severity of Coronary Artery Calcification. Clin. Appl. Thromb. Hemost. 2018, 24, 1042–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qing, P.; Li, X.-L.; Zhang, Y.; Li, Y.-L.; Xu, R.-X.; Guo, Y.-L.; Li, S.; Wu, N.-Q.; Li, J.-J. Association of Big Endothelin-1 with Coronary Artery Calcification. PLoS ONE 2015, 10, e0142458. [Google Scholar] [CrossRef]
- Rebić, D.; Rašić, S.; Hamzić-Mehmedbašić, A.; Džemidžić, J.; Kurtalić, E. Valvular calcification and left ventricular modifying in peritoneal dialysis patients. Ren. Fail. 2015, 37, 1316–1322. [Google Scholar] [CrossRef] [Green Version]
- Wedgwood, S.; Dettman, R.W.; Black, S.M. ET-1 stimulates pulmonary arterial smooth muscle cell proliferation via induction of reactive oxygen species. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 281, L1058–L1067. [Google Scholar] [CrossRef] [Green Version]
- Idris-Khodja, N.; Ouerd, S.; Trindade, M.; Gornitsky, J.; Rehman, A.; Barhoumi, T.; Offermanns, S.; Gonzalez, F.J.; Neves, M.F.; Paradis, P.; et al. Vascular smooth muscle cell peroxisome proliferator-activated receptor γ protects against endothelin-1-induced oxidative stress and inflammation. J. Hypertens. 2017, 35, 1390–1401. [Google Scholar] [CrossRef]
- Trindade, M.; Oigman, W.; Fritsch Neves, M. Potential Role of Endothelin in Early Vascular Aging. Curr. Hypertens. Rev. 2017, 13, 33–40. [Google Scholar] [CrossRef]
- Lin, C.-C.; Lin, W.-N.; Hou, W.-C.; Hsiao, L.-D.; Yang, C.-M. Endothelin-1 induces VCAM-1 expression-mediated inflammation via receptor tyrosine kinases and Elk/p300 in human tracheal smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L211–L225. [Google Scholar] [CrossRef] [PubMed]
- Kim, F.Y.; Barnes, E.A.; Ying, L.; Chen, C.; Lee, L.; Alvira, C.M.; Cornfield, D.N. Pulmonary artery smooth muscle cell endothelin-1 expression modulates the pulmonary vascular response to chronic hypoxia. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, L368–L377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, F.; Mao, Z.; Xia, J.; Zhu, S.; Wu, Z. Fluoxetine protects against big endothelin-1 induced anti-apoptosis by rescuing Kv1.5 channels in human pulmonary arterial smooth muscle cells. Yonsei Med. J. 2012, 53, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Sauter, G.; Wolf, S.; Risler, T.; Brehm, B. Influence of endothelin receptor antagonism on smooth muscle cell proliferation after chronic renal failure. J. Cardiovasc. Pharmacol. 2004, 44 (Suppl. 1), S165–S167. [Google Scholar] [CrossRef] [Green Version]
- Bouallegue, A.; Vardatsikos, G.; Srivastava, A.K. Role of insulin-like growth factor 1 receptor and c-Src in endothelin-1- and angiotensin II-induced PKB phosphorylation, and hypertrophic and proliferative responses in vascular smooth muscle cells. Can. J. Physiol. Pharmacol. 2009, 87, 1009–1018. [Google Scholar] [CrossRef]
- Sihvola, R.K.; Pulkkinen, V.P.; Koskinen, P.K.; Lemström, K.B. Crosstalk of endothelin-1 and platelet-derived growth factor in cardiac allograft arteriosclerosis. J. Am. Coll. Cardiol. 2002, 39, 710–717. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-J.; Juan, C.-C.; Kwok, C.-F.; Hsu, Y.-P.; Shih, K.-C.; Chen, C.-C.; Ho, L.-T. Endothelin-1 exacerbates development of hypertension and atherosclerosis in modest insulin resistant syndrome. Biochem. Biophys. Res. Commun. 2015, 460, 497–503. [Google Scholar] [CrossRef]
- Ivey, M.E.; Osman, N.; Little, P.J. Endothelin-1 signalling in vascular smooth muscle: Pathways controlling cellular functions associated with atherosclerosis. Atherosclerosis 2008, 199, 237–247. [Google Scholar] [CrossRef]
- Little, P.J.; Ivey, M.E.; Osman, N. Endothelin-1 actions on vascular smooth muscle cell functions as a target for the prevention of atherosclerosis. Curr. Vasc. Pharmacol. 2008, 6, 195–203. [Google Scholar] [CrossRef]
- Bouabdallah, J.; Zibara, K.; Issa, H.; Lenglet, G.; Kchour, G.; Caus, T.; Six, I.; Choukroun, G.; Kamel, S.; Bennis, Y. Endothelial cells exposed to phosphate and indoxyl sulphate promote vascular calcification through interleukin-8 secretion. Nephrol. Dial. Transplant. 2019, 34, 1125–1134. [Google Scholar] [CrossRef]
- Raaz-Schrauder, D.; Klinghammer, L.; Baum, C.; Frank, T.; Lewczuk, P.; Achenbach, S.; Cicha, I.; Stumpf, C.; Wiltfang, J.; Kornhuber, J.; et al. Association of systemic inflammation markers with the presence and extent of coronary artery calcification. Cytokine 2012, 57, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Gauss, S.; Klinghammer, L.; Steinhoff, A.; Raaz-Schrauder, D.; Marwan, M.; Achenbach, S.; Garlichs, C.D. Association of systemic inflammation with epicardial fat and coronary artery calcification. Inflamm. Res. 2015, 64, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Hastings, N.E.; Feaver, R.E.; Lee, M.Y.; Wamhoff, B.R.; Blackman, B.R. Human IL-8 regulates smooth muscle cell VCAM-1 expression in response to endothelial cells exposed to atheroprone flow. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 725–731. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Fan, F.; Zhao, Y.; Cui, Y.; Wei, X.; Kohama, K.; Gordon, J.R.; Li, F.; Gao, Y. Recombinant human CXCL8(3-72)K11R/G31P regulates smooth muscle cell proliferation and migration through blockage of interleukin-8 receptor. IUBMB Life 2013, 65, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Zhu, H.; Li, C.; Wang, J.; Zhao, D.; Li, S.; Ma, L.; Sun, G.; Li, F.; Zhao, Y.; et al. Inhibition of IL-8-mediated endothelial adhesion, VSMCs proliferation and migration by siRNA-TMEM98 suggests TMEM98′s emerging role in atherosclerosis. Oncotarget 2017, 8, 88043–88058. [Google Scholar] [CrossRef] [Green Version]
- Schelski, N.; Luong, T.T.; Lang, F.; Pieske, B.; Voelkl, J.; Alesutan, I. SGK1-Dependent Stimulation of Vascular Smooth Muscle Cell Osteo-/Chondrogenic Transdifferentiation by Interleukin-18. Available online: https://pubmed.ncbi.nlm.nih.gov/30706178/?from_term=il-18+AND+calcification&from_size=50&from_pos=7 (accessed on 19 March 2020).
- Zhang, K.; Zhang, Y.; Feng, W.; Chen, R.; Chen, J.; Touyz, R.M.; Wang, J.; Huang, H. Interleukin-18 Enhances Vascular Calcification and Osteogenic Differentiation of Vascular Smooth Muscle Cells through TRPM7 Activation. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1933–1943. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Wei, G.; Liu, B.; Zhou, X.; Xiao, H.; Dong, N.; Li, F. The Role of High Mobility Group Box 1 Protein in Interleukin-18-Induced Myofibroblastic Transition of Valvular Interstitial Cells. CRD 2016, 135, 168–178. [Google Scholar] [CrossRef]
- Porażko, T.; Kuźniar, J.; Kusztal, M.; Kuźniar, T.J.; Weyde, W.; Kuriata-Kordek, M.; Klinger, M. IL-18 Is Involved in Vascular Injury in End-Stage Renal Disease Patients. Available online: https://pubmed.ncbi.nlm.nih.gov/18775894/?from_term=il-18+AND+calcification&from_size=50&from_pos=9 (accessed on 19 March 2020).
- Kiu Weber, C.I.; Duchateau-Nguyen, G.; Solier, C.; Schell-Steven, A.; Hermosilla, R.; Nogoceke, E.; Block, G. Cardiovascular Risk Markers Associated with Arterial Calcification in Patients with Chronic Kidney Disease Stages 3 and 4. Available online: https://pubmed.ncbi.nlm.nih.gov/24683472/?from_term=il-18+AND+calcification&from_size=50&from_pos=17 (accessed on 19 March 2020).
- Venkatesan, B.; Valente, A.J.; Reddy, V.S.; Siwik, D.A.; Chandrasekar, B. Resveratrol blocks interleukin-18-EMMPRIN cross-regulation and smooth muscle cell migration. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H874–H886. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Menocal, L.; Faridi, M.H.; Martinez, L.; Shehadeh, L.A.; Duque, J.C.; Wei, Y.; Mesa, A.; Pena, A.; Gupta, V.; Pham, S.M.; et al. Macrophage-derived IL-18 and increased fibrinogen deposition are age-related inflammatory signatures of vascular remodeling. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H641–H653. [Google Scholar] [CrossRef]
- Sahar, S.; Dwarakanath, R.S.; Reddy, M.A.; Lanting, L.; Todorov, I.; Natarajan, R. Angiotensin II enhances interleukin-18 mediated inflammatory gene expression in vascular smooth muscle cells: A novel cross-talk in the pathogenesis of atherosclerosis. Circ. Res. 2005, 96, 1064–1071. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekar, B.; Mummidi, S.; Valente, A.J.; Patel, D.N.; Bailey, S.R.; Freeman, G.L.; Hatano, M.; Tokuhisa, T.; Jensen, L.E. The pro-atherogenic cytokine interleukin-18 induces CXCL16 expression in rat aortic smooth muscle cells via MyD88, interleukin-1 receptor-associated kinase, tumor necrosis factor receptor-associated factor 6, c-Src, phosphatidylinositol 3-kinase, Akt, c-Jun N-terminal kinase, and activator protein-1 signaling. J. Biol. Chem. 2005, 280, 26263–26277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.-C.; Chiang, C.-H.; Chang, L.-T.; Sun, C.-K.; Leu, S.; Shao, P.-L.; Hsieh, M.-C.; Tsai, T.-H.; Chua, S.; Chung, S.-Y.; et al. Simvastatin attenuates the additive effects of TNF-α and IL-18 on the connexin 43 up-regulation and over-proliferation of cultured aortic smooth muscle cells. Cytokine 2013, 62, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, B.; Mummidi, S.; Mahimainathan, L.; Patel, D.N.; Bailey, S.R.; Imam, S.Z.; Greene, W.C.; Valente, A.J. Interleukin-18-induced human coronary artery smooth muscle cell migration is dependent on NF-kappaB- and AP-1-mediated matrix metalloproteinase-9 expression and is inhibited by atorvastatin. J. Biol. Chem. 2006, 281, 15099–15109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valente, A.J.; Yoshida, T.; Izadpanah, R.; Delafontaine, P.; Siebenlist, U.; Chandrasekar, B. Interleukin-18 enhances IL-18R/Nox1 binding, and mediates TRAF3IP2-dependent smooth muscle cell migration. Inhibition by simvastatin. Cell. Signal. 2013, 25, 1447–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elhage, R.; Jawien, J.; Rudling, M.; Ljunggren, H.-G.; Takeda, K.; Akira, S.; Bayard, F.; Hansson, G.K. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovasc. Res. 2003, 59, 234–240. [Google Scholar] [CrossRef]
- Tenger, C.; Sundborger, A.; Jawien, J.; Zhou, X. IL-18 accelerates atherosclerosis accompanied by elevation of IFN-gamma and CXCL16 expression independently of T cells. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 791–796. [Google Scholar] [CrossRef] [Green Version]
- Bessueille, L.; Fakhry, M.; Hamade, E.; Badran, B.; Magne, D. Glucose stimulates chondrocyte differentiation of vascular smooth muscle cells and calcification: A possible role for IL-1β. FEBS Lett. 2015, 589, 2797–2804. [Google Scholar] [CrossRef]
- Ceneri, N.; Zhao, L.; Young, B.D.; Healy, A.; Coskun, S.; Vasavada, H.; Yarovinsky, T.O.; Ike, K.; Pardi, R.; Qin, L.; et al. Rac2 Modulates Atherosclerotic Calcification by Regulating Macrophage IL-1β Production. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 328–340. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Zhang, Y.; Zhang, M.; Guo, L.; Wang, J.; Zeng, F.; Xu, D.; Yin, Z.; Xu, Y.; Wang, D.; et al. Interleukin-1β-Induced Senescence Promotes Osteoblastic Transition of Vascular Smooth Muscle Cells. Kidney Blood Press. Res. 2020, 45, 314–330. [Google Scholar] [CrossRef]
- Dautova, Y.; Kapustin, A.N.; Pappert, K.; Epple, M.; Okkenhaug, H.; Cook, S.J.; Shanahan, C.M.; Bootman, M.D.; Proudfoot, D. Calcium phosphate particles stimulate interleukin-1β release from human vascular smooth muscle cells: A role for spleen tyrosine kinase and exosome release. J. Mol. Cell. Cardiol. 2018, 115, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Agharazii, M.; St-Louis, R.; Gautier-Bastien, A.; Ung, R.-V.; Mokas, S.; Larivière, R.; Richard, D.E. Inflammatory cytokines and reactive oxygen species as mediators of chronic kidney disease-related vascular calcification. Am. J. Hypertens. 2015, 28, 746–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awan, Z.; Denis, M.; Roubtsova, A.; Essalmani, R.; Marcinkiewicz, J.; Awan, A.; Gram, H.; Seidah, N.G.; Genest, J. Reducing Vascular Calcification by Anti-IL-1β Monoclonal Antibody in a Mouse Model of Familial Hypercholesterolemia. Angiology 2016, 67, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Kraśniak, A.; Drozdz, M.; Pasowicz, M.; Chmiel, G.; Michałek, M.; Szumilak, D.; Podolec, P.; Klimeczek, P.; Konieczyńska, M.; Wicher-Muniak, E.; et al. Factors involved in vascular calcification and atherosclerosis in maintenance haemodialysis patients. Nephrol. Dial. Transplant. 2007, 22, 515–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, K.; Souma, Y.; Akakabe, Y.; Kitamura, Y.; Matsuo, K.; Shimoda, Y.; Ueyama, T.; Matoba, S.; Yamada, H.; Okigaki, M.; et al. Macrophages play a unique role in the plaque calcification by enhancing the osteogenic signals exerted by vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2012, 425, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Hoare, G.S.; Marczin, N.; Chester, A.H.; Yacoub, M.H. Role of oxidant stress in cytokine-induced activation of NF-kappaB in human aortic smooth muscle cells. Am. J. Physiol. 1999, 277, H1975–H1984. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.R.; Murgai, M.; Moehle, C.W.; Owens, G.K. Interleukin-1β modulates smooth muscle cell phenotype to a distinct inflammatory state relative to PDGF-DD via NF-κB-dependent mechanisms. Physiol. Genom. 2012, 44, 417–429. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.H.; Um, H.J.; Park, J.-W.; Lee, I.-K.; Kwon, T.K. Interleukin-1beta promotes the expression of monocyte chemoattractant protein-1 in human aorta smooth muscle cells via multiple signaling pathways. Exp. Mol. Med. 2009, 41, 757–764. [Google Scholar] [CrossRef]
- Geng, Y.J.; Henderson, L.E.; Levesque, E.B.; Muszynski, M.; Libby, P. Fas is expressed in human atherosclerotic intima and promotes apoptosis of cytokine-primed human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2200–2208. [Google Scholar] [CrossRef]
- Geng, Y.J.; Wu, Q.; Muszynski, M.; Hansson, G.K.; Libby, P. Apoptosis of vascular smooth muscle cells induced by in vitro stimulation with interferon-gamma, tumor necrosis factor-alpha, and interleukin-1 beta. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 19–27. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Liu, J.; Yan, T.; Wu, G.; Xia, Y.; Zong, G.; Li, F. In vivo treatment of rat arterial adventitia with interleukin-1β induces intimal proliferation via the JAK2/STAT3 signaling pathway. Mol. Med. Rep. 2016, 13, 3451–3458. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Kim, M.Y.; Hwang, J.S.; Kim, H.J.; Lee, J.H.; Chang, K.C.; Kim, J.-H.; Han, C.W.; Kim, J.-H.; Seo, H.G. PPARdelta inhibits IL-1beta-stimulated proliferation and migration of vascular smooth muscle cells via up-regulation of IL-1Ra. Cell. Mol. Life Sci. 2010, 67, 2119–2130. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kong, L.; Kang, J.; Vaughn, D.M.; Bush, G.D.; Walding, A.L.; Grigorian, A.A.; Robinson, J.S.; Nakayama, D.K. Interleukin-lβ induces migration of rat arterial smooth muscle cells through a mechanism involving increased matrix metalloproteinase-2 activity. J. Surg. Res. 2011, 169, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Rheaume, E.; Tardif, J.-C. Examining the Role of and Treatment Directed at IL-1β in Atherosclerosis. Curr. Atheroscler. Rep. 2018, 20, 53. [Google Scholar] [CrossRef] [PubMed]
- Deuell, K.A.; Callegari, A.; Giachelli, C.M.; Rosenfeld, M.E.; Scatena, M. RANKL enhances macrophage paracrine pro-calcific activity in high phosphate-treated smooth muscle cells: Dependence on IL-6 and TNF-α. J. Vasc. Res. 2012, 49, 510–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callegari, A.; Coons, M.L.; Ricks, J.L.; Rosenfeld, M.E.; Scatena, M. Increased Calcification in Osteoprotegerin Deficient Smooth Muscle Cells: Dependence on Receptor Activator of NF-κB Ligand and Interleukin-6. J. Vasc. Res. 2014, 51, 118–131. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Watson, A.D.; Ji, S.; Boström, K.I. Heat shock protein 70 enhances vascular bone morphogenetic protein-4 signaling by binding matrix Gla protein. Circ. Res. 2009, 105, 575–584. [Google Scholar] [CrossRef]
- Sun, M.; Chang, Q.; Xin, M.; Wang, Q.; Li, H.; Qian, J. Endogenous bone morphogenetic protein 2 plays a role in vascular smooth muscle cell calcification induced by interleukin 6 in vitro. Int. J. Immunopathol. Pharmacol. 2017, 30, 227–237. [Google Scholar] [CrossRef]
- Xu, D.; Zeng, F.; Han, L.; Wang, J.; Yin, Z.; Lv, L.; Guo, L.; Wang, D.; Xu, Y.; Zhou, H. The synergistic action of phosphate and interleukin-6 enhances senescence-associated calcification in vascular smooth muscle cells depending on p53. Mech. Ageing Dev. 2019, 182, 111124. [Google Scholar] [CrossRef]
- Kurozumi, A.; Nakano, K.; Yamagata, K.; Okada, Y.; Nakayamada, S.; Tanaka, Y. IL-6 and sIL-6R induces STAT3-dependent differentiation of human VSMCs into osteoblast-like cells through JMJD2B-mediated histone demethylation of RUNX2. Bone 2019, 124, 53–61. [Google Scholar] [CrossRef]
- Lee, G.L.; Yeh, C.C.; Wu, J.Y.; Lin, H.C.; Wang, Y.F.; Kuo, Y.Y.; Hsieh, Y.T.; Hsu, Y.J.; Kuo, C.C. TLR2 Promotes Vascular Smooth Muscle Cell Chondrogenic Differentiation and Consequent Calcification via the Concerted Actions of Osteoprotegerin Suppression and IL-6–Mediated RANKL Induction. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 432–445. [Google Scholar] [CrossRef]
- Shavelle, D.M.; Katz, R.; Takasu, J.; Lima, J.A.; Jenny, N.S.; Budoff, M.J.; O’Brien, K.D. Soluble intercellular adhesion molecule-1 (sICAM-1) and aortic valve calcification in the multi-ethnic study of atherosclerosis (MESA). J. Heart Valve Dis. 2008, 17, 388–395. [Google Scholar] [PubMed]
- Takasu, J.; Katz, R.; Shavelle, D.M.; O’Brien, K.; Mao, S.; Carr, J.J.; Cushman, M.; Budoff, M.J. Inflammation and descending thoracic aortic calcification as detected by computed tomography: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2008, 199, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Qureshi, A.R.; Parini, P.; Hurt-Camejo, E.; Ripsweden, J.; Brismar, T.B.; Barany, P.; Jaminon, A.M.; Schurgers, L.J.; Heimbürger, O.; et al. Does statins promote vascular calcification in chronic kidney disease? Eur. J. Clin. Investig. 2017, 47, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Barreto, D.V.; Barreto, F.C.; Liabeuf, S.; Temmar, M.; Lemke, H.-D.; Tribouilloy, C.; Choukroun, G.; Vanholder, R.; Massy, Z.A.; European Uremic Toxin Work Group (EUTox). Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int. 2010, 77, 550–556. [Google Scholar] [CrossRef]
- He, J.; Reilly, M.; Yang, W.; Chen, J.; Go, A.S.; Lash, J.P.; Rahman, M.; DeFilippi, C.; Gadegbeku, C.; Kanthety, R.; et al. Risk factors for coronary artery calcium among patients with chronic kidney disease (from the Chronic Renal Insufficiency Cohort Study). Am. J. Cardiol. 2012, 110, 1735–1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bundy, J.D.; Chen, J.; Yang, W.; Budoff, M.; Go, A.S.; Grunwald, J.E.; Kallem, R.R.; Post, W.S.; Reilly, M.P.; Ricardo, A.C.; et al. Risk factors for progression of coronary artery calcification in patients with chronic kidney disease: The CRIC study. Atherosclerosis 2018, 271, 53–60. [Google Scholar] [CrossRef]
- Liu, Y.; Shanahan, C.M. Signalling pathways and vascular calcification. Front. Biosci. (Landmark Ed.) 2011, 16, 1302–1314. [Google Scholar] [CrossRef] [Green Version]
- Wassmann, S.; Stumpf, M.; Strehlow, K.; Schmid, A.; Schieffer, B.; Böhm, M.; Nickenig, G. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ. Res. 2004, 94, 534–541. [Google Scholar] [CrossRef] [Green Version]
- Den Dekker, W.K.; Tempel, D.; Bot, I.; Biessen, E.A.; Joosten, L.A.; Netea, M.G.; Van der Meer, J.W.M.; Cheng, C.; Duckers, H.J. Mast cells induce vascular smooth muscle cell apoptosis via a toll-like receptor 4 activation pathway. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1960–1969. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Shu, C.; Su, J.; Li, X. A crosstalk triggered by hypoxia and maintained by MCP-1/miR-98/IL-6/p38 regulatory loop between human aortic smooth muscle cells and macrophages leads to aortic smooth muscle cells apoptosis via Stat1 activation. Int. J. Clin. Exp. Pathol. 2015, 8, 2670–2679. [Google Scholar]
- Knobloch, J.; Yanik, S.D.; Körber, S.; Stoelben, E.; Jungck, D.; Koch, A. TNFα-induced airway smooth muscle cell proliferation depends on endothelin receptor signaling, GM-CSF and IL-6. Biochem. Pharmacol. 2016, 116, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Dong, N.-G.; Liu, J.-P.; Wang, Y.; Shi, J.-W.; Wei, Z.-J.; Hu, X.-J.; Gong, L. Inhibitory effects of suppressor of cytokine signaling 3 on inflammatory cytokine expression and migration and proliferation of IL-6/IFN-γ-induced vascular smooth muscle cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2013, 33, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-L.; Wu, J.-Y.; Tsai, C.-S.; Lin, C.-Y.; Tsai, Y.-T.; Lin, C.-S.; Wang, Y.-F.; Yet, S.-F.; Hsu, Y.-J.; Kuo, C.-C. TLR4-Activated MAPK-IL-6 Axis Regulates Vascular Smooth Muscle Cell Function. Int. J. Mol. Sci. 2016, 17, 1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrillo-López, N.; Panizo, S.; Alonso-Montes, C.; Martínez-Arias, L.; Avello, N.; Sosa, P.; Dusso, A.S.; Cannata-Andía, J.B.; Naves-Díaz, M. High-serum phosphate and parathyroid hormone distinctly regulate bone loss and vascular calcification in experimental chronic kidney disease. Nephrol. Dial. Transplant. 2019, 34, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Patidar, A.; Singh, D.K.; Winocour, P.; Farrington, K.; Baydoun, A.R. Human uraemic serum displays calcific potential in vitro that increases with advancing chronic kidney disease. Clin. Sci. 2013, 125, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Graciolli, F.G.; Neves, K.R.; dos Reis, L.M.; Graciolli, R.G.; Noronha, I.L.; Moysés, R.M.A.; Jorgetti, V. Phosphorus overload and PTH induce aortic expression of Runx2 in experimental uraemia. Nephrol. Dial. Transplant. 2009, 24, 1416–1421. [Google Scholar] [CrossRef]
- Neves, K.R.; Graciolli, F.G.; dos Reis, L.M.; Graciolli, R.G.; Neves, C.L.; Magalhães, A.O.; Custódio, M.R.; Batista, D.G.; Jorgetti, V.; Moysés, R.M.A. Vascular calcification: Contribution of parathyroid hormone in renal failure. Kidney Int. 2007, 71, 1262–1270. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.-L.; Shao, J.-S.; Halstead, L.R.; Distelhorst, K.; Sierra, O.; Towler, D.A. Activation of vascular smooth muscle parathyroid hormone receptor inhibits Wnt/beta-catenin signaling and aortic fibrosis in diabetic arteriosclerosis. Circ. Res. 2010, 107, 271–282. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.-S.; Cheng, S.-L.; Charlton-Kachigian, N.; Loewy, A.P.; Towler, D.A. Teriparatide (human parathyroid hormone (1-34)) inhibits osteogenic vascular calcification in diabetic low density lipoprotein receptor-deficient mice. J. Biol. Chem. 2003, 278, 50195–50202. [Google Scholar] [CrossRef] [Green Version]
- Malluche, H.H.; Blomquist, G.; Monier-Faugere, M.-C.; Cantor, T.L.; Davenport, D.L. High Parathyroid Hormone Level and Osteoporosis Predict Progression of Coronary Artery Calcification in Patients on Dialysis. J. Am. Soc. Nephrol. 2015, 26, 2534–2544. [Google Scholar] [CrossRef] [Green Version]
- Gulcicek, S.; Zoccali, C.; Olgun, D.Ç.; Tripepi, G.; Alagoz, S.; Yalın, S.F.; Trabulus, S.; Altiparmak, M.R.; Seyahi, N. Long-Term Progression of Coronary Artery Calcification Is Independent of Classical Risk Factors, C-Reactive Protein, and Parathyroid Hormone in Renal Transplant Patients. Cardiorenal Med. 2017, 7, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Hewitson, T.D.; Kelynack, K.J.; Martic, M.; Tait, M.G.; Becker, G.J. Parathyroid hormone has a prosclerotic effect on vascular smooth muscle cells. Kidney Blood Press. Res. 2003, 26, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, H.; Motomura, N.; Chung, U.-I.; Sata, M.; Takai, D.; Saito, A.; Ono, M.; Takamoto, S. Growth-associated hyperphosphatemia in young recipients accelerates aortic allograft calcification in a rat model. J. Thorac. Cardiovasc. Surg. 2013, 145, 522–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawada, S.; Nagasawa, Y.; Kawabe, M.; Ohyama, H.; Kida, A.; Kato-Kogoe, N.; Nanami, M.; Hasuike, Y.; Kuragano, T.; Kishimoto, H.; et al. Iron-induced calcification in human aortic vascular smooth muscle cells through interleukin-24 (IL-24), with/without TNF-alpha. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shioi, A.; Katagi, M.; Okuno, Y.; Mori, K.; Jono, S.; Koyama, H.; Nishizawa, Y. Induction of bone-type alkaline phosphatase in human vascular smooth muscle cells: Roles of tumor necrosis factor-alpha and oncostatin M derived from macrophages. Circ. Res. 2002, 91, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.-L.; Woo, K.M.; Ryoo, H.-M.; Baek, J.-H. Tumor necrosis factor-alpha increases alkaline phosphatase expression in vascular smooth muscle cells via MSX2 induction. Biochem. Biophys. Res. Commun. 2010, 391, 1087–1092. [Google Scholar] [CrossRef]
- Aghagolzadeh, P.; Bachtler, M.; Bijarnia, R.; Jackson, C.; Smith, E.R.; Odermatt, A.; Radpour, R.; Pasch, A. Calcification of vascular smooth muscle cells is induced by secondary calciprotein particles and enhanced by tumor necrosis factor-α. Atherosclerosis 2016, 251, 404–414. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Bian, W.; Fu, F.; Hu, J.; Liu, H. Selenoprotein S inhibits inflammation-induced vascular smooth muscle cell calcification. J. Biol. Inorg. Chem. 2018, 23, 739–751. [Google Scholar] [CrossRef]
- Zickler, D.; Luecht, C.; Willy, K.; Chen, L.; Witowski, J.; Girndt, M.; Fiedler, R.; Storr, M.; Kamhieh-Milz, J.; Schoon, J.; et al. Tumour necrosis factor-alpha in uraemic serum promotes osteoblastic transition and calcification of vascular smooth muscle cells via extracellular signal-regulated kinases and activator protein 1/c-FOS-mediated induction of interleukin 6 expression. Nephrol. Dial. Transplant. 2018, 33, 574–585. [Google Scholar] [CrossRef]
- García-Miguel, M.; Riquelme, J.A.; Norambuena-Soto, I.; Morales, P.E.; Sanhueza-Olivares, F.; Nuñez-Soto, C.; Mondaca-Ruff, D.; Cancino-Arenas, N.; San Martín, A.; Chiong, M. Autophagy mediates tumor necrosis factor-α-induced phenotype switching in vascular smooth muscle A7r5 cell line. PLoS ONE 2018, 13, e0197210. [Google Scholar] [CrossRef] [Green Version]
- Freise, C.; Querfeld, U. The lignan (+)-episesamin interferes with TNF-α-induced activation of VSMC via diminished activation of NF-ĸB, ERK1/2 and AKT and decreased activity of gelatinases. Acta Physiol. 2015, 213, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Dikalova, A.; Stark, R.J.; Lamb, F.S. c-Jun N-terminal kinase attenuates TNFα signaling by reducing Nox1-dependent endosomal ROS production in vascular smooth muscle cells. Free Radic. Biol. Med. 2015, 86, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Jiang, Y.; Shi, J.; Xu, C.; Liu, X.; Yang, T.; Fu, P.; Niu, T. Artemisinin inhibits the proliferation, migration, and inflammatory reaction induced by tumor necrosis factor-α in vascular smooth muscle cells through nuclear factor kappa B pathway. J. Surg. Res. 2015, 194, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Kazama, K.; Usui, T.; Okada, M.; Hara, Y.; Yamawaki, H. Omentin plays an anti-inflammatory role through inhibition of TNF-α-induced superoxide production in vascular smooth muscle cells. Eur. J. Pharmacol. 2012, 686, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Fang, J. TNF-α regulates apoptosis of human vascular smooth muscle cells through gap junctions. Mol. Med. Rep. 2017, 15, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Tang, V.; Dhirapong, A.; Yabes, A.P.; Weiss, R.H. TNF-alpha-mediated apoptosis in vascular smooth muscle cells requires p73. Am. J. Physiol. Cell Physiol. 2005, 289, C199–C206. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-H.; Kim, S.-A. 2-Methoxycinnamaldehyde inhibits the TNF-α-induced proliferation and migration of human aortic smooth muscle cells. Int. J. Mol. Med. 2017, 39, 191–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Liang, J.; Castrillon, D.H.; DePinho, R.A.; Olson, E.N.; Liu, Z.-P. FoxO4 regulates tumor necrosis factor alpha-directed smooth muscle cell migration by activating matrix metalloproteinase 9 gene transcription. Mol. Cell. Biol. 2007, 27, 2676–2686. [Google Scholar] [CrossRef] [Green Version]
- Goetze, S.; Xi, X.P.; Kawano, Y.; Kawano, H.; Fleck, E.; Hsueh, W.A.; Law, R.E. TNF-alpha-induced migration of vascular smooth muscle cells is MAPK dependent. Hypertension 1999, 33, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Kleinbongard, P.; Heusch, G.; Schulz, R. TNFalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol. Ther. 2010, 127, 295–314. [Google Scholar] [CrossRef]
- Brånén, L.; Hovgaard, L.; Nitulescu, M.; Bengtsson, E.; Nilsson, J.; Jovinge, S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2137–2142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Campenhout, A.; Moran, C.S.; Parr, A.; Clancy, P.; Rush, C.; Jakubowski, H.; Golledge, J. Role of homocysteine in aortic calcification and osteogenic cell differentiation. Atherosclerosis 2009, 202, 557–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Lin, J.; Ju, T.; Chu, L.; Zhang, L. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves matrix metalloproteinase-2 modulation by homocysteine. Mol. Cell. Biochem. 2015, 406, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chai, S.; Tang, C.; Du, J. Homocysteine potentiates calcification of cultured rat aortic smooth muscle cells. Life Sci. 2003, 74, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.-L.; Hou, Y.-L.; Ni, X.-Q.; Zhu, Q.; Chen, Y.; Zhang, L.-S.; Liu, X.; Xue, C.-D.; Wu, N.; Yu, Y.-R.; et al. Intermedin1-53 Ameliorates Homocysteine-Promoted Atherosclerotic Calcification by Inhibiting Endoplasmic Reticulum Stress. J. Cardiovasc. Pharmacol. Ther. 2020, 25, 251–264. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, N.; Yan, R.; Yang, W.; Cong, G.; Yan, N.; Ma, W.; Hou, J.; Yang, L.; Jia, S. Hyperhomocysteinemia induces vascular calcification by activating the transcription factor RUNX2 via Krüppel-like factor 4 up-regulation in mice. J. Biol. Chem. 2019, 294, 19465–19474. [Google Scholar] [CrossRef]
- Kim, B.J.; Kim, B.S.; Kang, J.H. Plasma homocysteine and coronary artery calcification in Korean men. Eur. J. Prev. Cardiol. 2015, 22, 478–485. [Google Scholar] [CrossRef]
- Peña-Duque, M.A.; Baños-González, M.A.; Valente-Acosta, B.; Rodríguez-Lobato, L.G.; Martínez-Ríos, M.A.; Cardoso-Saldaña, G.; Barragán-García, R.; Herrera-Alarcón, V.; Linares-López, C.; Delgado-Granados, H.; et al. Homocysteine is related to aortic mineralization in patients with ischemic heart disease. J. Atheroscler. Thromb. 2012, 19, 292–297. [Google Scholar] [CrossRef] [Green Version]
- Kronenberg, F.; Mündle, M.; Längle, M.; Neyer, U. Prevalence and progression of peripheral arterial calcifications in patients with ESRD. Am. J. Kidney Dis. 2003, 41, 140–148. [Google Scholar] [CrossRef]
- Rasouli, M.L.; Nasir, K.; Blumenthal, R.S.; Park, R.; Aziz, D.C.; Budoff, M.J. Plasma homocysteine predicts progression of atherosclerosis. Atherosclerosis 2005, 181, 159–165. [Google Scholar] [CrossRef]
- Kullo, I.J.; Li, G.; Bielak, L.F.; Bailey, K.R.; Sheedy, P.F.; Peyser, P.A.; Turner, S.T.; Kardia, S.L.R. Association of plasma homocysteine with coronary artery calcification in different categories of coronary heart disease risk. Mayo Clin. Proc. 2006, 81, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Baber, U.; de Lemos, J.A.; Khera, A.; McGuire, D.K.; Omland, T.; Toto, R.D.; Hedayati, S.S. Non-traditional risk factors predict coronary calcification in chronic kidney disease in a population-based cohort. Kidney Int. 2008, 73, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Held, C.; Sumner, G.; Sheridan, P.; McQueen, M.; Smith, S.; Dagenais, G.; Yusuf, S.; Lonn, E. Correlations between plasma homocysteine and folate concentrations and carotid atherosclerosis in high-risk individuals: Baseline data from the Homocysteine and Atherosclerosis Reduction Trial (HART). Vasc. Med. 2008, 13, 245–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karger, A.B.; Steffen, B.T.; Nomura, S.O.; Guan, W.; Garg, P.K.; Szklo, M.; Budoff, M.J.; Tsai, M.Y. Association between Homocysteine and Vascular Calcification Incidence, Prevalence, and Progression in the MESA Cohort. J. Am. Heart Assoc. 2020, 9, e013934. [Google Scholar] [CrossRef]
- Novaro, G.M.; Aronow, H.D.; Mayer-Sabik, E.; Griffin, B.P. Plasma homocysteine and calcific aortic valve disease. Heart 2004, 90, 802–803. [Google Scholar] [CrossRef] [Green Version]
- de Menezes, F.L.; Koch-Nogueira, P.C.; do Val, M.L.D.M.; Pestana, J.O.M.; Jorgetti, V.; Dos Reis, M.A.; Dos Reis Monteiro, M.L.G.; Leite, H.P. Is arterial calcification in children and adolescents with end-stage renal disease a rare finding? Nephrology 2019, 24, 696–702. [Google Scholar] [CrossRef]
- Godsland, I.F.; Elkeles, R.S.; Feher, M.D.; Nugara, F.; Rubens, M.B.; Richmond, W.; Khan, M.; Donovan, J.; Anyaoku, V.; Flather, M.D.; et al. Coronary calcification, homocysteine, C-reactive protein and the metabolic syndrome in Type 2 diabetes: The Prospective Evaluation of Diabetic Ischaemic Heart Disease by Coronary Tomography (PREDICT) Study. Diabet. Med. 2006, 23, 1192–1200. [Google Scholar] [CrossRef]
- Krajnc, M.; Pečovnik Balon, B.; Krajnc, I. Non-traditional risk factors for coronary calcification and its progression in patients with type 2 diabetes: The impact of postprandial glycemia and fetuin-A. J. Int. Med. Res. 2019, 47, 846–858. [Google Scholar] [CrossRef]
- Ke, X.D.; Foucault-Bertaud, A.; Genovesio, C.; Dignat-George, F.; Lamy, E.; Charpiot, P. Homocysteine modulates the proteolytic potential of human arterial smooth muscle cells through a reactive oxygen species dependant mechanism. Mol. Cell. Biochem. 2010, 335, 203–210. [Google Scholar] [CrossRef]
- Chang, L.; Xu, J.; Zhao, J.; Pang, Y.; Tang, C.; Qi, Y. Taurine antagonized oxidative stress injury induced by homocysteine in rat vascular smooth muscle cells. Acta Pharmacol. Sin. 2004, 25, 341–346. [Google Scholar]
- Zhang, L.; Jin, M.; Hu, X.-S.; Zhu, J.-H. Homocysteine stimulates nuclear factor kappaB activity and interleukin-6 expression in rat vascular smooth muscle cells. Cell Biol. Int. 2006, 30, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Liu, J.; Zhao, J.; Mao, J.; Zhang, X.; Feng, L.; Han, C.; Li, M.; Wang, S.; Wu, D. Homocysteine induces the expression of C-reactive protein via NMDAr-ROS-MAPK-NF-κB signal pathway in rat vascular smooth muscle cells. Atherosclerosis 2014, 236, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Wang, S.; Hunag, Y.; Jinag, Y. A comparative study on pathogenic effects of homocysteine and cysteine on atherosclerosis. Wei Sheng Yan Jiu 2009, 38, 43–46. [Google Scholar] [PubMed]
- Buemi, M.; Marino, D.; Di Pasquale, G.; Floccari, F.; Ruello, A.; Aloisi, C.; Corica, F.; Senatore, M.; Romeo, A.; Frisina, N. Effects of homocysteine on proliferation, necrosis, and apoptosis of vascular smooth muscle cells in culture and influence of folic acid. Thromb. Res. 2001, 104, 207–213. [Google Scholar] [CrossRef]
- Rasmussen, L.M.; Hansen, P.R.; Ledet, T. Homocysteine and the production of collagens, proliferation and apoptosis in human arterial smooth muscle cells. APMIS 2004, 112, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-P.; Wang, Y.-H.; Cao, C.-J.; Yang, X.-M.; Ma, S.-C.; Han, X.-B.; Yang, X.-L.; Yang, A.-N.; Tian, J.; Xu, H.; et al. A regulatory circuit involving miR-143 and DNMT3a mediates vascular smooth muscle cell proliferation induced by homocysteine. Mol. Med. Rep. 2016, 13, 483–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.-C.; Zhang, H.-P.; Jiao, Y.; Wang, Y.-H.; Zhang, H.; Yang, X.-L.; Yang, A.-N.; Jiang, Y.-D. Homocysteine-induced proliferation of vascular smooth muscle cells occurs via PTEN hypermethylation and is mitigated by Resveratrol. Mol. Med. Rep. 2018, 17, 5312–5319. [Google Scholar] [CrossRef] [Green Version]
- Bao, X.; Zheng, H. Atorvastatin attenuates homocysteine-induced migration of smooth muscle cells through mevalonate pathway involving reactive oxygen species and p38 MAPK. Clin. Exp. Pharmacol. Physiol. 2015, 42, 865–873. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, H.; Zhang, W.; Kong, W.; Zhu, Y.; Zhang, H.; Xu, Q.; Li, Y.; Wang, X. Homocysteine promotes vascular smooth muscle cell migration by induction of the adipokine resistin. Am. J. Physiol. Cell Physiol. 2009, 297, C1466–C1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Chen, H.; Liu, N.; Chen, J.; Gu, Y.; Chen, J.; Yang, K. Role of Hyperhomocysteinemia and Hyperuricemia in Pathogenesis of Atherosclerosis. J. Stroke Cerebrovasc. Dis. 2017, 26, 2695–2699. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, R.; Zhang, G.; Yu, Q.; Jia, M.; Zheng, C.; Wang, Y.; Xu, C.; Zhang, Y.; Liu, E. Hypercysteinemia promotes atherosclerosis by reducing protein S-nitrosylation. Biomed. Pharmacother. 2015, 70, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Zhang, H.; Liu, T.; Zhang, H.; Teng, J.; Ji, J.; Ding, X. Indoxyl Sulfate Enhance the Hypermethylation of Klotho and Promote the Process of Vascular Calcification in Chronic Kidney Disease. Int. J. Biol. Sci. 2016, 12, 1236–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Chen, J.; Shen, Z.; Gu, Y.; Xu, L.; Hu, J.; Zhang, X.; Ding, X. Indoxyl sulfate accelerates vascular smooth muscle cell calcification via microRNA-29b dependent regulation of Wnt/β-catenin signaling. Toxicol. Lett. 2018, 284, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Han, X.; Wang, L.; Diao, Z.; Liu, W. Indoxyl sulfate promotes vascular smooth muscle cell calcification via the JNK/Pit-1 pathway. Ren. Fail. 2016, 38, 1702–1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opdebeeck, B.; Maudsley, S.; Azmi, A.; De Maré, A.; De Leger, W.; Meijers, B.; Verhulst, A.; Evenepoel, P.; D’Haese, P.C.; Neven, E. Indoxyl Sulfate and p-Cresyl Sulfate Promote Vascular Calcification and Associate with Glucose Intolerance. J. Am. Soc. Nephrol. JASN 2019, 30, 751–766. [Google Scholar] [CrossRef]
- Asami, M.; Tanabe, K.; Ito, S.; Yoshida, E.; Aoki, J.; Tanimoto, S.; Horiuchi, Y.; Yoshida, M. Impact of Indoxyl Sulfate on Coronary Plaques in Patients on Hemodialysis. Int. Heart J. 2018, 59, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Barreto, F.C.; Barreto, D.V.; Liabeuf, S.; Meert, N.; Glorieux, G.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A.; European Uremic Toxin Work Group (EUTox). Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1551–1558. [Google Scholar] [CrossRef] [Green Version]
- Taki, K.; Takayama, F.; Tsuruta, Y.; Niwa, T. Oxidative stress, advanced glycation end product, and coronary artery calcification in hemodialysis patients. Kidney Int. 2006, 70, 218–224. [Google Scholar] [CrossRef] [Green Version]
- Muteliefu, G.; Enomoto, A.; Jiang, P.; Takahashi, M.; Niwa, T. Indoxyl sulphate induces oxidative stress and the expression of osteoblast-specific proteins in vascular smooth muscle cells. Nephrol. Dial. Transplant. 2009, 24, 2051–2058. [Google Scholar] [CrossRef] [Green Version]
- Muteliefu, G.; Shimizu, H.; Enomoto, A.; Nishijima, F.; Takahashi, M.; Niwa, T. Indoxyl sulfate promotes vascular smooth muscle cell senescence with upregulation of p53, p21, and prelamin A through oxidative stress. Am. J. Physiol. Cell Physiol. 2012, 303, C126–C134. [Google Scholar] [CrossRef] [Green Version]
- Adelibieke, Y.; Yisireyili, M.; Ng, H.-Y.; Saito, S.; Nishijima, F.; Niwa, T. Indoxyl sulfate induces IL-6 expression in vascular endothelial and smooth muscle cells through OAT3-mediated uptake and activation of AhR/NF-κB pathway. Nephron Exp. Nephrol. 2014, 128, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yisireyili, M.; Saito, S.; Abudureyimu, S.; Adelibieke, Y.; Ng, H.-Y.; Nishijima, F.; Takeshita, K.; Murohara, T.; Niwa, T. Indoxyl sulfate-induced activation of (pro)renin receptor promotes cell proliferation and tissue factor expression in vascular smooth muscle cells. PLoS ONE 2014, 9, e109268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, H.; Tsuruoka, S.; Ioka, T.; Ando, H.; Ito, C.; Akimoto, T.; Fujimura, A.; Asano, Y.; Kusano, E. Indoxyl sulfate stimulates proliferation of rat vascular smooth muscle cells. Kidney Int. 2006, 69, 1780–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, H.-Y.; Bolati, W.; Lee, C.-T.; Chien, Y.-S.; Yisireyili, M.; Saito, S.; Pei, S.-N.; Nishijima, F.; Niwa, T. Indoxyl Sulfate Downregulates Mas Receptor via Aryl Hydrocarbon Receptor/Nuclear Factor-kappa B, and Induces Cell Proliferation and Tissue Factor Expression in Vascular Smooth Muscle Cells. Nephron 2016, 133, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Hirose, Y.; Nishijima, F.; Tsubakihara, Y.; Miyazaki, H. ROS and PDGF-beta [corrected] receptors are critically involved in indoxyl sulfate actions that promote vascular smooth muscle cell proliferation and migration. Am. J. Physiol. Cell Physiol. 2009, 297, C389–C396. [Google Scholar] [CrossRef]
- Shimizu, H.; Hirose, Y.; Goto, S.; Nishijima, F.; Zrelli, H.; Zghonda, N.; Niwa, T.; Miyazaki, H. Indoxyl sulfate enhances angiotensin II signaling through upregulation of epidermal growth factor receptor expression in vascular smooth muscle cells. Life Sci. 2012, 91, 172–177. [Google Scholar] [CrossRef]
- Taki, K.; Tsuruta, Y.; Niwa, T. Indoxyl sulfate and atherosclerotic risk factors in hemodialysis patients. Am. J. Nephrol. 2007, 27, 30–35. [Google Scholar] [CrossRef]
- Parhami, F.; Tintut, Y.; Ballard, A.; Fogelman, A.M.; Demer, L.L. Leptin enhances the calcification of vascular cells: Artery wall as a target of leptin. Circ. Res. 2001, 88, 954–960. [Google Scholar] [CrossRef] [Green Version]
- Dubey, L.; Hesong, Z. Role of leptin in atherogenesis. Exp. Clin. Cardiol. 2006, 11, 269–275. [Google Scholar]
- Zeadin, M.G.; Butcher, M.K.; Shaughnessy, S.G.; Werstuck, G.H. Leptin promotes osteoblast differentiation and mineralization of primary cultures of vascular smooth muscle cells by inhibiting glycogen synthase kinase (GSK)-3β. Biochem. Biophys. Res. Commun. 2012, 425, 924–930. [Google Scholar] [CrossRef]
- Zeadin, M.; Butcher, M.; Werstuck, G.; Khan, M.; Yee, C.K.; Shaughnessy, S.G. Effect of leptin on vascular calcification in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 2069–2075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, N.X.; O’Neill, K.; Akl, N.K.; Moe, S.M. Adipocyte induced arterial calcification is prevented with sodium thiosulfate. Biochem. Biophys. Res. Commun. 2014, 449, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Iribarren, C.; Husson, G.; Go, A.S.; Lo, J.C.; Fair, J.M.; Rubin, G.D.; Hlatky, M.A.; Fortmann, S.P. Plasma leptin levels and coronary artery calcification in older adults. J. Clin. Endocrinol. Metab. 2007, 92, 729–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szulc, P.; Amri, E.Z.; Varennes, A.; Panaia-Ferrari, P.; Fontas, E.; Goudable, J.; Chapurlat, R.; Breuil, V. Positive Association of High Leptin Level and Abdominal Aortic Calcification in Men—The Prospective MINOS Study. Circ. J. 2018, 82, 2954–2961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, R.B.; Liabeuf, S.; Okazaki, H.; Lenglet, A.; Desjardins, L.; Lemke, H.-D.; Vanholder, R.; Choukroun, G.; Massy, Z.A.; European Uremic Toxin Work Group (EUTox). The clinical impact of plasma leptin levels in a cohort of chronic kidney disease patients. Clin. Kidney J. 2013, 6, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Reilly, M.P.; Iqbal, N.; Schutta, M.; Wolfe, M.L.; Scally, M.; Localio, A.R.; Rader, D.J.; Kimmel, S.E. Plasma leptin levels are associated with coronary atherosclerosis in type 2 diabetes. J. Clin. Endocrinol. Metab. 2004, 89, 3872–3878. [Google Scholar] [CrossRef]
- Liu, G.-Y.; Liang, Q.-H.; Cui, R.-R.; Liu, Y.; Wu, S.-S.; Shan, P.-F.; Yuan, L.-Q.; Liao, E.-Y. Leptin promotes the osteoblastic differentiation of vascular smooth muscle cells from female mice by increasing RANKL expression. Endocrinology 2014, 155, 558–567. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Mamputu, J.-C.; Wiernsperger, N.; Renier, G. Signaling pathways involved in human vascular smooth muscle cell proliferation and matrix metalloproteinase-2 expression induced by leptin: Inhibitory effect of metformin. Diabetes 2005, 54, 2227–2234. [Google Scholar] [CrossRef] [Green Version]
- Ghantous, C.M.; Azrak, Z.; Hanache, S.; Abou-Kheir, W.; Zeidan, A. Differential Role of Leptin and Adiponectin in Cardiovascular System. Int. J. Endocrinol. 2015, 2015. [Google Scholar] [CrossRef]
- Huang, F.; Xiong, X.; Wang, H.; You, S.; Zeng, H. Leptin-induced vascular smooth muscle cell proliferation via regulating cell cycle, activating ERK1/2 and NF-kappaB. Acta Biochim. Biophys. Sin. 2010, 42, 325–331. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Zhu, S.; Chen, Y.; Wu, X.; Ni, W.; Chen, Y.; Zhao, L. The effects of leptin on apoptosis of airway smooth muscle cells via the PI3K/Akt signaling pathway. Zhonghua Jie He He Hu Xi Za Zhi 2012, 35, 915–918. [Google Scholar] [PubMed]
- Schroeter, M.R.; Leifheit-Nestler, M.; Hubert, A.; Schumann, B.; Glückermann, R.; Eschholz, N.; Krüger, N.; Lutz, S.; Hasenfuss, G.; Konstantinides, S.; et al. Leptin promotes neointima formation and smooth muscle cell proliferation via NADPH oxidase activation and signalling in caveolin-rich microdomains. Cardiovasc. Res. 2013, 99, 555–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohlen, F.; Kratzsch, J.; Mueller, M.; Seidel, B.; Friedman-Einat, M.; Witzigmann, H.; Teupser, D.; Koerner, A.; Storck, M.; Thiery, J. Leptin inhibits cell growth of human vascular smooth muscle cells. Vasc. Pharmacol. 2007, 46, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Oda, A.; Taniguchi, T.; Yokoyama, M. Leptin stimulates rat aortic smooth muscle cell proliferation and migration. Kobe J. Med. Sci. 2001, 47, 141–150. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Leu, S.-Y.; Peng, Y.-J.; Lee, Y.-M.; Hsu, C.-H.; Chou, S.-C.; Yen, M.-H.; Cheng, P.-Y. Genistein suppresses leptin-induced proliferation and migration of vascular smooth muscle cells and neointima formation. J. Cell. Mol. Med. 2017, 21, 422–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneiderman, J.; Schaefer, K.; Kolodgie, F.D.; Savion, N.; Kotev-Emeth, S.; Dardik, R.; Simon, A.J.; Halak, M.; Pariente, C.; Engelberg, I.; et al. Leptin locally synthesized in carotid atherosclerotic plaques could be associated with lesion instability and cerebral emboli. J. Am. Heart Assoc. 2012, 1, e001727. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Jiang, Y.; Liu, N.; Ren, L.; Zhu, Y.; An, Y.; Chen, D. Advanced glycation end-product Nε-carboxymethyl-Lysine accelerates progression of atherosclerotic calcification in diabetes. Atherosclerosis 2012, 221, 387–396. [Google Scholar] [CrossRef]
- Li, L.H.; Ye, F.; Fu, X.L.; Xu, S.N.; Bao, Z.Y.; Sun, Z.; Yan, J.C.; Wu, J.N.; Wang, Z.Q. Association between serum Nε-carboxymethyl-lysine level and anterior tibial arterial plaque calcification in patients with diabetic foot post foot amputation. Zhonghua Xin Xue Guan Bing Za Zhi 2017, 45, 958–962. [Google Scholar] [CrossRef]
- Kislinger, T.; Fu, C.; Huber, B.; Qu, W.; Taguchi, A.; Du Yan, S.; Hofmann, M.; Yan, S.F.; Pischetsrieder, M.; Stern, D.; et al. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J. Biol. Chem. 1999, 274, 31740–31749. [Google Scholar] [CrossRef] [Green Version]
- Meloche, J.; Paulin, R.; Courboulin, A.; Lambert, C.; Barrier, M.; Bonnet, P.; Bisserier, M.; Roy, M.; Sussman, M.A.; Agharazii, M.; et al. RAGE-Dependent Activation of the Oncoprotein Pim1 Plays a Critical Role in Systemic Vascular Remodeling Processes. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2114–2124. [Google Scholar] [CrossRef] [Green Version]
- Liabeuf, S.; Barreto, D.V.; Barreto, F.C.; Meert, N.; Glorieux, G.; Schepers, E.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A.; et al. Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol. Dial. Transplant. 2010, 25, 1183–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, H.; Miyamoto, Y.; Enoki, Y.; Ishima, Y.; Kadowaki, D.; Kotani, S.; Nakajima, M.; Tanaka, M.; Matsushita, K.; Mori, Y.; et al. p-Cresyl sulfate, a uremic toxin, causes vascular endothelial and smooth muscle cell damages by inducing oxidative stress. Pharmacol. Res. Perspect. 2015, 3, e00092. [Google Scholar] [CrossRef] [PubMed]
- Gross, P.; Massy, Z.A.; Henaut, L.; Boudot, C.; Cagnard, J.; March, C.; Kamel, S.; Drueke, T.B.; Six, I. Para-cresyl sulfate acutely impairs vascular reactivity and induces vascular remodeling. J. Cell. Physiol. 2015, 230, 2927–2935. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Chen, Y.; Zhu, Z.; Su, X.; Ni, J.; Du, R.; Zhang, R.; Jin, W. p-Cresyl sulfate promotes the formation of atherosclerotic lesions and induces plaque instability by targeting vascular smooth muscle cells. Front. Med. 2016, 10, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Lu, L.; Hua, Y.; Huang, K.; Wang, I.; Huang, L.; Fu, Q.; Chen, A.; Chan, P.; Fan, H.; et al. Vasculopathy in the setting of cardiorenal syndrome: Roles of protein-bound uremic toxins. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H1–H13. [Google Scholar] [CrossRef] [Green Version]
- Ciceri, P.; Volpi, E.; Brenna, I.; Elli, F.; Borghi, E.; Brancaccio, D.; Cozzolino, M. The combination of lanthanum chloride and the calcimimetic calindol delays the progression of vascular smooth muscle cells calcification. Biochem. Biophys. Res. Commun. 2012, 418, 770–773. [Google Scholar] [CrossRef]
- Sinha-Hikim, I.; Shen, R.; Paul Lee, W.-N.N.; Crum, A.; Vaziri, N.D.; Norris, K.C. Effects of a novel cystine-based glutathione precursor on oxidative stress in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2010, 299, C638–C642. [Google Scholar] [CrossRef]
- Facchiano, F.; D’Arcangelo, D.; Riccomi, A.; Lentini, A.; Beninati, S.; Capogrossi, M.C. Transglutaminase activity is involved in polyamine-induced programmed cell death. Exp. Cell Res. 2001, 271, 118–129. [Google Scholar] [CrossRef]
- Sinha-Hikim, I.; Shen, R.; Kovacheva, E.; Crum, A.; Vaziri, N.D.; Norris, K.C. Inhibition of apoptotic signalling in spermine-treated vascular smooth muscle cells by a novel glutathione precursor. Cell Biol. Int. 2010, 34, 503–511. [Google Scholar] [CrossRef] [Green Version]
- Nishida, K.; Abiko, T.; Ishihara, M.; Tomikawa, M. Arterial injury-induced smooth muscle cell proliferation in rats is accompanied by increase in polyamine synthesis and level. Atherosclerosis 1990, 83, 119–125. [Google Scholar] [CrossRef]
- Zhu, L.; Xiao, R.; Zhang, X.; Lang, Y.; Liu, F.; Yu, Z.; Zhang, J.; Su, Y.; Lu, Y.; Wang, T.; et al. Spermine on Endothelial Extracellular Vesicles Mediates Smoking-Induced Pulmonary Hypertension Partially Through Calcium-Sensing Receptor. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 482–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, C.; Li, H.-Z.; Wang, Y.-H.; Peng, X.; Shao, H.-J.; Li, H.-X.; Bai, S.-Z.; Lu, X.-X.; Wu, L.-Y.; Wang, R.; et al. Exogenous spermine inhibits the proliferation of human pulmonary artery smooth muscle cells caused by chemically-induced hypoxia via the suppression of the ERK1/2- and PI3K/AKT-associated pathways. Int. J. Mol. Med. 2016, 37, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schepers, E.; Glorieux, G.; Dou, L.; Cerini, C.; Gayrard, N.; Louvet, L.; Maugard, C.; Preus, P.; Rodriguez-Ortiz, M.; Argiles, A.; et al. Guanidino compounds as cause of cardiovascular damage in chronic kidney disease: An in vitro evaluation. Blood Purif. 2010, 30, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.J.; Berkheimer, S.B.; Merchant, M.; Krishnaswami, A. Asymmetric dimethylarginine and coronary artery calcium scores are increased in patients infected with human immunodeficiency virus. Atherosclerosis 2011, 217, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Iribarren, C.; Husson, G.; Sydow, K.; Wang, B.-Y.; Sidney, S.; Cooke, J.P. Asymmetric dimethyl-arginine and coronary artery calcification in young adults entering middle age: The CARDIA Study. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Oka, M.; Maesato, K.; Ikee, R.; Mano, T.; Hidekazu, M.; Ohtake, T. Coronary Artery Calcification, ADMA, and Insulin Resistance in CKD Patients. CJASN 2008, 3, 1289–1295. [Google Scholar] [CrossRef] [Green Version]
- Kiani, A.N.; Mahoney, J.A.; Petri, M. Asymmetric dimethylarginine is a marker of poor prognosis and coronary calcium in systemic lupus erythematosus. J. Rheumatol. 2007, 34, 1502–1505. [Google Scholar]
- Krzanowski, M.; Krzanowska, K.; Gajda, M.; Dumnicka, P.; Kopeć, G.; Guzik, B.; Woziwodzka, K.; Dziewierz, A.; Litwin, J.A.; Sułowicz, W. Asymmetric dimethylarginine as a useful risk marker of radial artery calcification in patients with advanced kidney disease. Pol. Arch. Intern. Med. 2018, 128, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Coen, G.; Mantella, D.; Sardella, D.; Beraldi, M.P.; Ferrari, I.; Pierantozzi, A.; Lippi, B.; Di Giulio, S. Asymmetric dimethylarginine, vascular calcifications and parathyroid hormone serum levels in hemodialysis patients. J. Nephrol. 2009, 22, 616–622. [Google Scholar]
- Yuan, Q.; Jiang, D.-J.; Chen, Q.-Q.; Wang, S.; Xin, H.-Y.; Deng, H.-W.; Li, Y.-J. Role of asymmetric dimethylarginine in homocysteine-induced apoptosis of vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2007, 356, 880–885. [Google Scholar] [CrossRef]
- Pekarova, M.; Koudelka, A.; Kolarova, H.; Ambrozova, G.; Klinke, A.; Cerna, A.; Kadlec, J.; Trundova, M.; Sindlerova Svihalkova, L.; Kuchta, R.; et al. Asymmetric dimethyl arginine induces pulmonary vascular dysfunction via activation of signal transducer and activator of transcription 3 and stabilization of hypoxia-inducible factor 1-alpha. Vasc. Pharmacol. 2015, 73, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-H.; Peng, J.; Tan, N.; Wu, W.-H.; Li, T.-T.; Shi, R.-Z.; Li, Y.-J. Involvement of asymmetric dimethylarginine and Rho kinase in the vascular remodeling in monocrotaline-induced pulmonary hypertension. Vasc. Pharmacol. 2010, 53, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lan, X.; Guo, H.; Zhang, Y.; Ma, L.; Cao, J. Rho/ROCK signal cascade mediates asymmetric dimethylarginine-induced vascular smooth muscle cells migration and phenotype change. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Zhang, T.; Yu, X.; Xin, W.; Lan, X.; Zhang, D.; Huang, C.; Du, G. Asymmetric dimethylarginine confers the communication between endothelial and smooth muscle cells and leads to VSMC migration through p38 and ERK1/2 signaling cascade. FEBS Lett. 2011, 585, 2727–2734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alesutan, I.; Musculus, K.; Castor, T.; Alzoubi, K.; Voelkl, J.; Lang, F. Inhibition of Phosphate-Induced Vascular Smooth Muscle Cell Osteo-/Chondrogenic Signaling and Calcification by Bafilomycin A1 and Methylamine. Kidney Blood Press. Res. 2015, 40, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.; Solé, M.; Boada, M.; Unzeta, M. Soluble semicarbazide sensitive amine oxidase (SSAO) catalysis induces apoptosis in vascular smooth muscle cells. Biochim. Biophys. Acta 2006, 1763, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Hao, W.; Yang, R.; Yang, Y.; Jin, S.; Li, Y.; Yuan, F.; Guo, Q.; Xiao, L.; Wang, X.; Wang, F.; et al. Stellate ganglion block ameliorates vascular calcification by inhibiting endoplasmic reticulum stress. Life Sci. 2018, 193, 1–8. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, M.-C.; Yang, Y.-A.; Chen, E.-Q.; Xu, H.-T.; Wu, K.-Y.; Zhang, S.-M. Norepinephrine reversibly regulates the proliferation and phenotypic transformation of vascular smooth muscle cells. Exp. Mol. Pathol. 2008, 85, 196–200. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, X.; Liu, R.; Qiao, H.; Wang, P.; Jiang, C.; Zhang, Q.; Cao, Y.; Yu, H.; Qu, L. alpha1A-adrenoceptor is involved in norepinephrine-induced proliferation of pulmonary artery smooth muscle cells via CaMKII signaling. J. Cell. Biochem. 2019, 120, 9345–9355. [Google Scholar] [CrossRef]
- Bell, L.; Madri, J.A. Effect of platelet factors on migration of cultured bovine aortic endothelial and smooth muscle cells. Circ. Res. 1989, 65, 1057–1065. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Facemire, C.S.; Banes, A.J.; Faber, J.E. Different alpha-adrenoceptors mediate migration of vascular smooth muscle cells and adventitial fibroblasts in vitro. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H2364–H2370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Li, Y.; Yang, P.; Liu, X.; Lu, L.; Chen, Y.; Zhong, X.; Li, Z.; Liu, H.; Ou, C.; et al. Trimethylamine-N-Oxide Promotes Vascular Calcification Through Activation of NLRP3 (Nucleotide-Binding Domain, Leucine-Rich-Containing Family, Pyrin Domain-Containing-3) Inflammasome and NF-κB (Nuclear Factor κB) Signals. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.A.; Benton, T.Z.; Bennett, B.J.; Jacobs, D.R.; Lloyd-Jones, D.M.; Gross, M.D.; Carr, J.J.; Gordon-Larsen, P.; Zeisel, S.H. Microbiota-Dependent Metabolite Trimethylamine N-Oxide and Coronary Artery Calcium in the Coronary Artery Risk Development in Young Adults Study (CARDIA). J. Am. Heart Assoc. 2016, 5, e003970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide Promotes Vascular Inflammation through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-κB. J. Am. Heart Assoc. 2016, 5, e002767. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Zhao, Y.; Wang, X.; Xu, M.-J. Secondary hyperuricemia in chronic renal failure promotes vascular calcification in rats. Sheng Li Xue Bao 2016, 68, 709–715. [Google Scholar]
- Yan, B.; Liu, D.; Zhu, J.; Pang, X. The effects of hyperuricemia on the differentiation and proliferation of osteoblasts and vascular smooth muscle cells are implicated in the elevated risk of osteopenia and vascular calcification in gout: An in vivo and in vitro analysis. J. Cell. Biochem. 2019, 120, 19660–19672. [Google Scholar] [CrossRef]
- Jun, J.E.; Lee, Y.-B.; Lee, S.-E.; Ahn, J.Y.; Kim, G.; Jin, S.-M.; Hur, K.Y.; Lee, M.-K.; Kang, M.R.; Kim, J.H. Elevated serum uric acid predicts the development of moderate coronary artery calcification independent of conventional cardiovascular risk factors. Atherosclerosis 2018, 272, 233–239. [Google Scholar] [CrossRef]
- Grossman, C.; Shemesh, J.; Koren-Morag, N.; Bornstein, G.; Ben-Zvi, I.; Grossman, E. Serum uric acid is associated with coronary artery calcification. J. Clin. Hypertens. 2014, 16, 424–428. [Google Scholar] [CrossRef] [Green Version]
- Calvo, R.Y.; Araneta, M.R.G.; Kritz-Silverstein, D.; Laughlin, G.A.; Barrett-Connor, E. Relation of serum uric acid to severity and progression of coronary artery calcium in postmenopausal White and Filipino women (from the Rancho Bernardo study). Am. J. Cardiol. 2014, 113, 1153–1158. [Google Scholar] [CrossRef]
- D’Marco, L.; García, I.; Vega, C. Uric acid, atherosclerosis and vascular calcifications in chronic kidney disease. Investig. Clin. 2012, 53, 52–59. [Google Scholar]
- Malik, R.; Aneni, E.C.; Shahrayar, S.; Freitas, W.M.; Ali, S.S.; Veledar, E.; Latif, M.A.; Aziz, M.; Ahmed, R.; Khan, S.A.; et al. Elevated serum uric acid is associated with vascular inflammation but not coronary artery calcification in the healthy octogenarians: The Brazilian study on healthy aging. Aging Clin. Exp. Res. 2016, 28, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Corry, D.B.; Eslami, P.; Yamamoto, K.; Nyby, M.D.; Makino, H.; Tuck, M.L. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J. Hypertens. 2008, 26, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Xu, Y.; Wei, Y.; He, X. Uric acid induces the expression of TNF-α via the ROS-MAPK-NF-κB signaling pathway in rat vascular smooth muscle cells. Mol. Med. Rep. 2017, 16, 6928–6933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oğuz, N.; Kırça, M.; Çetin, A.; Yeşilkaya, A. Effect of uric acid on inflammatory COX-2 and ROS pathways in vascular smooth muscle cells. J. Recept. Signal Transduct. 2017, 37, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Cao, Y.; Zhang, Z.; Vallurupalli, S.; Mehta, J.L. Xanthine Oxidase Induces Foam Cell Formation through LOX-1 and NLRP3 Activation. Cardiovasc. Drugs Ther. 2017, 31, 19–27. [Google Scholar] [CrossRef]
- Fu, X.; Niu, N.; Li, G.; Xu, M.; Lou, Y.; Mei, J.; Liu, Q.; Sui, Z.; Sun, J.; Qu, P. Blockage of macrophage migration inhibitory factor (MIF) suppressed uric acid-induced vascular inflammation, smooth muscle cell de-differentiation, and remodeling. Biochem. Biophys. Res. Commun. 2019, 508, 440–444. [Google Scholar] [CrossRef]
- Rao, G.N.; Corson, M.A.; Berk, B.C. Uric acid stimulates vascular smooth muscle cell proliferation by increasing platelet-derived growth factor A-chain expression. J. Biol. Chem. 1991, 266, 8604–8608. [Google Scholar]
- Kırça, M.; Oğuz, N.; Çetin, A.; Uzuner, F.; Yeşilkaya, A. Uric acid stimulates proliferative pathways in vascular smooth muscle cells through the activation of p38 MAPK, p44/42 MAPK and PDGFRβ. J. Recept. Signal Transduct. Res. 2017, 37, 167–173. [Google Scholar] [CrossRef]
- Kang, D.-H.; Park, S.-K.; Lee, I.-K.; Johnson, R.J. Uric acid-induced C-reactive protein expression: Implication on cell proliferation and nitric oxide production of human vascular cells. J. Am. Soc. Nephrol. 2005, 16, 3553–3562. [Google Scholar] [CrossRef] [Green Version]
- Krohn, J.B.; Hutcheson, J.D.; Martínez-Martínez, E.; Aikawa, E. Extracellular vesicles in cardiovascular calcification: Expanding current paradigms. J. Physiol. 2016, 594, 2895–2903. [Google Scholar] [CrossRef] [Green Version]
- Pfaltzgraff, E.R.; Bader, D.M. Heterogeneity in vascular smooth muscle cell embryonic origin in relation to adult structure, physiology, and disease. Dev. Dyn. 2015, 244, 410–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gayrard, N.; Muyor, K.; Notarnicola, C.; Duranton, F.; Jover, B.; Argilés, À. Optimisation of cell and ex vivo culture conditions to study vascular calcification. PLoS ONE 2020, 15, e0230201. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Glorieux, G.; Thornalley, P.; Schepers, E.; Meert, N.; Jankowski, J.; Jankowski, V.; Argiles, A.; Anderstam, B.; Brunet, P.; et al. Review on uraemic toxins III: Recommendations for handling uraemic retention solutes in vitro—Towards a standardized approach for research on uraemia. Nephrol. Dial. Transplant. 2007, 22, 3381–3390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, P.C.; Huang, L.L.; Panrucker, D.E.; Church, R.B.; Lorscheider, F.L. Distribution of bovine fetuin and albumin in plasma, allantoic and amniotic fluids during development. J. Reprod. Fertil. 1981, 63, 53–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Häusler, M.; Schäfer, C.; Osterwinter, C.; Jahnen-Dechent, W. The Physiologic Development of Fetuin—A Serum Concentrations in Children. Pediatr. Res. 2009, 66, 660–664. [Google Scholar] [CrossRef] [Green Version]
- Basnakian, A.G.; Shah, S.V.; Ok, E.; Altunel, E.; Apostolov, E.O. Carbamylated LDL. Adv. Clin. Chem. 2010, 51, 25–52. [Google Scholar] [CrossRef]
- Shah, S.V.; Shukla, A.M.; Bose, C.; Basnakian, A.G.; Rajapurkar, M. Recent advances in understanding the pathogenesis of atherosclerosis in CKD patients. J. Ren. Nutr. 2015, 25, 205–208. [Google Scholar] [CrossRef]
- Hawkins, C.L. Protein carbamylation: A key driver of vascular calcification during chronic kidney disease. Kidney Int. 2018, 94, 12–14. [Google Scholar] [CrossRef]
- Kukla, A.; Adulla, M.; Pascual, J.; Samaniego, M.; Nanovic, L.; Becker, B.N.; Djamali, A. CKD stage-to-stage progression in native and transplant kidney disease. Nephrol. Dial. Transplant. 2008, 23, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Hill, A.B. The Environment and Disease: Association or Causation? Proc. R. Soc. Med. 1965, 58, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Schantl, A.E.; Ivarsson, M.E.; Leroux, J.-C. Investigational Pharmacological Treatments for Vascular Calcification. Adv. Ther. 2019, 2, 1800094. [Google Scholar] [CrossRef] [Green Version]
- Nelson, A.J.; Raggi, P.; Wolf, M.; Gold, A.M.; Chertow, G.M.; Roe, M.T. Targeting Vascular Calcification in Chronic Kidney Disease. J. Am. Coll. Cardiol. Basic Transl. Sci. 2020, 5, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Raggi, P.; Bellasi, A.; Bushinsky, D.; Bover, J.; Rodriguez, M.; Ketteler, M.; Sinha, S.; Salcedo, C.; Gillotti, K.; Padgett, C.; et al. Slowing Progression of Cardiovascular Calcification with SNF472 in Patients on Hemodialysis. Circulation 2020, 141, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Cranenburg, E.C.M.; Vermeer, C. Matrix Gla-protein: The calcification inhibitor in need of vitamin K. Thromb. Haemost. 2008, 100, 593–603. [Google Scholar] [PubMed]
- Dai, L.; Qureshi, A.R.; Witasp, A.; Lindholm, B.; Stenvinkel, P. Early Vascular Ageing and Cellular Senescence in Chronic Kidney Disease. Comput. Struct. Biotechnol. J. 2019, 17, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, J.; Liu, H.; Tang, Y.; Zhan, Q.; Lai, W.; Ao, L.; Meng, X.; Ren, H.; Xu, D.; et al. The intestinal microbiota associated with cardiac valve calcification differs from that of coronary artery disease. Atherosclerosis 2019, 284, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Moludi, J.; Maleki, V.; Jafari-Vayghyan, H.; Vaghef-Mehrabany, E.; Alizadeh, M. Metabolic endotoxemia and cardiovascular disease: A systematic review about potential roles of prebiotics and probiotics. Clin. Exp. Pharmacol. Physiol. 2020, 47, 927–939. [Google Scholar] [CrossRef] [Green Version]
- Willems, B.A.; Furmanik, M.; Caron, M.M.J.; Chatrou, M.L.L.; Kusters, D.H.M.; Welting, T.J.M.; Stock, M.; Rafael, M.S.; Viegas, C.S.B.; Simes, D.C.; et al. Ucma/GRP inhibits phosphate-induced vascular smooth muscle cell calcification via SMAD-dependent BMP signalling. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Furmanik, M.; Chatrou, M.; van Gorp, R.H.; Akbulut, A.; Willems, B.; Schmidt, H.H.; van Eys, G.; Bochaton-Piallat, M.-L.; Proudfoot, D.; Biessen, E.A.; et al. Reactive Oxygen-Forming Nox5 Links Vascular Smooth Muscle Cell Phenotypic Switching and Extracellular Vesicle-Mediated Vascular Calcification. Circ. Res. 2020, 127, 911–927. [Google Scholar] [CrossRef]
- Viegas Carla, S.B.; Santos, L.; Macedo Anjos, L.; Matos António, A.; Silva Ana, P.; Neves Pedro, L.; Staes, A.; Gevaert, K.; Morais, R.; Vermeer, C.; et al. Chronic Kidney Disease Circulating Calciprotein Particles and Extracellular Vesicles Promote Vascular Calcification. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 575–587. [Google Scholar] [CrossRef] [Green Version]
- EUTox the European Uremic Toxins (EUTox) Database. Available online: http://eutoxdb.odeesoft.com/home.php (accessed on 15 January 2019).
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A.; on behalf of the European Uremic Toxin Work Group. Normal and Pathologic Concentrations of Uremic Toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
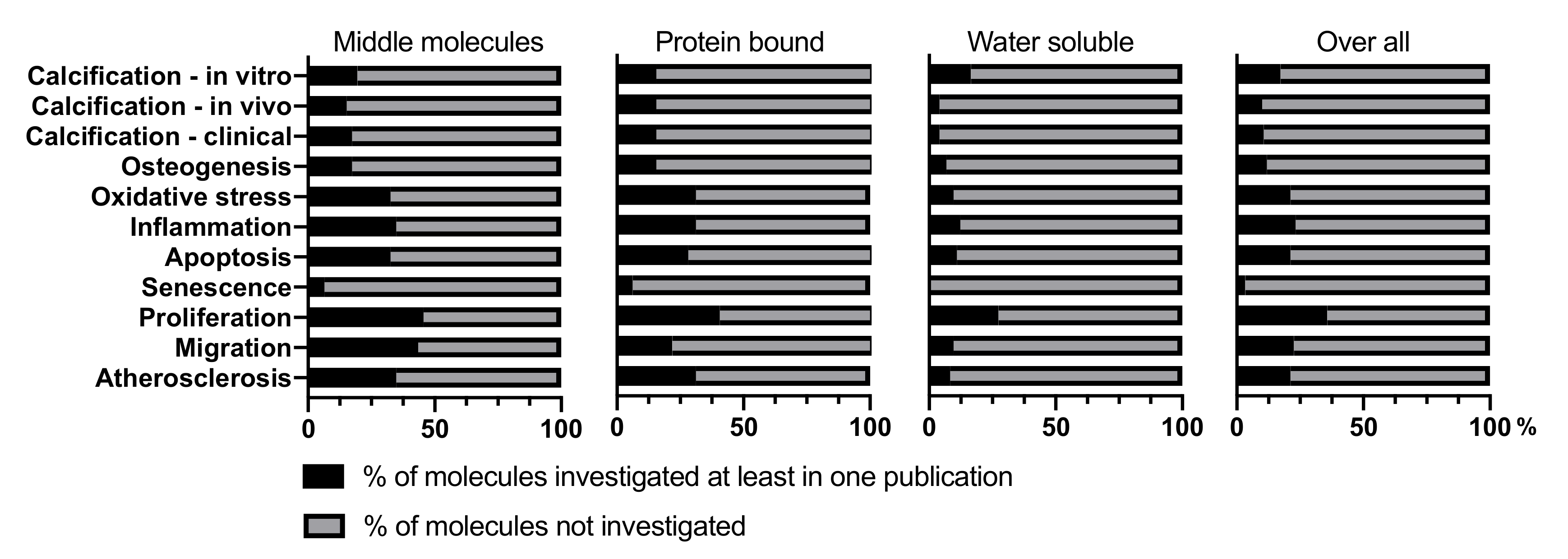
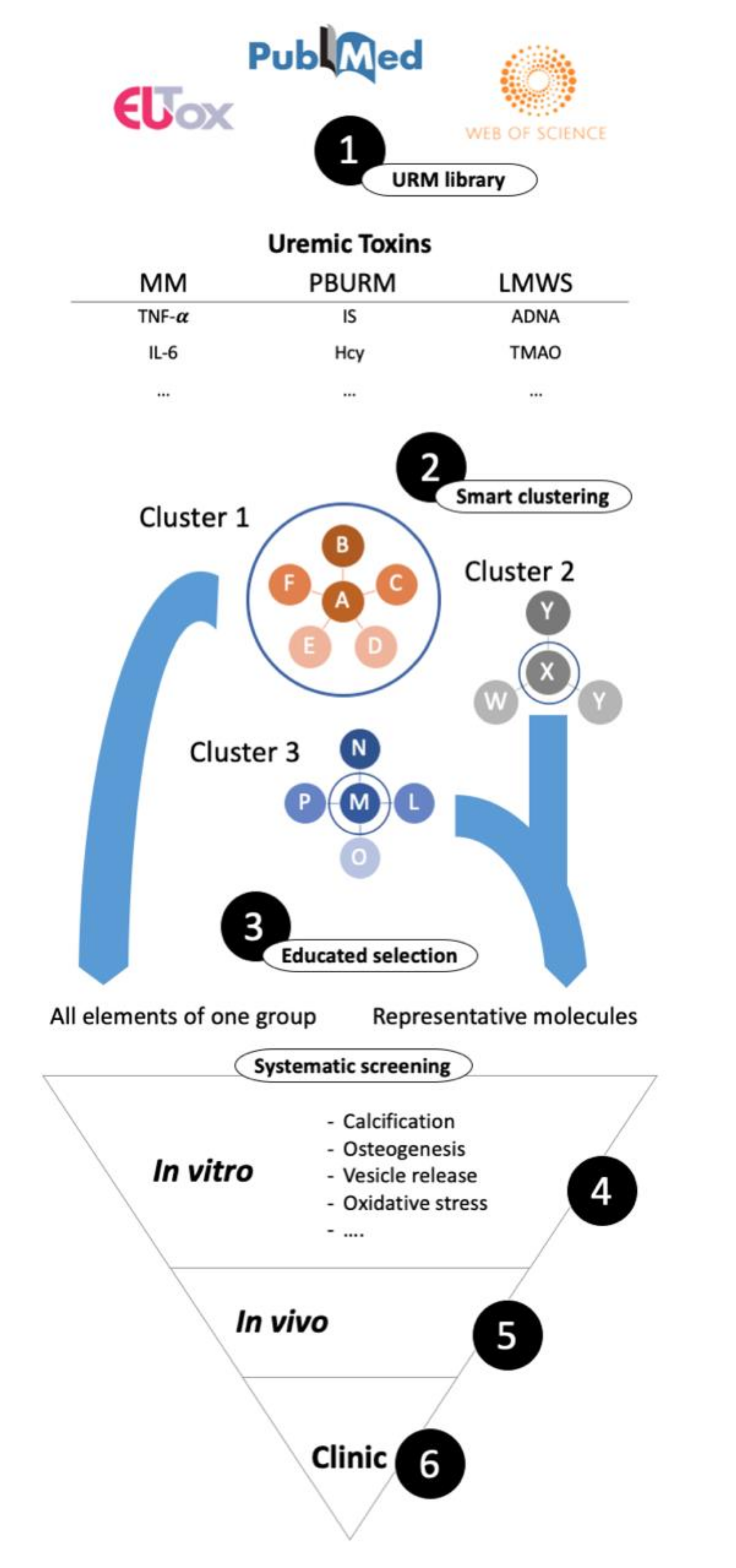
| Middle molecules | 46 (30,5) | # (%) | MW > 500 Da | e.g., TNF |
| Protein bound | 32 (21,2 | # (%) | - | e.g., Indoxyl sulfate |
| Water soluble | 73 (48,3) | # (%) | MW < 500 Da | e.g., Urea |
| Total | 151 (100) | #(%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapp, N.; Evenepoel, P.; Stenvinkel, P.; Schurgers, L. Uremic Toxins and Vascular Calcification–Missing the Forest for All the Trees. Toxins 2020, 12, 624. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12100624
Rapp N, Evenepoel P, Stenvinkel P, Schurgers L. Uremic Toxins and Vascular Calcification–Missing the Forest for All the Trees. Toxins. 2020; 12(10):624. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12100624
Chicago/Turabian StyleRapp, Nikolas, Pieter Evenepoel, Peter Stenvinkel, and Leon Schurgers. 2020. "Uremic Toxins and Vascular Calcification–Missing the Forest for All the Trees" Toxins 12, no. 10: 624. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12100624






