Uremic Apelin and Leucocytic Angiotensin-Converting Enzyme 2 in CKD Patients
Abstract
:1. Introduction
2. Results
2.1. Levels of Serum APLN Are Elevated in CKD3–5 and HD Patients and Correlate with Leucocytic Expression of APLNR and ACE2
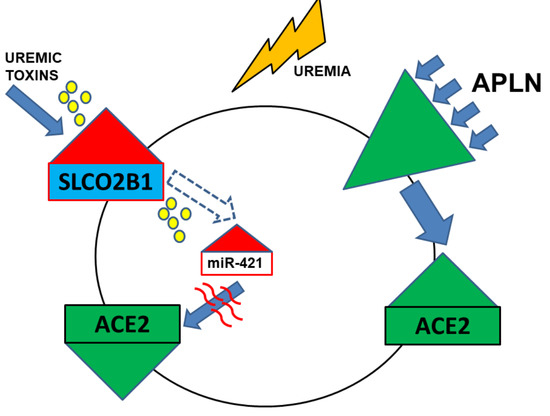
2.2. Leucocytic Organic Anion Transporter SLCO2B1 Is Increased in Uremic Patients and Correlates with Diminished ACE2
2.3. Uremic Toxins Mediated Increase of Intracellular miR-421 Leads to Monocytic Silencing of ACE2 and Elevation of Pro-Inflammatory TNFa and IL-6
2.4. Uremic Milieu and APLN Peptides Increase the Expression of APLNR In Vitro
2.5. APLN Peptides Dramatically Increase the Expression of ACE2 and APLNR
2.6. Uraemia-Mediated Decrease in Monocytic ACE2 Could Be Partially Re-Induced with APLN Peptides
2.7. APLN Peptides Decrease the Expression of TNFa and IL-6 In Vitro as well as AngII and Ang1–7 Receptors
2.8. APLN Peptides Affect Transmigration and Adhesion of the Cells In Vitro
3. Discussion
4. Materials and Methods
4.1. Patients, RNA, and Serum Isolation
4.2. Serum APLN Levels
4.3. Cell Culture and Treatments of THP-1 Monocytes
4.4. Real Time PCR
4.5. Transmigration Assay
4.6. Adhesion Assay
4.7. Statistics
4.8. Limitations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vickers, C.; Hales, P.; Kaushik, V.; Dick, L.; Gavin, J.; Tang, J.; Godbout, K.; Parsons, T.; Baronas, E.; Hsieh, F.; et al. Hydrolysis of Biological Peptides by Human Angiotensin-converting Enzyme-related Carboxypeptidase. J. Biol. Chem. 2002, 277, 14838–14843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Messari, S.; Iturrioz, X.; Fassot, C.; De Mota, N.; Roesch, D.; Llorens-Cortes, C. Functional dissociation of apelin receptor signaling and endocytosis: Implications for the effects of apelin on arterial blood pressure. J. Neurochem. 2004, 90, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; McKinnie, S.M.; Farhan, M.; Paul, M.; McDonald, T.; McLean, B.; Llorens-Cortes, C.; Hazra, S.; Murray, A.G.; Vederas, J.C.; et al. Angiotensin-Converting Enzyme 2 Metabolizes and Partially Inactivates Pyr-Apelin-13 and Apelin-17: Physiological Effects in the Cardiovascular System. Hypertension 2016, 68, 365–377. [Google Scholar] [CrossRef] [Green Version]
- O’Dowd, B.F.; Heiber, M.; Chan, A.; Heng, H.H.; Tsui, L.-C.; Kennedy, J.L.; Shi, X.; Petronis, A.; George, S.R.; Nguyen, T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene 1993, 136, 355–360. [Google Scholar] [CrossRef]
- Pitkin, S.L.; Maguire, J.J.; Bonner, T.I.; Davenport, A.P. International Union of Basic and Clinical Pharmacology. LXXIV. Apelin receptor nomenclature, distribution, pharmacology, and function. Pharmacol. Rev. 2010, 62, 331–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, M.; Hidaka, K.; Akiho, H.; Tada, S.; Okada, M.; Yamaguchi, T. Low stringency hybridization study of the dopamine D4 receptor revealed D4-like mRNA distribution of the orphan seven-transmembrane receptor, APJ, in human brain. Neurosci. Lett. 1996, 219, 119–122. [Google Scholar] [CrossRef]
- Medhurst, A.D.; Jennings, C.A.; Robbins, M.J.; Davis, R.P.; Ellis, C.; Winborn, K.Y.; Lawrie, K.W.M.; Hervieu, G.; Riley, G.; Bolaky, J.E.; et al. Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J. Neurochem. 2003, 84, 1162–1172. [Google Scholar] [CrossRef]
- Katugampola, S.D.; Maguire, J.J.; Matthewson, S.R.; Davenport, A.P. [125 I]-(Pyr1 )Apelin-13 is a novel radioligand for localizing the APJ orphan receptor in human and rat tissues with evidence for a vasoconstrictor role in man. Br. J. Pharmacol. 2001, 132, 1255–1260. [Google Scholar] [CrossRef] [Green Version]
- Kleinz, M.J.; Skepper, J.N.; Davenport, A.P. Immunocytochemical localisation of the apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells. Regul. Pept. 2005, 126, 233–240. [Google Scholar] [CrossRef]
- Wu, D.; He, L.; Li, L. Apelin/APJ system: A promising therapy target for hypertension. Mol. Biol. Rep. 2014, 41, 6691–6703. [Google Scholar] [CrossRef]
- Lu, L.; Wu, D.; Li, L.; Chen, L. Apelin/APJ system: A bifunctional target for cardiac hypertrophy. Int. J. Cardiol. 2017, 230, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, J.; Chen, L.; Jiang, Z. Apelin/APJ system: A novel promising therapy target for thrombotic diseases. Acta Biochim. Biophys. Sin. 2016, 48, 589–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Chen, L.; Li, L. Apelin/APJ system: A novel promising therapy target for pathological angiogenesis. Clin. Chim. Acta 2017, 466, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Soltani-Hekmat, A.; Najafipour, H.; Nekooeian, A.A.; Esmaeili-Mahani, S.; Javanmardi, K. Cardiovascular responses to apelin in two-kidney–one-clip hypertensive rats and its receptor expression in ischemic and non-ischemic kidneys. Regul. Pept. 2011, 172, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Najafipour, H.; Hekmat, A.S.; Nekooian, A.A.; Esmaeili-Mahani, S. Apelin receptor expression in ischemic and non- ischemic kidneys and cardiovascular responses to apelin in chronic two-kidney–one-clip hypertension in rats. Regul. Pept. 2012, 178, 43–50. [Google Scholar] [CrossRef]
- Siddiquee, K.; Hampton, J.; Khan, S.; Zadory, D.; Gleaves, L.; Vaughan, D.E.; Smith, L.H. Apelin protects against angiotensin II-induced cardiovascular fibrosis and decreases plasminogen activator inhibitor type-1 production. J. Hypertens. 2011, 29, 724–731. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, T.; Kihara, M.; Imai, N.; Yoshida, S.-I.; Shimoyamada, H.; Yasuzaki, H.; Ishida, J.; Toya, Y.; Kiuchi, Y.; Hirawa, N.; et al. Requirement of Apelin-Apelin Receptor System for Oxidative Stress-Linked Atherosclerosis. Am. J. Pathol. 2007, 171, 1705–1712. [Google Scholar] [CrossRef] [Green Version]
- Daviaud, D.; Boucher, J.; Gesta, S.; Dray, C.; Guigne, C.; Quilliot, D.; Ayav, A.; Ziegler, O.; Carpene, C.; Saulnier-Blache, J.-S.; et al. TNFalpha up-regulates apelin expression in human and mouse adipose tissue. FASEB J. 2006, 20, 1528–1530. [Google Scholar] [CrossRef]
- Melgar-Lesmes, P.; Pauta, M.; Reichenbach, V.; Casals, G.; Ros, J.; Bataller, R.; Morales-Ruiz, M.; Jiménez, W. Hypoxia and proinflammatory factors upregulate apelin receptor expression in human stellate cells and hepatocytes. Gut 2011, 60, 1404–1411. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, X.; Liang, G.-X.; Cui, R.-R.; Liu, Y.; Wu, S.-S.; Liang, Q.-H.; Liu, G.-Y.; Jiang, Y.; Liao, X.-B.; et al. Apelin-APJ induces ICAM-1, VCAM-1 and MCP-1 expression via NF-kappaB/JNK signal pathway in human umbilical vein endothelial cells. Amino Acids 2012, 43, 2125–2136. [Google Scholar] [CrossRef]
- Vanholder, R.; Massy, Z.; Argiles, A.; Spasovski, G.; Verbeke, F.; Lameire, N. Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol. Dial. Transplant. 2005, 20, 1048–1056. [Google Scholar] [CrossRef] [Green Version]
- Stenvinkel, P. Inflammation in End-Stage Renal Disease – A Fire that Burns within. Contrib. Nephrol. 2005, 149, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Trojanowicz, B.; Ulrich, C.; Kohler, F.; Bode, V.; Seibert, E.; Fiedler, R.; Girndt, M. Monocytic angiotensin-converting enzyme 2 relates to atherosclerosis in patients with chronic kidney disease. Nephrol. Dial. Transplant. 2017, 32, 287–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trojanowicz, B.; Ulrich, C.; Seibert, E.; Fiedler, R.; Girndt, M. Uremic Conditions Drive Human Monocytes to Pro-Atherogenic Differentiation via an Angiotensin-Dependent Mechanism. PLoS ONE 2014, 9, e102137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulrich, C.; Seibert, E.; Heine, G.H.; Fliser, D.; Girndt, M. Monocyte Angiotensin Converting Enzyme Expression May Be Associated with Atherosclerosis Rather than Arteriosclerosis in Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 505–511. [Google Scholar] [CrossRef] [Green Version]
- Chun, H.J.; Ali, Z.A.; Kojima, Y.; Kundu, R.K.; Sheikh, A.Y.; Agrawal, R.; Zheng, L.; Leeper, N.J.; Pearl, N.E.; Patterson, A.J.; et al. Apelin signaling antagonizes Ang II effects in mouse models of atherosclerosis. J. Clin. Investig. 2008, 118, 3343–3354. [Google Scholar] [CrossRef] [Green Version]
- Trojanowicz, B.; Imdahl, T.; Ulrich, C.; Fiedler, R.; Girndt, M. Circulating miR-421 Targeting Leucocytic Angiotensin Converting Enzyme 2 Is Elevated in Patients with Chronic Kidney Disease. Nephron 2018, 141, 61–74. [Google Scholar] [CrossRef]
- Sato, T.; Suzuki, T.; Watanabe, H.; Kadowaki, A.; Fukamizu, A.; Liu, P.P.; Kimura, A.; Ito, H.; Penninger, J.M.; Imai, Y.; et al. Apelin is a positive regulator of ACE2 in failing hearts. J. Clin. Investig. 2013, 123, 5203–5211. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Shen, M.; Fischer, C.; Basu, R.; Hazra, S.; Couvineau, P.; Paul, M.; Wang, F.; Toth, S.; Mix, D.S.; et al. Apelin protects against abdominal aortic aneurysm and the therapeutic role of neutral endopeptidase resistant apelin analogs. Proc. Natl. Acad. Sci. USA 2019, 116, 13006–13015. [Google Scholar] [CrossRef] [Green Version]
- Judge, P.; Haynes, R.; Landray, M.J.; Baigent, C. Neprilysin inhibition in chronic kidney disease. Nephrology, dialysis, transplantation. Nephrol. Dial. Transplant. 2015, 30, 738–743. [Google Scholar] [CrossRef] [Green Version]
- Gutta, S.; Grobe, N.; Kumbaji, M.; Osman, H.; Saklayen, M.; Li, G.; Elased, K.M. Increased urinary angiotensin converting enzyme 2 and neprilysin in patients with type 2 diabetes. Am. J. Physiol. Physiol. 2018, 315, F263–F274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schepers, E.; Meert, N.; Glorieux, G.; Goeman, J.; Van der Eycken, J.; Vanholder, R. P-cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol. Dial. Transplant. 2007, 22, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Saito, S.; Higashiyama, Y.; Nishijima, F.; Niwa, T. CREB, NF-κB, and NADPH oxidase coordinately upregulate indoxyl sulfate-induced angiotensinogen expression in proximal tubular cells. Am. J. Physiol. Physiol. 2013, 304, C685–C692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.-Y.; Chang, S.-C.; Wu, M.-S. Uremic Toxins Induce Kidney Fibrosis by Activating Intrarenal Renin–Angiotensin–Aldosterone System Associated Epithelial-to-Mesenchymal Transition. PLoS ONE 2012, 7, e34026. [Google Scholar] [CrossRef] [Green Version]
- Pletinck, A.; Glorieux, G.; Schepers, E.; Cohen, G.; Gondouin, B.; Van Landschoot, M.; Eloot, S.; Rops, A.; Van De Voorde, J.; De Vriese, A.; et al. Protein-Bound Uremic Toxins Stimulate Crosstalk between Leukocytes and Vessel Wall. J. Am. Soc. Nephrol. 2013, 24, 1981–1994. [Google Scholar] [CrossRef] [Green Version]
- Nakano, T.; Katsuki, S.; Chen, M.; Decano, J.L.; Halu, A.; Lee, L.H.; Pestana, D.V.S.; Kum, A.S.T.; Kuromoto, R.K.; Golden, W.S.; et al. Uremic Toxin Indoxyl Sulfate Promotes Proinflammatory Macrophage Activation Via the Interplay of OATP2B1 and Dll4-Notch Signaling. Circulation 2019, 139, 78–96. [Google Scholar] [CrossRef]
- Galon-Tilleman, H.; Yang, H.; Bednarek, M.A.; Spurlock, S.M.; Paavola, K.J.; Ko, B.; To, C.; Luo, J.; Tian, H.; Jermutus, L.; et al. Apelin-36 Modulates Blood Glucose and Body Weight Independently of Canonical APJ Receptor Signaling. J. Biol. Chem. 2016, 292, 1925–1933. [Google Scholar] [CrossRef] [Green Version]





| Parameters | NP (n = 32) | HD (n = 66) | CKD3–5 (n = 24) |
|---|---|---|---|
| Age (years) | 53.1 ± 6.84 | 63.02 ± 14.69 | 74.54 ± 10.72 |
| Male/female | 8/24 | 44/22 | 8/16 |
| Body mass index (kg/m2) | 24.50 ± 4.01 | 25.91 ± 4.73 | 28.56 ± 6.42 |
| Hypertension (%) | 6.25 | 92.42 | 91.67 |
| Diabetes (%) | 3.12 | 37.88 | 33.3 |
| Ever smoker (%) | 46.88 | 57.58 | 41.67 |
| Dialysis vintage HD (years) | 0 | 8.40 ± 5.11 | 0 |
| History of cardiovascular disease (%) | 0 | 45.45 | 66.67 |
| Creatinine (µmol/L) | 69.88 ± 12.94 | 763.58 ± 290.65 | 157.71 ± 67.28 |
| Urea (mmol/L) | 4.54 ± 1.36 | 21.57 ± 6.95 | 15.33 ± 17.48 |
| Albumin (g/dL) | 4.10 ± 0.30 | 3.88 ± 0.48 | N/A |
| C-reactive protein (mg/L) | 1.73 ± 1.36 | 13.38 ± 23.84 | 11.03 ± 15.13 |
| Leucocytes (G/L) | 6.15 ± 1.59 | 7.69 ± 3.44 | 6.71 ± 1.75 |
| Monocytes (% leukocytes) | 8.22 ± 2.41 | 8.48 ± 2.99 | N/A |
| HDL (mmol/L) | 1.78 ± 0.45 | 1.32 ± 0.63 | N/A |
| LDL (mmol/L) | 2.42 ± 0.92 | 3.78 ± 0.86 | N/A |
| Cholesterol (mmol/L) | 6.17 ± 0.83 | 4.37 ± 1.26 | N/A |
| Triglyceride (mmol/L) | 1.40 ± 1.14 | 2.20 ± 2.36 | N/A |
| 25(OH) vit. D (%) | 0 | 90.91 | 25.00 |
| ACEi (%) | 0 | 24.24 | 33.33 |
| ARB (%) | 0 | 42.42 | 45.83 |
| β-blockers (%) | 0 | 77.27 | 75.00 |
| Statins (%) | 0 | 37.88 | 58.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trojanowicz, B.; Ulrich, C.; Girndt, M. Uremic Apelin and Leucocytic Angiotensin-Converting Enzyme 2 in CKD Patients. Toxins 2020, 12, 742. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12120742
Trojanowicz B, Ulrich C, Girndt M. Uremic Apelin and Leucocytic Angiotensin-Converting Enzyme 2 in CKD Patients. Toxins. 2020; 12(12):742. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12120742
Chicago/Turabian StyleTrojanowicz, Bogusz, Christof Ulrich, and Matthias Girndt. 2020. "Uremic Apelin and Leucocytic Angiotensin-Converting Enzyme 2 in CKD Patients" Toxins 12, no. 12: 742. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12120742