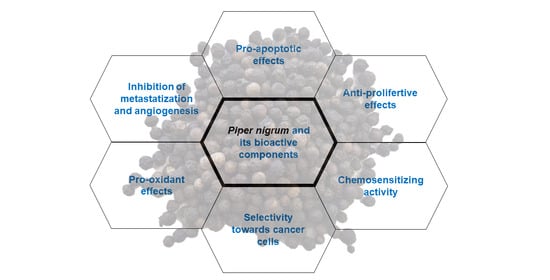
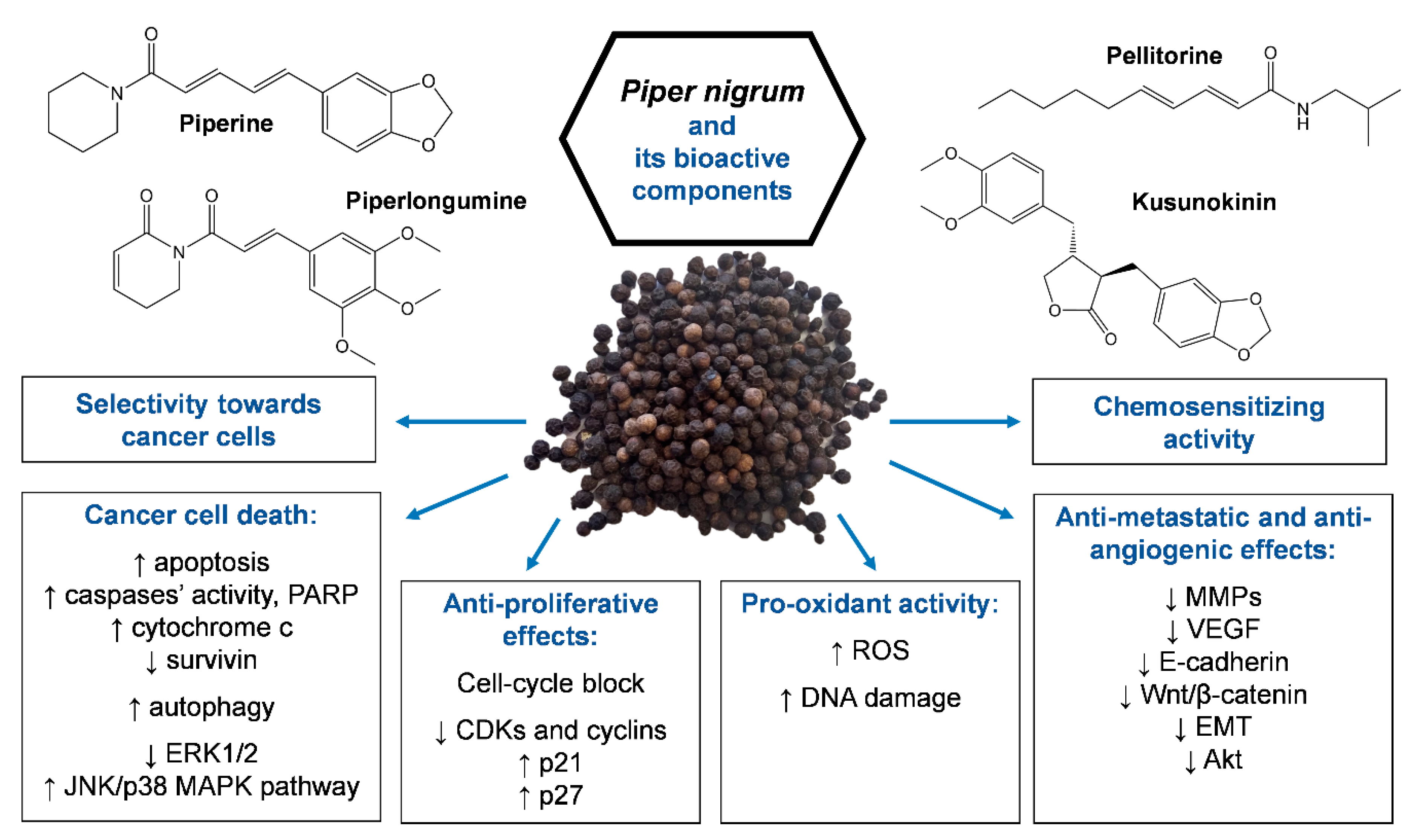
Overview of the Anticancer Potential of the “King of Spices” Piper nigrum and Its Main Constituent Piperine
Abstract
:1. Introduction
2. Anticancer Activity of Piper nigrum Extracts
3. In Vitro and In Vivo Anticancer Activity of Piperine
3.1. In Vitro Studies
3.1.1. Piperine Induces Apoptosis and Inhibits Cell Proliferation in Cancer Cells
Induction of Cell Death
Pro-Oxidant Activity and Induction of Endoplasmic Reticulum (ER) Stress
Induction of Cell-Cycle Arrest
3.1.2. Piperine Inhibits Cancer Metastasization and Neoangiogenesis
Inhibition of Invasion/Migration and Epithelial Mesenchymal Transition
Inhibition of Angiogenesis
3.2. In Vivo Studies
3.3. Selectivity of Piperine towards Cancer Cells
3.4. Basic Aspects of Piperine Pharmacokinetics
3.5. Chemosensitizing Activity of Piperine
4. Other Compounds from Piper nigrum with Anticancer Potential
5. Toxicological Studies
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 26 August 2019).
- Pritchard, J.R.; Lauffenburger, D.A.; Hemann, M.T. Understanding resistance to combination chemotherapy. Drug Resist. Updates 2012, 15, 249–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahomoodally, M.F. A systematic review on black pepper (Piper nigrum L.): From folk uses to pharmacological applications. Crit. Rev. Food Sci. Nutr. 2019, 59, S210–S243. [Google Scholar] [CrossRef] [PubMed]
- Meghwal, M.; Goswami, T.K. Piper nigrum and piperine: An update. Phytother. Res. 2013, 27, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K. Antioxidant potential of spices and their active constituents. Crit. Rev. Food Sci. Nutr. 2014, 54, 352–372. [Google Scholar] [CrossRef]
- Smilkov, K.; Ackova, D.G.; Cvetkovski, A.; Ruskovska, T.; Vidovic, B.; Atalay, M. Piperine: Old Spice and New Nutraceutical? Curr. Pharm. Des. 2019, 25, 1729–1739. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Morris-Natschke, S.L.; Yang, J.; Niu, H.-M.; Long, C.-L.; Lee, K.-H. Anticancer principles from medicinal Piper (胡椒 Hú Jiāo) plants. J. Tradit. Complement. Med. 2014, 4, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E.; et al. Piper Species: A Comprehensive Review on Their Phytochemistry, Biological Activities and Applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef] [Green Version]
- Chavarria, D.; Silva, T.; Magalhães e Silva, D.; Remião, F.; Borges, F. Lessons from black pepper: Piperine and derivatives thereof. Expert Opin. Pat. 2016, 26, 245–264. [Google Scholar] [CrossRef]
- Manayi, A.; Nabavi, S.M.; Setzer, W.N.; Jafari, S. Piperine as a Potential Anti-cancer Agent: A Review on Preclinical Studies. Curr. Med. Chem. 2018, 25, 4918–4928. [Google Scholar] [CrossRef]
- Ahmad, N.; Fazal, H.; Abbasi, B.H.; Farooq, S.; Ali, M.; Khan, M.A. Biological role of Piper nigrum L. (Black pepper): A review. Asian Pac. J. Trop. Biomed. 2012, 2, S1945–S1953. [Google Scholar] [CrossRef]
- Prashant, A.; Rangaswamy, C.; Yadav, A.K.; Reddy, V.; Sowmya, M.N.; Madhunapantula, S. In vitro anticancer activity of ethanolic extracts of Piper nigrum against colorectal carcinoma cell lines. Int. J. Appl. Basic Med. Res 2017, 7, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tammina, S.K.; Mandal, B.K.; Ranjan, S.; Dasgupta, N. Cytotoxicity study of Piper nigrum seed mediated synthesized SnO(2) nanoparticles towards colorectal (HCT116) and lung cancer (A549) cell lines. J. PhotoChem. PhotoBiol. B 2017, 166, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Grinevicius, V.M.; Kviecinski, M.R.; Santos Mota, N.S.; Ourique, F.; Porfirio Will Castro, L.S.; Andreguetti, R.R.; Gomes Correia, J.F.; Filho, D.W.; Pich, C.T.; Pedrosa, R.C. Piper nigrum ethanolic extract rich in piperamides causes ROS overproduction, oxidative damage in DNA leading to cell cycle arrest and apoptosis in cancer cells. J. Ethnopharmacol. 2016, 189, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Grinevicius, V.M.; Andrade, K.S.; Ourique, F.; Micke, G.A.; Ferreira, S.R.; Pedrosa, R.C. Antitumor activity of conventional and supercritical extracts from Piper nigrum L. cultivar Bragantina through cell cycle arrest and apoptosis induction. J. Supercrit. Fluids 2017, 128, 94–101. [Google Scholar] [CrossRef]
- Grinevicius, V.M.; Andrade, K.S.; Mota, N.; Bretanha, L.C.; Felipe, K.B.; Ferreira, S.R.S.; Pedrosa, R.C. CDK2 and Bcl-xL inhibitory mechanisms by docking simulations and anti-tumor activity from piperine enriched supercritical extract. Food Chem. Toxicol. 2019, 132, 110644. [Google Scholar] [CrossRef] [PubMed]
- Sriwiriyajan, S.; Ninpesh, T.; Sukpondma, Y.; Nasomyon, T.; Graidist, P. Cytotoxicity screening of plants of genus Piper in breast cancer cell lines. Trop. J. Pharm. Res. 2014, 13, 921–928. [Google Scholar] [CrossRef] [Green Version]
- Sriwiriyajan, S.; Tedasen, A.; Lailerd, N.; Boonyaphiphat, P.; Nitiruangjarat, A.; Deng, Y.; Graidist, P. Anticancer and Cancer Prevention Effects of Piperine-Free Piper nigrum Extract on N-nitrosomethylurea-Induced Mammary Tumorigenesis in Rats. Cancer Prev. Res. 2016, 9, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Sriwiriyajan, S.; Tedasen, A.; Hiransai, P.; Graidist, P. Anti-cancer effects of Piper nigrum via inducing multiple molecular signaling in vivo and in vitro. J. Ethnopharmacol. 2016, 188, 87–95. [Google Scholar] [CrossRef]
- Ee, G.; Lim, C.; Lim, C.; Rahmani, M.; Shaari, K.; Bong, C. Alkaloids from Piper sarmentosum and Piper nigrum. Nat. Prod. Res. 2009, 23, 1416–1423. [Google Scholar] [CrossRef]
- Yu, W.; Yang, L.; Li, T.; Zhang, Y. Cadherin Signaling in Cancer: Its Functions and Role as a Therapeutic Target. Front. Oncol. 2019, 9, 989. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.C.; Lobkovsky, E.; Gange, A.C.; Singh, S.K.; Prakash, S. Piperine production by endophytic fungus Periconia sp. isolated from Piper longum L. J. Antibiot. 2011, 64, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Chithra, S.; Jasim, B.; Sachidanandan, P.; Jyothis, M.; Radhakrishnan, E.K. Piperine production by endophytic fungus Colletotrichum gloeosporioides isolated from Piper nigrum. Phytomedicine 2014, 21, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.H.; Fu, Q.H.; Liu, Y.; Jiang, K.; Guo, Q.M.; Chen, Q.Y.; Yan, B.; Wang, Q.Q.; Shen, J.G. Piperine suppresses tumor growth and metastasis in vitro and in vivo in a 4T1 murine breast cancer model. Acta Pharm. Sin. 2012, 33, 523–530. [Google Scholar] [CrossRef]
- Do, M.T.; Kim, H.G.; Choi, J.H.; Khanal, T.; Park, B.H.; Tran, T.P.; Jeong, T.C.; Jeong, H.G. Antitumor efficacy of piperine in the treatment of human HER2-overexpressing breast cancer cells. Food Chem. 2013, 141, 2591–2599. [Google Scholar] [CrossRef]
- Abdelhamed, S.; Yokoyama, S.; Refaat, A.; Ogura, K.; Yagita, H.; Awale, S.; Saiki, I. Piperine enhances the efficacy of TRAIL-based therapy for triple-negative breast cancer cells. Anticancer Res. 2014, 34, 1893–1899. [Google Scholar]
- Greenshields, A.L.; Doucette, C.D.; Sutton, K.M.; Madera, L.; Annan, H.; Yaffe, P.B.; Knickle, A.F.; Dong, Z.; Hoskin, D.W. Piperine inhibits the growth and motility of triple-negative breast cancer cells. Cancer Lett. 2015, 357, 129–140. [Google Scholar] [CrossRef]
- Talib, W.H. Regressions of Breast Carcinoma Syngraft Following Treatment with Piperine in Combination with Thymoquinone. Sci. Pharm. 2017, 85, 27. [Google Scholar] [CrossRef] [Green Version]
- Khamis, A.A.A.; Ali, E.M.M.; El-Moneim, M.A.A.; Abd-Alhaseeb, M.M.; El-Magd, M.A.; Salim, E.I. Hesperidin, piperine and bee venom synergistically potentiate the anticancer effect of tamoxifen against breast cancer cells. Biomed. Pharm. 2018, 105, 1335–1343. [Google Scholar] [CrossRef]
- Marques da Fonseca, L.; Jacques da Silva, L.R.; Santos Dos Reis, J.; Rodrigues da Costa Santos, M.A.; de Sousa Chaves, V.; Monteiro da Costa, K.; Sa-Diniz, J.N.; Freire de Lima, C.G.; Morrot, A.; Nunes Franklim, T.; et al. Piperine Inhibits TGF-β Signaling Pathways and Disrupts EMT-Related Events in Human Lung Adenocarcinoma Cells. Medicines 2020, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, D.Y.; Zeng, L.H.; Pan, H.; Xu, L.H.; Wang, Y.; Liu, K.P.; He, X.H. Piperine inhibits the proliferation of human prostate cancer cells via induction of cell cycle arrest and autophagy. Food Chem. Toxicol. 2013, 60, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Samykutty, A.; Shetty, A.V.; Dakshinamoorthy, G.; Bartik, M.M.; Johnson, G.L.; Webb, B.; Zheng, G.; Chen, A.; Kalyanasundaram, R.; Munirathinam, G. Piperine, a Bioactive Component of Pepper Spice Exerts Therapeutic Effects on Androgen Dependent and Androgen Independent Prostate Cancer Cells. PLoS ONE 2013, 8, e65889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ba, Y.; Malhotra, A. Potential of piperine in modulation of voltage-gated K+ current and its influences on cell cycle arrest and apoptosis in human prostate cancer cells. Eur. Rev. Med. Pharm. Sci. 2018, 22, 8999–9011. [Google Scholar] [CrossRef]
- George, K.; Thomas, N.S.; Malathi, R. Piperine blocks voltage gated K(+) current and inhibits proliferation in androgen sensitive and insensitive human prostate cancer cell lines. Arch. Biochem. Biophys. 2019, 667, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Yang, Y. Piperine depresses the migration progression via downregulating the Akt/mTOR/MMP-9 signaling pathway in DU145 cells. Mol. Med. Rep. 2018, 17, 6363–6370. [Google Scholar] [CrossRef] [Green Version]
- Duessel, S.; Heuertz, R.M.; Ezekiel, U.R. Growth inhibition of human colon cancer cells by plant compounds. Clin. Lab. Sci. 2008, 21, 151–157. [Google Scholar]
- Yaffe, P.B.; Power Coombs, M.R.; Doucette, C.D.; Walsh, M.; Hoskin, D.W. Piperine, an alkaloid from black pepper, inhibits growth of human colon cancer cells via G1 arrest and apoptosis triggered by endoplasmic reticulum stress. Mol. Carcinog. 2015, 54, 1070–1085. [Google Scholar] [CrossRef]
- De Almeida, G.C.; Oliveira, L.F.S.; Predes, D.; Fokoue, H.H.; Kuster, R.M.; Oliveira, F.L.; Mendes, F.A.; Abreu, J.G. Piperine suppresses the Wnt/β-catenin pathway and has anti-cancer effects on colorectal cancer cells. Sci. Rep. 2020, 10, 11681. [Google Scholar] [CrossRef]
- Song, L.; Wang, Y.; Zhen, Y.; Li, D.; He, X.; Yang, H.; Zhang, H.; Liu, Q. Piperine inhibits colorectal cancer migration and invasion by regulating STAT3/Snail-mediated epithelial-mesenchymal transition. Biotechnol. Lett. 2020. [Google Scholar] [CrossRef]
- Yaffe, P.B.; Doucette, C.D.; Walsh, M.; Hoskin, D.W. Piperine impairs cell cycle progression and causes reactive oxygen species-dependent apoptosis in rectal cancer cells. Exp. Mol. Pathol. 2013, 94, 109–114. [Google Scholar] [CrossRef]
- Pradeep, C.R.; Kuttan, G. Effect of piperine on the inhibition of lung metastasis induced B16F-10 melanoma cells in mice. Clin. Exp. Metastasis 2002, 19, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Selvendiran, K.; Banu, S.M.; Sakthisekaran, D. Protective effect of piperine on benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice. Clin. Chim. Acta 2004, 350, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Selvendiran, K.; Prince Vijeya Singh, J.; Sakthisekaran, D. In vivo effect of piperine on serum and tissue glycoprotein levels in benzo(a)pyrene induced lung carcinogenesis in Swiss albino mice. Pulm. Pharm. 2006, 19, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xu, J.; Liao, H.; Li, L.; Pan, L. Piperine induces apoptosis of lung cancer A549 cells via p53-dependent mitochondrial signaling pathway. Tumour Biol. 2014, 35, 3305–3310. [Google Scholar] [CrossRef] [PubMed]
- Tawani, A.; Amanullah, A.; Mishra, A.; Kumar, A. Evidences for Piperine inhibiting cancer by targeting human G-quadruplex DNA sequences. Sci. Rep. 2016, 6, 39239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fofaria, N.M.; Kim, S.H.; Srivastava, S.K. Piperine causes G1 phase cell cycle arrest and apoptosis in melanoma cells through checkpoint kinase-1 activation. PLoS ONE 2014, 9, e94298. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.S.; Choo, G.S.; Kim, S.H.; Woo, J.S.; Kim, H.J.; Park, Y.S.; Kim, B.S.; Kim, S.K.; Park, B.K.; Cho, S.D.; et al. Antitumor and Apoptosis-inducing Effects of Piperine on Human Melanoma Cells. Anticancer Res. 2019, 39, 1883–1892. [Google Scholar] [CrossRef]
- Gunasekaran, V.; Elangovan, K.; Niranjali Devaraj, S. Targeting hepatocellular carcinoma with piperine by radical-mediated mitochondrial pathway of apoptosis: An in vitro and in vivo study. Food Chem. Toxicol. 2017, 105, 106–118. [Google Scholar] [CrossRef]
- Si, L.; Yang, R.; Lin, R.; Yang, S. Piperine functions as a tumor suppressor for human ovarian tumor growth via activation of JNK/p38 MAPK-mediated intrinsic apoptotic pathway. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [Green Version]
- Qiu, M.; Xue, C.; Zhang, L. Piperine alkaloid induces anticancer and apoptotic effects in cisplatin resistant ovarian carcinoma by inducing G2/M phase cell cycle arrest, caspase activation and inhibition of cell migration and PI3K/Akt/GSK3β signalling pathway. J. BU ON 2019, 24, 2316–2321. [Google Scholar]
- Zhang, J.; Zhu, X.; Li, H.; Li, B.; Sun, L.; Xie, T.; Zhu, T.; Zhou, H.; Ye, Z. Piperine inhibits proliferation of human osteosarcoma cells via G2/M phase arrest and metastasis by suppressing MMP-2/-9 expression. Int. Immunopharmacol. 2015, 24, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.B.; Yang, W.; Si, M.; Nie, L. Wnt/β-catenin signaling modulates piperine-mediated antitumor effects on human osteosarcoma cells. Mol. Med. Rep. 2020, 21, 2202–2208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, Y.P.; Yun, H.J.; Kim, H.G.; Han, E.H.; Choi, J.H.; Chung, Y.C.; Jeong, H.G. Suppression of phorbol-12-myristate-13-acetate-induced tumor cell invasion by piperine via the inhibition of PKCα/ERK1/2-dependent matrix metalloproteinase-9 expression. Toxicol. Lett. 2011, 203, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Ahamad, M.S.; Jafri, A.; Afzal, M.; Arshad, M. Piperine Triggers Apoptosis of Human Oral Squamous Carcinoma Through Cell Cycle Arrest and Mitochondrial Oxidative Stress. Nutr. Cancer 2017, 69, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Jafri, A.; Siddiqui, S.; Rais, J.; Ahmad, M.S.; Kumar, S.; Jafar, T.; Afzal, M.; Arshad, M. Induction of apoptosis by piperine in human cervical adenocarcinoma via ROS mediated mitochondrial pathway and caspase-3 activation. EXCLI J. 2019, 18, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wen, S.; Chen, G.; Wang, S. Antiproliferative potential of piperine and curcumin in drug-resistant human leukemia cancer cells are mediated via autophagy and apoptosis induction, S-phase cell cycle arrest and inhibition of cell invasion and migration. J. BU ON 2020, 25, 401–406. [Google Scholar]
- Fulda, S.; Debatin, K.M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006, 25, 4798–4811. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Duan, N.; Zhang, C.; Zhang, W. Survivin and Tumorigenesis: Molecular Mechanisms and Therapeutic Strategies. J. Cancer 2016, 7, 314–323. [Google Scholar] [CrossRef]
- Rivlin, N.; Brosh, R.; Oren, M.; Rotter, V. Mutations in the p53 Tumor Suppressor Gene: Important Milestones at the Various Steps of Tumorigenesis. Genes Cancer 2011, 2, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Menendez, J.A.; Mehmi, I.; Verma, V.A.; Teng, P.K.; Lupu, R. Pharmacological inhibition of fatty acid synthase (FAS): A novel therapeutic approach for breast cancer chemoprevention through its ability to suppress Her-2/neu (erbB-2) oncogene-induced malignant transformation. Mol. Carcinog. 2004, 41, 164–178. [Google Scholar] [CrossRef]
- Roxo, C.; Kotkowiak, W.; Pasternak, A. G-Quadruplex-Forming Aptamers-Characteristics, Applications, and Perspectives. Molecules 2019, 24, 3781. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, C.; Seimiya, H. G-quadruplex in cancer biology and drug discovery. Biochem. Biophys. Res. Commun. 2020, 531, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Zeh, H.; Lotze, M.; Tang, D. The Multifaceted Effects of Autophagy on the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1225, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Rather, R.A.; Bhagat, M. Cancer Chemoprevention and Piperine: Molecular Mechanisms and Therapeutic Opportunities. Front. Cell Dev. Biol. 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.; Das, I.; Chandhok, D.; Saha, T. Redox regulation in cancer: A double-edged sword with therapeutic potential. Oxid. Med. Cell. Longev. 2010, 3, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Warren, S.; Adjemian, S.; Agostinis, P.; Martinez, A.B.; Chan, T.A.; Coukos, G.; Demaria, S.; Deutsch, E.; et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimri, G.P.; Nakanishi, M.; Desprez, P.Y.; Smith, J.R.; Campisi, J. Inhibition of E2F activity by the cyclin-dependent protein kinase inhibitor p21 in cells expressing or lacking a functional retinoblastoma protein. Mol. Cell. Biol. 1996, 16, 2987–2997. [Google Scholar] [CrossRef] [Green Version]
- Kastan, M.B.; Bartek, J. Cell-cycle checkpoints and cancer. Nature 2004, 432, 316–323. [Google Scholar] [CrossRef]
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-dimensional cell culture: A powerful tool in tumor research and drug discovery. Oncol. Lett. 2017, 14, 6999–7010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alison, M.R.; Lin, W.R.; Lim, S.M.; Nicholson, L.J. Cancer stem cells: In the line of fire. Cancer Treat. Rev. 2012, 38, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Cioce, M.; Gherardi, S.; Viglietto, G.; Strano, S.; Blandino, G.; Muti, P.; Ciliberto, G. Mammosphere-forming cells from breast cancer cell lines as a tool for the identification of CSC-like- and early progenitor-targeting drugs. Cell Cycle 2010, 9, 2878–2887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Dontu, G.; Wicha, M.S. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 2005, 7, 86–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakarala, M.; Brenner, D.E.; Korkaya, H.; Cheng, C.; Tazi, K.; Ginestier, C.; Liu, S.; Dontu, G.; Wicha, M.S. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res. Treat. 2010, 122, 777–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouadid-Ahidouch, H.; Ahidouch, A. K+ channel expression in human breast cancer cells: Involvement in cell cycle regulation and carcinogenesis. J. Membr. Biol. 2008, 221, 1–6. [Google Scholar] [CrossRef]
- Serrano-Novillo, C.; Capera, J.; Colomer-Molera, M.; Condom, E.; Ferreres, J.C.; Felipe, A. Implication of Voltage-Gated Potassium Channels in Neoplastic Cell Proliferation. Cancers 2019, 11, 287. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.G.; Sanders, A.J.; Katoh, M.; Ungefroren, H.; Gieseler, F.; Prince, M.; Thompson, S.; Zollo, M.; Spano, D.; Dhawan, P. Tissue invasion and metastasis: Molecular, biological and clinical perspectives. Semin. Cancer Biol. 2015, 35, S244–S275. [Google Scholar] [CrossRef]
- Pellikainen, J.M.; Ropponen, K.M.; Kataja, V.V.; Kellokoski, J.K.; Eskelinen, M.J.; Kosma, V.M. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER2, and prognosis. Clin. Cancer Res. 2004, 10, 7621–7628. [Google Scholar] [CrossRef] [Green Version]
- Balduyck, M.; Zerimech, F.; Gouyer, V.; Lemaire, R.; Hemon, B.; Grard, G.; Thiebaut, C.; Lemaire, V.; Dacquembronne, E.; Duhem, T.; et al. Specific expression of matrix metalloproteinases 1, 3, 9 and 13 associated with invasiveness of breast cancer cells in vitro. Clin. Exp. Metastasis 2000, 18, 171–178. [Google Scholar] [CrossRef]
- Murphy, G. Tissue inhibitors of metalloproteinases. Genome Biol. 2011, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Abdulghani, J.; Gu, L.; Dagvadorj, A.; Lutz, J.; Leiby, B.; Bonuccelli, G.; Lisanti, M.P.; Zellweger, T.; Alanen, K.; Mirtti, T.; et al. Stat3 promotes metastatic progression of prostate cancer. Am. J. Pathol. 2008, 172, 1717–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Y.; Khoi, P.N.; Yoon, H.J.; Lian, S.; Joo, Y.E.; Chay, K.O.; Kim, K.K.; Jung, Y.D. Piperine inhibits IL-1β-induced IL-6 expression by suppressing p38 MAPK and STAT3 activation in gastric cancer cells. Mol. Cell. Biochem. 2015, 398, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Garcia, E.; Diaz-Garcia, C.V.; Garcia-Ruiz, I.; Agullo-Ortuno, M.T. Epithelial-to-mesenchymal transition in tumor progression. Med. Oncol. 2017, 34, 122. [Google Scholar] [CrossRef]
- Yang, H.; Zhan, L.; Yang, T.; Wang, L.; Li, C.; Zhao, J.; Lei, Z.; Li, X.; Zhang, H.T. Ski prevents TGF-β-induced EMT and cell invasion by repressing SMAD-dependent signaling in non-small cell lung cancer. Oncol. Rep. 2015, 34, 87–94. [Google Scholar] [CrossRef]
- Pai, S.G.; Carneiro, B.A.; Mota, J.M.; Costa, R.; Leite, C.A.; Barroso-Sousa, R.; Kaplan, J.B.; Chae, Y.K.; Giles, F.J. Wnt/beta-catenin pathway: Modulating anticancer immune response. J. Hematol. Oncol. 2017, 10, 101. [Google Scholar] [CrossRef] [Green Version]
- Folkman, J. What is the evidence that tumors are angiogenesis dependent? J. Natl. Cancer Inst. 1990, 82, 4–6. [Google Scholar] [CrossRef] [Green Version]
- Doucette, C.D.; Hilchie, A.L.; Liwski, R.; Hoskin, D.W. Piperine, a dietary phytochemical, inhibits angiogenesis. J. Nutr. Biochem. 2013, 24, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wuhrer, M.; Holst, S. Serum sialylation changes in cancer. Glycoconj. J. 2018, 35, 139–160. [Google Scholar] [CrossRef] [Green Version]
- Hanigan, M.H. gamma-Glutamyl transpeptidase, a glutathionase: Its expression and function in carcinogenesis. Chem. Biol. Interact. 1998, 111–112, 333–342. [Google Scholar] [CrossRef]
- Kim, H.S.; Kwack, S.J.; Lee, B.M. Lipid peroxidation, antioxidant enzymes, and benzo[a]pyrene-quinones in the blood of rats treated with benzo[a]pyrene. Chem. Biol. Interact. 2000, 127, 139–150. [Google Scholar] [CrossRef]
- Thomas, T.; Thomas, T.J. Polyamines in cell growth and cell death: Molecular mechanisms and therapeutic applications. Cell Mol. Life Sci. 2001, 58, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Lennicke, C.; Rahn, J.; Lichtenfels, R.; Wessjohann, L.A.; Seliger, B. Hydrogen peroxide—Production, fate and role in redox signaling of tumor cells. Cell Commun. Signal. 2015, 13, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butt, M.S.; Pasha, I.; Sultan, M.T.; Randhawa, M.A.; Saeed, F.; Ahmed, W. Black pepper and health claims: A comprehensive treatise. Crit. Rev. Food Sci. Nutr. 2013, 53, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Bhat, B.G.; Chandrasekhara, N. Metabolic disposition of piperine in the rat. Toxicology 1987, 44, 99–106. [Google Scholar] [CrossRef]
- Bajad, S.; Singla, A.K.; Bedi, K.L. Liquid chromatographic method for determination of piperine in rat plasma: Application to pharmacokinetics. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 776, 245–249. [Google Scholar] [CrossRef]
- Sahu, P.K.; Sharma, A.; Rayees, S.; Kour, G.; Singh, A.; Khullar, M.; Magotra, A.; Paswan, S.K.; Gupta, M.; Ahmad, I. Pharmacokinetic study of piperine in Wistar rats after oral and intravenous administration. Int. J. Drug Deliv. 2014, 6, 82. [Google Scholar]
- Li, C.; Wang, Q.; Ren, T.; Zhang, Y.; Lam, C.W.K.; Chow, M.S.S.; Zuo, Z. Non-linear pharmacokinetics of piperine and its herb-drug interactions with docetaxel in Sprague-Dawley rats. J. Pharm. Biomed. Anal. 2016, 128, 286–293. [Google Scholar] [CrossRef]
- Wang, X.; Peng, W.; Zhang, Q.; Yang, J.; Zhu, R.; Zhang, J.; Cai, L. Pharmacokinetics of piperine capsules in healthy volunteers. Zhongnan Yaoxue 2010, 8, 513–516. [Google Scholar]
- Jumpa-ngern, P.; Kietinun, S.; Sakpakdeejaroen, I.; Cheomung, A.; Na-Bangchang, K. Pharmacokinetics of piperine following single dose administration of benjakul formulation in healthy Thai subjects. Afr. J. Pharm. Pharmacol. 2013, 7, 560–566. [Google Scholar] [CrossRef]
- Kathawala, R.J.; Gupta, P.; Ashby, C.R., Jr.; Chen, Z.S. The modulation of ABC transporter-mediated multidrug resistance in cancer: A review of the past decade. Drug Resist. Updates 2015, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Vinod, B.S.; Maliekal, T.T.; Anto, R.J. Phytochemicals as chemosensitizers: From molecular mechanism to clinical significance. Antioxid. Redox Signal. 2013, 18, 1307–1348. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H. ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int. 2005, 5, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, U.K.; Singh, A.; Chakraborty, A.K. Role of piperine as a bioavailability enhancer. Int. J. Recent Adv. Pharm. Res. 2011, 4, 16–23. [Google Scholar]
- Atal, N.; Bedi, K. Bioenhancers: Revolutionary concept to market. J. Ayurveda Integr. Med. 2010, 1, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhardwaj, R.K.; Glaeser, H.; Becquemont, L.; Klotz, U.; Gupta, S.K.; Fromm, M.F. Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4. J. Pharm. Exp. 2002, 302, 645–650. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Lei, Y.; Jia, Y.; Li, N.; Wink, M.; Ma, Y. Piperine, a piperidine alkaloid from Piper nigrum re-sensitizes P-gp, MRP1 and BCRP dependent multidrug resistant cancer cells. Phytomedicine 2011, 19, 83–87. [Google Scholar] [CrossRef]
- Li, H.; Krstin, S.; Wang, S.; Wink, M. Capsaicin and Piperine Can Overcome Multidrug Resistance in Cancer Cells to Doxorubicin. Molecules 2018, 23, 557. [Google Scholar] [CrossRef] [Green Version]
- Alzahrani, A.M.; Rajendran, P. The Multifarious Link between Cytochrome P450s and Cancer. Oxid. Med. Cell Longev. 2020, 2020, 3028387. [Google Scholar] [CrossRef]
- Makhov, P.; Golovine, K.; Canter, D.; Kutikov, A.; Simhan, J.; Corlew, M.M.; Uzzo, R.G.; Kolenko, V.M. Co-administration of piperine and docetaxel results in improved anti-tumor efficacy via inhibition of CYP3A4 activity. Prostate 2012, 72, 661–667. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Wang, Z.; Wang, Q.; Ka Yan Ho, R.L.; Huang, Y.; Chow, M.S.S.; Kei Lam, C.W.; Zuo, Z. Enhanced anti-tumor efficacy and mechanisms associated with docetaxel-piperine combination- in vitro and in vivo investigation using a taxane-resistant prostate cancer model. Oncotarget 2018, 9, 3338–3352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Z.; Wei, Y.; Xu, J.; Lei, J.; Yu, J. Alkaloids from Piper nigrum Synergistically Enhanced the Effect of Paclitaxel against Paclitaxel-Resistant Cervical Cancer Cells through the Downregulation of Mcl-1. J. Agric. Food Chem. 2019, 67, 5159–5168. [Google Scholar] [CrossRef] [PubMed]
- Motiwala, M.; Rangari, V. Combined effect of paclitaxel and piperine on a MCF-7 breast cancer cell line in vitro: Evidence of a synergistic interaction. Synergy 2015, 2, 1–6. [Google Scholar] [CrossRef]
- Pal, M.K.; Jaiswar, S.P.; Srivastav, A.K.; Goyal, S.; Dwivedi, A.; Verma, A.; Singh, J.; Pathak, A.K.; Sankhwar, P.L.; Ray, R.S. Synergistic effect of piperine and paclitaxel on cell fate via cyt-c, Bax/Bcl-2-caspase-3 pathway in ovarian adenocarcinomas SKOV-3 cells. Eur. J. Pharm. 2016, 791, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.S.; Muntimadugu, E.; Rafeeqi, T.A.; Domb, A.J.; Khan, W. Co-delivery of rapamycin- and piperine-loaded polymeric nanoparticles for breast cancer treatment. Drug Deliv. 2016, 23, 2608–2616. [Google Scholar] [CrossRef]
- Singh, A.; Thotakura, N.; Singh, B.; Lohan, S.; Negi, P.; Chitkara, D.; Raza, K. Delivery of Docetaxel to Brain Employing Piperine-Tagged PLGA-Aspartic Acid Polymeric Micelles: Improved Cytotoxic and Pharmacokinetic Profiles. AAPS Pharmscitech. 2019, 20, 220. [Google Scholar] [CrossRef]
- Raza, K.; Kumar, D.; Kiran, C.; Kumar, M.; Guru, S.K.; Kumar, P.; Arora, S.; Sharma, G.; Bhushan, S.; Katare, O.P. Conjugation of Docetaxel with Multiwalled Carbon Nanotubes and Codelivery with Piperine: Implications on Pharmacokinetic Profile and Anticancer Activity. Mol. Pharm. 2016, 13, 2423–2432. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Wang, C.; Gao, M.; Xu, Y.; Ma, X.; Wu, J.; Li, L. Soluplus(®)/TPGS mixed micelles for co-delivery of docetaxel and piperine for combination cancer therapy. Pharm. Dev. Technol. 2020, 25, 107–115. [Google Scholar] [CrossRef]
- Han, S.Z.; Liu, H.X.; Yang, L.Q.; Cui, L.D.; Xu, Y. Piperine (PP) enhanced mitomycin-C (MMC) therapy of human cervical cancer through suppressing Bcl-2 signaling pathway via inactivating STAT3/NF-κB. Biomed. Pharm. 2017, 96, 1403–1410. [Google Scholar] [CrossRef]
- Dimberg, L.Y.; Anderson, C.K.; Camidge, R.; Behbakht, K.; Thorburn, A.; Ford, H.L. On the TRAIL to successful cancer therapy? Predicting and counteracting resistance against TRAIL-based therapeutics. Oncogene 2013, 32, 1341–1350. [Google Scholar] [CrossRef] [Green Version]
- Piska, K.; Gunia-Krzyżak, A.; Koczurkiewicz, P.; Wójcik-Pszczoła, K.; Pękala, E. Piperlongumine (piplartine) as a lead compound for anticancer agents—Synthesis and properties of analogues: A mini-review. Eur. J. Med. Chem. 2018, 156, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, D.P.; Pessoa, C.; de Moraes, M.O.; Saker-Neto, N.; Silveira, E.R.; Costa-Lotufo, L.V. Overview of the therapeutic potential of piplartine (piperlongumine). Eur. J. Pharm Sci. 2013, 48, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Ma, Y.; Guo, Z.; Liu, L.; Yang, Y.; Wang, Y.; Pan, B.; Wu, L.; Hui, Y.; Yang, W. Two Natural Alkaloids Synergistically Induce Apoptosis in Breast Cancer Cells by Inhibiting STAT3 Activation. Molecules 2020, 25, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ee, G.C.; Lim, C.M.; Rahmani, M.; Shaari, K.; Bong, C.F. Pellitorine, a potential anti-cancer lead compound against HL6 and MCT-7 cell lines and microbial transformation of piperine from Piper Nigrum. Molecules 2010, 15, 2398–2404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sriwiriyajan, S.; Sukpondma, Y.; Srisawat, T.; Madla, S.; Graidist, P. (-)-Kusunokinin and piperloguminine from Piper nigrum: An alternative option to treat breast cancer. Biomed. Pharm. 2017, 92, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Rattanaburee, T.; Tipmanee, V.; Tedasen, A.; Thongpanchang, T.; Graidist, P. Inhibition of CSF1R and AKT by (±)-kusunokinin hinders breast cancer cell proliferation. Biomed. Pharm. 2020, 129, 110361. [Google Scholar] [CrossRef]
- Tedasen, A.; Dokduang, S.; Sukpondma, Y.; Lailerd, N.; Madla, S.; Sriwiriyajan, S.; Rattanaburee, T.; Tipmanee, V.; Graidist, P. (-)-Kusunokinin inhibits breast cancer in N-nitrosomethylurea-induced mammary tumor rats. Eur. J. Pharm. 2020, 173311. [Google Scholar] [CrossRef]
- Piyachaturawat, P.; Glinsukon, T.; Toskulkao, C. Acute and subacute toxicity of piperine in mice, rats and hamsters. Toxicol. Lett. 1983, 16, 351–359. [Google Scholar] [CrossRef]
- Chunlaratthanaphorn, S.; Lertprasertsuke, N.; Ngamjariyawat USATA, S.N.; Jaijoy, K. Acute and subchronic toxicity study of the water extract from dried fruits of Piper nigrum L. in rats. Health 2007, 29, 109–124. [Google Scholar]
- Sponchiado, G.; Adam, M.L.; Silva, C.D.; Soley, B.S.; de Mello-Sampayo, C.; Cabrini, D.A.; Correr, C.J.; Otuki, M.F. Quantitative genotoxicity assays for analysis of medicinal plants: A systematic review. J. Ethnopharmacol. 2016, 178, 289–296. [Google Scholar] [CrossRef]
- Karekar, V.R.; Mujumdar, A.M.; Joshi, S.S.; Dhuley, J.; Shinde, S.L.; Ghaskadbi, S. Assessment of genotoxic effect of piperine using Salmonella typhimurium and somatic and somatic and germ cells of Swiss albino mice. Arzneimittelforschung 1996, 46, 972–975. [Google Scholar] [PubMed]
- Thiel, A.; Buskens, C.; Woehrle, T.; Etheve, S.; Schoenmakers, A.; Fehr, M.; Beilstein, P. Black pepper constituent piperine: Genotoxicity studies in vitro and in vivo. Food Chem. Toxicol. 2014, 66, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Cardoso, V.; Vermelho, A.B.; Ribeiro de Lima, C.A.; Mendes de Oliveira, J.; Freire de Lima, M.E.; Pinto da Silva, L.H.; Direito, G.M.; Miranda Danelli, M.D. Antigenotoxic Effect of Piperine in Broiler Chickens Intoxicated with Aflatoxin B1. Toxins 2016, 8, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wongpa, S.; Himakoun, L.; Soontornchai, S.; Temcharoen, P. Antimutagenic effects of piperine on cyclophosphamide-induced chromosome aberrations in rat bone marrow cells. Asian Pac. J. Cancer Prev. 2007, 8, 623–627. [Google Scholar]
- Reen, R.K.; Wiebel, F.J.; Singh, J. Piperine inhibits aflatoxin B1-induced cytotoxicity and genotoxicity in V79 Chinese hamster cells genetically engineered to express rat cytochrome P4502B1. J. Ethnopharmacol. 1997, 58, 165–173. [Google Scholar] [CrossRef]
- Zarev, Y.; Naessens, T.; Theunis, M.; Elgorashi, E.; Apers, S.; Ionkova, I.; Verschaeve, L.; Pieters, L.; Hermans, N.; Foubert, K. In vitro antigenotoxic activity, in silico ADME prediction and protective effects against aflatoxin B(1) induced hepatotoxicity in rats of an Erythrina latissima stem bark extract. Food Chem. Toxicol. 2020, 135, 110768. [Google Scholar] [CrossRef]
- Selvendiran, K.; Padmavathi, R.; Magesh, V.; Sakthisekaran, D. Preliminary study on inhibition of genotoxicity by piperine in mice. Fitoterapia 2005, 76, 296–300. [Google Scholar] [CrossRef]
- Singh, J.; Reen, R.K.; Wiebel, F.J. Piperine, a major ingredient of black and long peppers, protects against AFB1-induced cytotoxicity and micronuclei formation in H4IIEC3 rat hepatoma cells. Cancer Lett. 1994, 86, 195–200. [Google Scholar] [CrossRef]
- Abo-Zeid, M.; Farghaly, A.A. The anti-mutagenic activity of piperine against mitomycine C induced sister chromatid exchanges and chromosomal aberrations in mice. Nat. Sci. 2009, 7, 72–78. [Google Scholar]
- Daware, M.B.; Mujumdar, A.M.; Ghaskadbi, S. Reproductive toxicity of piperine in Swiss albino mice. Planta Med. 2000, 66, 231–236. [Google Scholar] [CrossRef]
- Malini, T.; Manimaran, R.; Arunakaran, J.; Aruldhas, M.; Govindarajulu, P. Effects of piperine on testis of albino rats. J. Ethnopharmacol. 1999, 64, 219–225. [Google Scholar] [CrossRef]
- D’Cruz, S.C.; Vaithinathan, S.; Saradha, B.; Mathur, P.P. Piperine activates testicular apoptosis in adult rats. J. Biochem. Mol. Toxicol. 2008, 22, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Gagini, T.B.; Silva, R.E.; Castro, I.S.; Soares, B.A.; Lima, M.E.; Brito, M.F.; Mazur, C.; Direito, G.M.; Danelli, M. Oral administration of piperine for the control of aflatoxin intoxication in rats. Braz. J. Microbiol. 2010, 41, 345–348. [Google Scholar] [CrossRef] [Green Version]
- Dogra, R.K.; Khanna, S.; Shanker, R. Immunotoxicological effects of piperine in mice. Toxicology 2004, 196, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Pathak, N.; Khandelwal, S. Immunomodulatory role of piperine in cadmium induced thymic atrophy and splenomegaly in mice. Environ. Toxicol. Pharm. 2009, 28, 52–60. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on Flavouring Group Evaluation 86, Revision 2 (FGE.86Rev2): Consideration of aliphatic and arylalkyl amines and amides evaluated by JECFA (65th meeting). EFSA J. 2015, 13, 3998. [Google Scholar] [CrossRef] [Green Version]
- Helma, C. Lazy structure-activity relationships (lazar) for the prediction of rodent carcinogenicity and Salmonella mutagenicity. Mol. Divers. 2006, 10, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Cramer, G.M.; Ford, R.A.; Hall, R.L. Estimation of toxic hazard--a decision tree approach. Food Cosmet. Toxicol. 1978, 16, 255–276. [Google Scholar] [CrossRef]
- Norvegian Scietific Committee for Food Safety (VKM). Opinion of the Panel Food Additives, Flavourings, Processing Aids, Materials in Contact with Food and Cosmetics of the Norwegian Scientific Committee for Food Safety—Risk assessment of other substances—Piperine; VKM Report; Norvegian Scietific Committee for Food Safety (VKM): Oslo, Norway, 2016; Volume 31.
- Wang, Y.M.; Lin, W.; Chai, S.C.; Wu, J.; Ong, S.S.; Schuetz, E.G.; Chen, T. Piperine activates human pregnane X receptor to induce the expression of cytochrome P450 3A4 and multidrug resistance protein 1. Toxicol. Appl. Pharm. 2013, 272, 96–107. [Google Scholar] [CrossRef] [Green Version]
- Chinta, G.; B Syed, S.; Coumar, M.S.; Periyasamy, L. Piperine: A comprehensive review of pre-clinical and clinical investigations. Curr. Bioact. Compd. 2015, 11, 156–169. [Google Scholar] [CrossRef]
| Piper nigrum Extracts | Experimental Model | IC50 a or EC50 b (Time of Treatment) | Anticancer Effects and Molecular Targets | Reference |
|---|---|---|---|---|
| Seeds’ ethanolic extract (50% ethanol) | Colorectal cancer cells (HCT-116, HCT-15, HT-29) | IC50: HCT-116: 4.0 (24 h) 3.1 (48 h) 3.4 (72 h) μg/mL HCT-15: 3.2 (24 h) 2.9 (48 h) 1.9 (72 h) μg/mL HT-29: 7.9 (24 h) 6.1 (48 h) 7.4 (72 h) μg/mL | ↑ tumor cell death | [13] |
| Seeds’ extract, SnO2 nanoparticles | Colorectal cancer cells (HCT-116) and lung cancer cells (A549) | IC50: HCT-116: 165 µM A549: 135 µM | ↑ ROS c | [14] |
| Fruits’ ethanolic extract | Vitro: Breast cancer cells (MCF-7) and colon cancer cells (HT-29) (1–1000 µg/mL) Vivo: Ehrlich ascites carcinoma-bearing male Balb/c mice (intraperitoneal injection (i.p.), 100 mg/kg/day in saline containing 1% Tween 80, for 9 days) | EC50: MCF-7: 27.1 µg/mL (24 h) HT-29: 80.5 µg/mL (24 h) | Vitro: ↑ tumor cell death ↓ tumor cell proliferation ↑ ROS, ↑ DNA damage Vivo: ↓ tumor growth, ↑ mice survival ↑ apoptosis cell-cycle arrest at G1/S (↑ Bax, p53; ↓Bcl-xL, cyclin A) ↑ oxidative stress (↑ lipid peroxidation, protein carbonylation, GR d, SOD e, CAT f) | [15] |
| Supercritical fluid extract (SFE) of fruits’ ethanolic extract | Vitro: Breast cancer cells (MCF-7) (1–1000 µg/mL) Vivo: Ehrlich ascites carcinoma-bearing male Balb/c mice (i.p., 10 or 100 mg/kg/day in saline containing 1% Tween 80, for 9 days) | EC50: 14.40 μg/mL (72 h) IC50: 27.8 μg/mL (24 h) | Vitro: ↑ apoptosis Silico (docking study): Piperine interaction with CDK2 g, ATP binding site; cyclin A binding site and Bcl-xL binding site. Vivo: ↓ tumor growth, ↑ mice survival ↑ apoptosis cell-cycle arrest at G2/M (↑ Bax, p53; ↓Bcl-xL, ↓cyclin A, ↓CDK2) | [16,17] |
| Fruits’ (i) methanol crude extract or (ii) dichloromethane crude extract | Breast cancer cells (MCF-7, MDA-MB-231, MDA-MB-468) | IC50 (72 h) methanol crude extract: MCF-7: 20.25 µg/mL MDA-MB-231: 22.37 µg/mL MDA-MB-468: 9.04 µg/mL IC50 (72 h) dichloromethane crude extract: MCF-7: 23.46 µg/mL MDA-MB-231: 38.82 µg/mL MDA-MB-468: 7.94 µg/mL | ↑ tumor cell death | [18] |
| Piperine-free Piper nigrum fruits’ extract (PFPE) | Vitro: Breast cancer cells (MCF-7, MDA-MB-231, MDA-MB-468, ZR-75-1), colorectal cancer cells (HT-29, SW-620), lung cancer cells (H358, A549), neuroblastoma cells (LA-N-5, SK-N-SH). Vivo: Female ICR mice (oral administration (os) 5000 mg/kg b.w. in mixture of distilled water and Tween-80 (4:1 v/v) for acute oral toxicity studies) or NMU-treated female Sprague-Dawley treated orally with (i) 100 or 200 mg/kg b.w. in mixture of distilled water and Tween-80 (4:1 v/v) at 14 days after NMU application three times per week up to 76 days, or (ii) 100, 200, or 400 mg/kg b.w. PFPE after the first NMU-induced tumor every two days up to 30 days | IC50 (72 h): MCF-7: 7.45 µg/mL MDA-MB-231: 22.67 µg/mL MDA-MB-468: 18.19 µg/mL ZR-75-1: 13.85 µg/mLHT-29: 27.74 µg/mL SW-620: 29.56 µg/mL H358: 34.69 µg/mLA549: 30.77 µg/mL LA-N-5: 111.28 µg/mL SK-N-SH: 21.51 µg/mL | Vitro: ↓ cell proliferation ↑ apoptosis (↑ p53 and cytochrome c; ↓ topoisomerase II) Vivo: ↓ tumor bearing rats ↓ tumor size, ↑ cytochrome c in tumor tissues | [19] |
| Piperine-free Piper nigrum fruits’ extract | Vitro: Breast cancer cells (MCF-7) Vivo: NMU-treated female Sprague-Dawley rats. PFPE treatment regimen as previously described above | Vitro: ↓ E-cadherin, c-myc, VEGF h Vivo: ↑ p53 ↓ E-cadherin, MMP i-9, MMP-2, c-myc, and VEGF | [20] | |
| Root dried power crude (i) petroleum ether extract, (ii) chloroform extract, (iii) ethylacetate extract | Promyeolocytic leukemia cells (HL60) | IC50: petroleum ether extract (72 h): 11.2 µg/mL chloroform extract (72 h): 9.8 µg/mL ethylacetate extract (72 h): / | ↑ tumor cell death | [21] |
| Cancer Type | Experimental Models | Piperine | IC50 a | Anticancer Effects and Molecular Targets | Reference |
|---|---|---|---|---|---|
| Breast cancer | Vitro: 4T1 mouse mammary carcinoma cells Vivo: Female BALB/c mice syngeneic to 4T1 cells (4T1 cells transplanted subcutaneously) | Vitro: 35–280 µM Vivo: Intratumoral injection of 2.5 or 5 mg/kg every 3 days 3 times | 105 ± 1.08 µM (48 h) 78.52 ± 1.06 µM (72 h) | Vitro: ↑ apoptosis (↑ caspase-3 activity) ↓proliferation (↓ cyclin B1, cell-cycle block in G2/M phase) ↓ migration; ↓ MMP b-9 and MMP-13 Vivo: ↓ tumor growth ↓ lung metastasis | [25] |
| HER-overexpressing cells: SKBR3 and BT-474 Basal HER-expressing cells: MCF-7 and MDA-MB-231 | 10–200 µM | SKBR3 50 µM (48 h) MCF-7 > 200 µM (48 h) | ↑ apoptosis (↑ caspase-3 activity, cleaved-PARP c, DNA damage) ↓ HER2 d expression ↓ SREBP-1 e and fatty acid synthase via ERK1/2 f inhibition ↓ MMP-9 via inhibition of Akt and MAPK g signaling | [26] | |
| Vitro: MDA-MB-231, MDA-MB-468, murine 4T1 Vivo: BALB/c female mice orthotopically-inoculated 4T1 | Vitro: 25–200 µM Vivo: Oral administration (os) 50 mg/kg/day from day 7 to 21 | Vitro: ↓ proliferation (cell-cycle block in G2/M phase) ↓ survivin and p65 phosphorylation Vivo: ↓ tumor growth | [27] | ||
| MDA-MB-231, MDA-MB-468, T-47D, and MCF-7 | 50–150 µM | ↑ apoptosis (↑ Smac/DIABLO h, cytochrome c; ↓ IAPs i) ↓ cell-cycle progression (↑ p21; ↓ CDK j4, CDK1, cyclin D3, cyclin B, E2F1 k, CDC25 C l) ↓ mammospheres’ growth ↓ MMP-2, MMP-9 | [28] | ||
| Vitro: Mouse mammary EMT6/P cancer cells Vivo: Balb/C female mice with EMT6/P cells injected subcutaneously in the abdominal area | Vitro: 50–1200 µM Vivo: Intraperitoneal injection (i.p.) 25 mg/kg/day in PBS for 14 days | 870 µM (48h) | Vitro: ↑ apoptosis (↑ caspase-3 activity) ↓ VEGF m Vivo: ↓ tumor size ↑ apoptosis in tumor tissue ↓ ALT n, AST o, creatinine | [29] | |
| MCF-7, T-47D | 3–100 µM | MCF-7 37.34 µM (24 h) T-47D 61.05 µM (24 h) | ↑ apoptosis (↑ Bax, ↓ Bcl-2) ↓ proliferation (cell-cycle block in G2/M phase) | [30] | |
| MDA-MB-231 | 20–320 µM | 238 µM (72 h) | ↓ proliferation | [31] | |
| Prostate cancer | DU145, LNCaP, and PC3 | 20–320 µM | LNCaP 74.4 µM (24 h) DU145 226.6 µM (24 h) PC3 111.0 µM (24 h) | ↓ proliferation (cell-cycle block in G0/G1 phase, ↓cyclin D1 and cyclin A; ↑ p21 and p27) ↑ autophagy (↑ LC3B p-II and LC3B puncta formation) | [32] |
| Vitro: DU145, LNCaP, 22RV1, and PC3 Vivo: Nude mice (LNCaP or DU145 transplanted subcutaneously) | Vitro: 50–200 µM Vivo: I.p., 100 mg/kg/day in vegetable oil for 1 month os 10 mg/kg body weight (b.w.) for 1 month | LCNaP 60 µM (24 h) PC3 75 µM (24 h) 22Rv1 110 µM (24 h) DU145 160 µM (24 h) | Vitro: ↑ apoptosis (↑ caspase-3 activity and cleaved-PARP) ↓ migration (↓ STAT-3 q and NF-kB r) Vivo: ↓ tumor growth | [33] | |
| LNCaP and PC3 | 5–150 µM | LNCaP 39.91 µM (24 h) PC3 49.45 µM (24 h) | ↑ apoptosis ↓ proliferation (cell-cycle block in G0/G1) via voltage-gated K+ current blockade | [34] | |
| LNCaP ad PC3 | 0.1–100 µM | LNCaP 39.91 µM (24 h) PC3 49.45 µM (24 h) | ↑ apoptosis ↓ proliferation (cell-cycle block in G1 phase) via voltage-gated K+ current inhibition | [35] | |
| DU145 | 80–320 µM | ↑ apoptosis (↑ Bax, ↓ Bcl-2) ↓ proliferation ↓ migration (↓ MMP-9 via inhibition of Akt/mTOR signaling) | [36] | ||
| Colon cancer | DLD1 | 1–200 µM | ↓ proliferation | [37] | |
| HT-29, Caco-2, SW480, HCT-116 (p53+/+), and HCT-116 (p53−/−) | 10–150 µM | HT-29 53 ± 1 µM (72 h) Caco-2 54 ± 5 µM (72 h) SW480 126 ± 3 µM (72 h) HCT-116 (p53+/+) 109 ± 9 µM (72 h) HCT-116 (p53−/−) 118 ± 7 µM (72 h) | ↑ apoptosis (↑ loss of mitochondrial membrane potential, caspase activity, cleaved-PARP) ↑ ROS s ↑ endoplasmic reticulum stress (↑ IRE1α t, CHOP u, BiP v) ↓ survivin ↓ proliferation (cell-cycle block in G1 phase; ↓ cyclin D1 and cyclin D3, CDK4 and CDK6; ↑ p21 and p27) ↓ colony formation and spheroids’ growth | [38] | |
| HCT6, SW480, and DLD1 | 20–200 µM | ↓ proliferation ↓ migration ↓ Wnt/β-catenin and GSK3β w | [39] | ||
| SW480 and HCT-116 | 25–800 µM | ↓ migration and EMT x (↓ STAT-3/Snail, ↓ vimentin, ↑ E-cadherin) | [40] | ||
| Rectal cancer | HRT-18 | 10–150 µM | ↑ apoptosis ↓ proliferation (block cell-cycle progression) ↑ ROS | [41] | |
| Lung cancer | Vivo: C57BL/6 Mice lung metastasis from melanoma cells (B16F-10 lateral tail vein injection) | I.p., 200 μmol/kg b.w. in 0.1% gum acacia for 10 days | ↑ animal survival ↓ metastatic lung fibrosis, ↓ uronic acid and hexosamine in lung tissue ↓ serum level of sialic acid and GGT y | [42] | |
| Vivo: Swiss Albino mice benzo(a)pyrene induced lung cancer (os in corn oil 50 mg/kg b.w.) | Os 50 mg/kg b.w. in corn oil: (i) On alternate days for 16 weeks immediate after the first dose of carcinogen; (ii) piperine as (i), but starting from the sixth week of B(a)P till the end of the experiment | ↓ lipid peroxidation, protein carbonyls, nucleic acid content, and polyamine synthesis in lung | [43] | ||
| Vivo: Swiss Albino mice benzo(a)pyrene induced lung cancer (os in corn oil 50 mg/kg b.w.) | Os 50 mg/kg b.w. in corn oil for 16 weeks. Treatment: (i) Immediately after the first dose of benzo(a)pyrene; (ii) after the last dose of benzo(a)pyrene | ↓ hexose, hexosamine and sialic acid in serum, liver, and lung tissues | [44] | ||
| A549 | 25–400 µM | 122 µM (48 h) | ↑ apoptosis (↑ caspase3 and -9 activity, Bax/Bcl-2 ratio, p53 expression) ↓ Proliferation (cell-cycle block in G2/M phase) | [45] | |
| A549 | 100–500 µM | ↑ apoptosis (↓ c-myc) | [46] | ||
| A549 | 20–320 µM | 198 µM (72 h) | ↓ EMT (↓ fibronectin and N-caderin, ↑ E-cadherin) ↓ ERK 1/2 and SMAD z 2 ↓ migration (↓ MMP-2) | [31] | |
| Melanoma | SK MEL 28, A375 (human cells), and B16 F0 (murine cells) | 75–300 µM | SK MEL 28 221 µM (24 h) 172 µM (48 h) 136 µM (72 h) B16 F0 200 µM (24 h) 155 µM (48 h) 137 µM (72 h) A375 225 µM (24 h) 160 µM (48 h) 100 µM (72 h) | ↑ apoptosis (↑ p53; ↓ XIAP aa, Bid ab; ↑ Caspase-3 and cleaved-PARP) ↓ proliferation (cell-cycle block in G1 phase; ↓ cyclin D, E2F1, and Rb ac phosphorylation; ↑ p21, ATR ad, Chk ae 1) ↑ ROS ↑ DNA damage (↑ H2AX af phosphorylation) | [47] |
| Vitro: A375SM (highly metastatic), A375P (moderately metastatic) Vivo: BALB/c nude mice (nu/nu) (A375SM or A375P transplanted subcutaneously) | Vitro: 50–200 µM Vivo: Os 50 or 100 mg/kg b.w. in water 5 times per week for 4 weeks | Vitro: ↑ apoptosis (↑ Bax, cleaved-PARP, caspase-9, ↓ Bcl2) ↑ JNK/p38 MAPK phosphorylation, ↓ ERK1/2 Vivo: ↓ tumor growth ↑ apoptosis (↑ caspase-3) ↓ ERK1/2 | [48] | ||
| Hepatocellular cancer | Vitro: HepG2 Vivo: Male Wistar rats tumor induced using diethylnitrosamine (DEN, 0.01% of DEN in drinking water for 16 weeks) | Vitro: 5–100 µM Vivo: Os 5 mg/kg b.w. in corn oil for 6 weeks starting from the 10th week of the experimental period | 75 µM (24 h) 30 µM (48 h) | ↑ apoptosis (↑ cleaved caspase-3 and caspase-9, mitochondrial permeabilization, Bax, cytochrome c release, ↓ Bcl-2) ↓ proliferation ↑ ROS (↓ catalase) ↓ ERK1/2 and SMAD Vivo: ↓ AST, ALP ag, and ALT ↑ improvement in liver architecture ↓ Ki67 | [49] |
| HepG2 | 20–320 µM | 214 µM (72 h) | ↓ proliferation | [31] | |
| Ovarian | A2780 | 4–20 µM | ↑ apoptosis (↑ cytochrome c release, caspase-3 and caspase-9 activity, cleaved-PARP) ↑ JNK and p38 MAPK phosphorylation | [50] | |
| OVACAR-3 (ovarian cisplatin-resistant cells) | 3.12–200 µM | 28 µM (24 h) | ↑ apoptosis (↑ caspase-3, caspase-9, and Bax) Cell-cycle block in G2/M phase ↓ migration ↓ MAPK signaling (PI3 K ah/Akt/GSK3β) | [51] | |
| Osteosarcoma | HOS and U2OS | 25–200 µM | HOS 72 µM (72 h) H2OS 126 µM (72 h) | ↓ proliferation (cell-cycle block in G2/M phase, ↓ cyclin B1, ↑ CDK1, Chk2) ↓ Akt, ↑ c-JNK/p38 MAPK phosphorylation ↓ migration (↓ MMP-2 and MMP-9; ↑ TIMP1/2 ai) | [52] |
| U2OS and 143B | 50–150 µM | ↓ cell proliferation ↑ apoptosis ↓ invasion and angiogenesis (↓ MMP-2 and VEGF) ↓ Wnt/β-catenin and GSK3β (↓ cyclin D1, c-Myc, and COX-2 aj) | [53] | ||
| Fibrosarcoma | HT-1080 | ↓ MMP-9 | [54] | ||
| Oral squamous carcinoma | KB | 25–300 µM | 124 µM (24 h) | ↑ apoptosis (↑ caspase-3 activity, loss mitochondrial potential) ↑ ROS ↓ proliferation (cell-cycle arrest in G2/M phase) | [55] |
| Cervical adenocarcinoma | HeLa | 10–200 µM | ↑ apoptosis (↑ caspase-3 activity, loss mitochondrial potential) ↑ ROS ↑ DNA damage ↓ proliferation (cell-cycle arrest in G2/M phase) | [56] | |
| Leukemia | HL60 | 10–200 µM | 25 µM (24 h) | ↑ apoptosis (↑ Bax, ↓ Bcl-2) ↑ autophagy ↓ cell proliferation (cell-cycle arrest in S phase) ↓ migration | [57] |
| Experimental Model | Treatment Conditions | Selectivity (Compared to Cancer Cell Lines) | Reference |
|---|---|---|---|
| Murine fibroblasts (NIH3T3) | IC50 a 232 ± 1.15 µM (48 h) | + (4T1) | [25] |
| Human normal prostate epithelial cells (RWPE-1) | <160 µM (48 h) | + (LNCaP, DU145, PC3) | [32] |
| Human lung fibroblasts (WI38) | 25–400 µM (48 h) | ++ (A549) | [45] |
| Human osteoblasts (hFOB) | 25–200 µM (72 h) | + (HOS, U2OS) | [52] |
| Fibroblasts and human mammary epithelial cells | 50–150 µM (72 h) | ++ [Caco-2, SW480, HCT-116 (p53+/+), HCT-116 (p53−/−)] | [38] |
| Human mammary epithelial cells | 50–150 µM (72 h) | + (MDA-MB-231, MCF-7, T-47D, MDA-MB-468) | [28] |
| Primary monolayer cultures of adult rat hepatocytes | Up to 100 µM (48 h) | ++ (HepG2) | [49] |
| Human normal ovarian cells (OSE) | 0–20 µM (72 h) | + (A2780) | [50] |
| Human intestinal cells (IEC-6) | 20–200 µM (24, 48, 72 h) | ++ (HCT-116, SW480, DLD1) | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turrini, E.; Sestili, P.; Fimognari, C. Overview of the Anticancer Potential of the “King of Spices” Piper nigrum and Its Main Constituent Piperine. Toxins 2020, 12, 747. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12120747
Turrini E, Sestili P, Fimognari C. Overview of the Anticancer Potential of the “King of Spices” Piper nigrum and Its Main Constituent Piperine. Toxins. 2020; 12(12):747. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12120747
Chicago/Turabian StyleTurrini, Eleonora, Piero Sestili, and Carmela Fimognari. 2020. "Overview of the Anticancer Potential of the “King of Spices” Piper nigrum and Its Main Constituent Piperine" Toxins 12, no. 12: 747. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12120747