Bioactive Components of Pomegranate Oil and Their Influence on Mycotoxin Secretion
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Phenolic and Flavonoid Content
2.2. Tocochromanols Composition (Tocols)
2.3. Phytosterols
2.4. Carotenoids
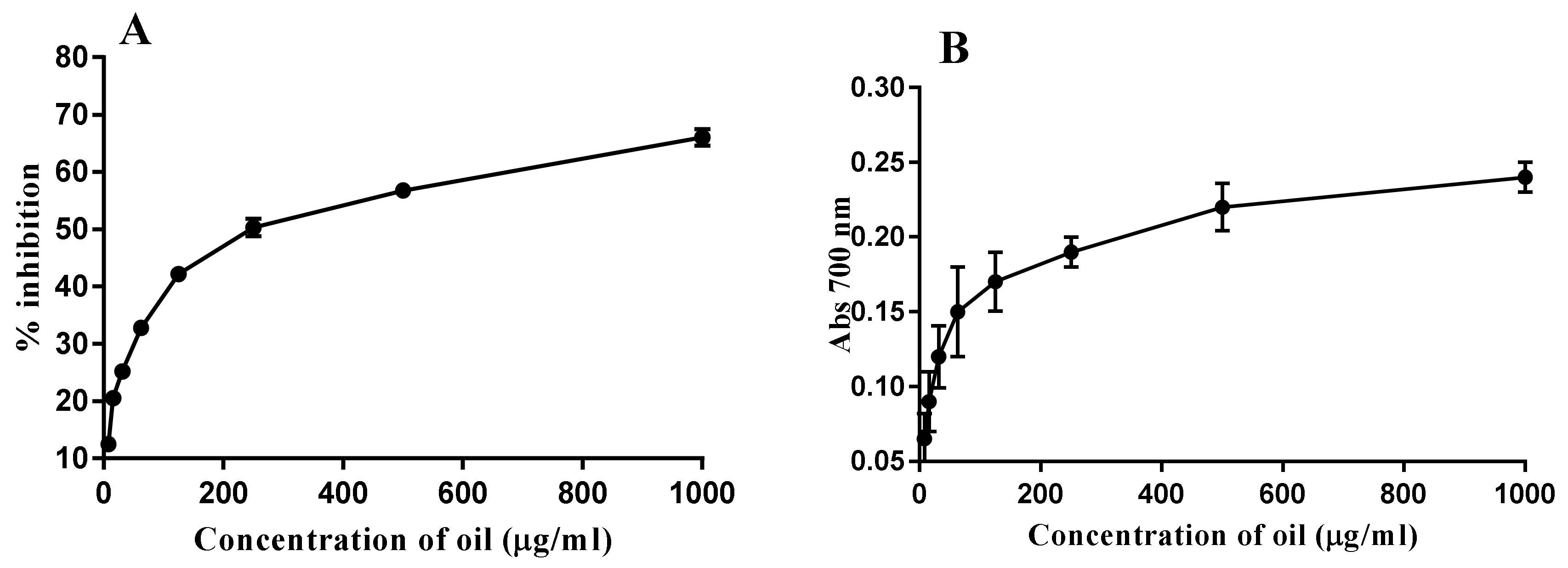
2.5. Antioxidant Activity
2.6. Phenolic Acid Composition
2.7. Fatty Acid Composition
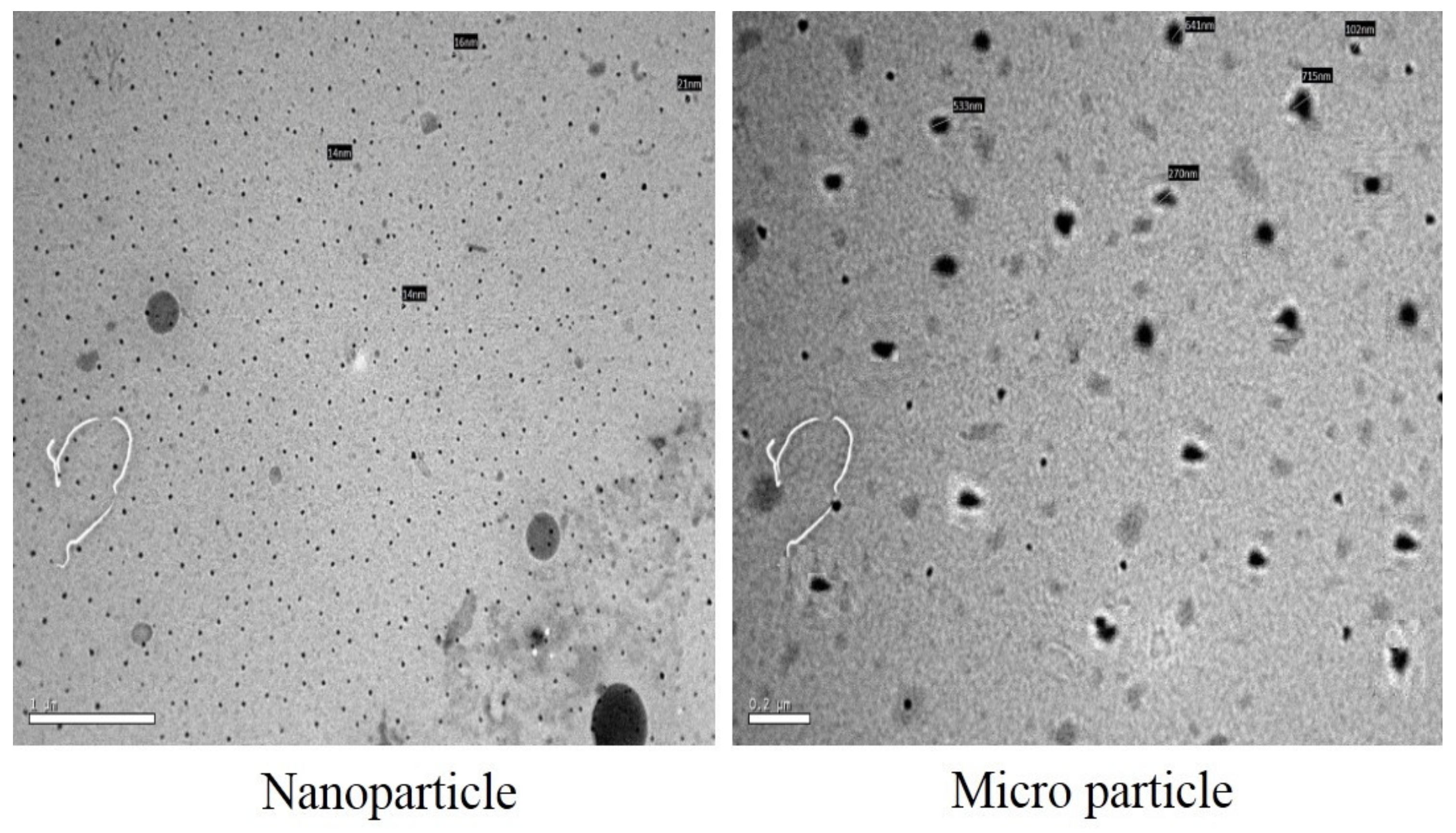
2.8. Encapsulation of the PGO
2.9. Antimicrobial Activity of the PGO Types
2.10. Mycotoxin Reducing of The PGO
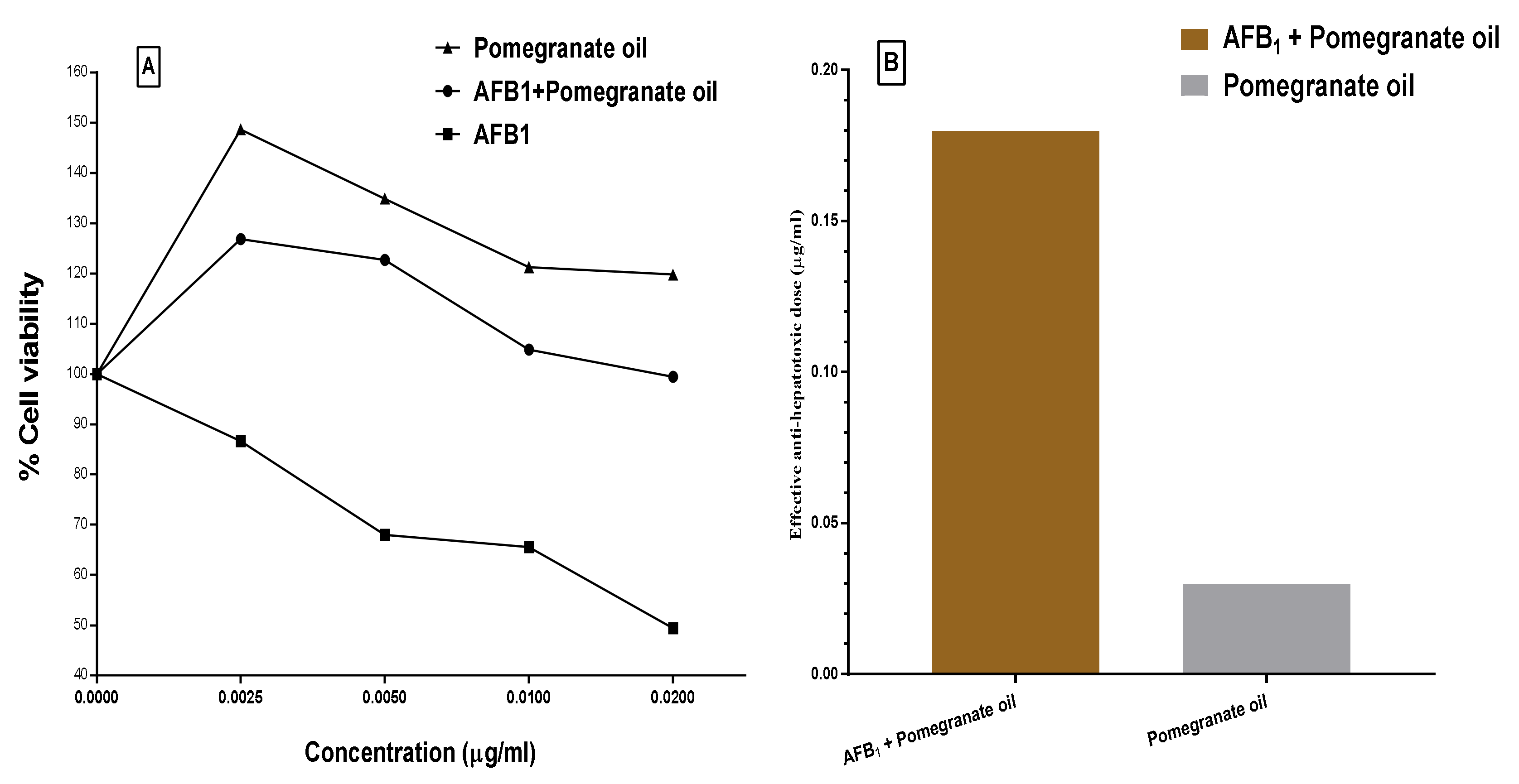
2.11. Cytotoxicity and Protection Effect of the PGO
3. Conclusions
4. Materials and Methods
4.1. Source and Extraction of Pomegranate Seed Oil
4.2. Determination of Total Phenolic Compounds (TPC)
4.3. Determination of Tocopherols and Tocotrienols Content
4.4. Determination of Sterols and Carotenoid Content
4.5. Antioxidant Activity Assays
4.5.1. Scavenging Activity of DPPH Radicals
4.5.2. Determination of Reducing Power
4.6. Determination of Phenolic Fractions
4.7. Determination of Fatty Acid Profile
4.8. The PGO Encapsulation
4.9. Determination of Antimicrobial and Antifungal Activity
4.10. Estimation of Antibacterial and Antifungal Activity
4.11. The PGO Impact on Mycotoxin Excretion in Liquid Media
4.12. Mycotoxin Determination
4.13. Detecting the PGO Prevention against The AFB1 Impact
- Ae: absorbance of exposed cells.
- Au: absorbance of unexposed control cells.
4.14. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Basu, A.; Penugonda, K. Pomegranate juice: A heart-healthy fruit juice. Nutr. Rev. 2009, 67, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Surek, E.; Nilufer-Erdil, D. Phenolic contents, antioxidant activities and potential bioaccessibilities of industrial pomegranate nectar processing wastes. Int. J. Food Sci. Technol. 2015, 51, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Hasnaoui, N.; Wathelet, B.; Jimenez-Araujo, A. Valorization of pomegranate peel from 12 cultivars: Dietary fibre composition, antioxidant capacity and functional properties. Food Chem. 2014, 160, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, S.; Sithul, H.; Muraleedharan, P.; Azeez, J.M.; Sreeharshan, S. Pomegranate Fruit as a Rich Source of Biologically Active Compounds. BioMed Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Numonov, S.; Qureshi, M.N.; Aisa, H.A. Development of HPLC Protocol and Simultaneous Quantification of Four Free Flavonoids from Dracocephalum heterophyllum Benth. Int. J. Anal. Chem. 2015, 2015, 1–5. [Google Scholar] [CrossRef] [Green Version]
- EEmri, T.; Szarvas, V.; Orosz, E.; Antal, K.; Park, H.; Han, K.-H.; Yu, J.-H.; Pócsi, I. Core oxidative stress response in Aspergillus nidulans. BMC Genom. 2015, 16, 478. [Google Scholar] [CrossRef] [Green Version]
- Türkyılmaz, M.; Tağı, Ş; Dereli, U.; Özkan, M. Effects of various pressing programs and yields on the antioxidant activity, antimicrobial activity, phenolic content and colour of pomegranate juices. Food Chem. 2013, 138, 1810–1818. [Google Scholar] [CrossRef]
- Topuz, O.K.; Yerlikaya, P.; Uçak, I.; Gümüş, B.; Buyukbenli, H.A.; Yerlıkaya, P. Effects of olive oil and olive oil–pomegranate juice sauces on chemical, oxidative and sensorial quality of marinated anchovy. Food Chem. 2014, 154, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Šonje, M.B.; Giacometti, J.; Abram, M. Antioxidant and antilisterial activity of olive oil, cocoa and rosemary extract polyphenols. Food Chem. 2011, 127, 1821–1827. [Google Scholar] [CrossRef]
- Beveridge, T.H.J.; Girard, B.; Kopp, A.T.; Drover, J.C.G. Yield and Composition of Grape Seed Oils Extracted by Supercritical Carbon Dioxide and Petroleum Ether: Varietal Effects. J. Agric. Food Chem. 2005, 53, 1799–1804. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Appelqvist, L.-Å. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef]
- Habibnia, M.; Ghavami, M.; Ansaripour, M.; Vosough, S. Chemical Evaluation of oils extracted from five different varieties of Iranian pomegranate seeds. J. Food Biosci. Technol. 2012, 2, 35–40. [Google Scholar]
- Pande, G.; Akoh, C.C. Antioxidant Capacity and Lipid Characterization of Six Georgia-Grown Pomegranate Cultivars. J. Agric. Food Chem. 2009, 57, 9427–9436. [Google Scholar] [CrossRef]
- Martins, C.M.; Fonseca, F.A.; Ballus, C.A.; Figueiredo-Neto, A.M.; Meinhart, A.D.; De Godoy, H.T.; Izar, M.C. Common sources and composition of phytosterols and their estimated intake by the population in the city of São Paulo, Brazil. Nutrition 2013, 29, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Lagarda, M.; Llatas, G.G.; Farré, R. Analysis of phytosterols in foods. J. Pharm. Biomed. Anal. 2006, 41, 1486–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshime, L.T.; De Melo, I.L.P.; Sattler, J.A.G.; Torres, R.P.; Mancini-Filho, J. Bioactive compounds and the antioxidant capacities of seed oils from pomegranate (Punica granatum L.) and bitter gourd (Momordica charantia L.). Food Sci. Technol. 2019, 39, 571–580. [Google Scholar] [CrossRef] [Green Version]
- Chirinos, R.; Zuloeta, G.; Pedreschi, R.; Mignolet, E.; Larondelle, Y.; Campos, D. Sacha inchi (Plukenetia volubilis): A seed source of polyunsaturated fatty acids, tocopherols, phytosterols, phenolic compounds and antioxidant capacity. Food Chem. 2013, 141, 1732–1739. [Google Scholar] [CrossRef]
- Koksal, E.; Bursal, E.; Dikici, E.; Tozoglu, F.; Gulcin, I. Antioxidant activity of Melissa officinalis leaves. J. Med. Plants Res. 2011, 5, 217–222. [Google Scholar]
- Kiralan, M.; Gölükcü, M.; Tokgöz, H. Oil and Conjugated Linolenic Acid Contents of Seeds from Important Pomegranate Cultivars (Punica granatum L.) Grown in Turkey. J. Am. Oil Chem. Soc. 2009, 86, 985–990. [Google Scholar] [CrossRef]
- Özgül-Yücel, S. Determination of conjugated linolenic acid content of selected oil seeds grown in Turkey. J. Am. Oil Chem. Soc. 2005, 82, 893–897. [Google Scholar] [CrossRef]
- Parry, J.; Hao, Z.; Luther, M.; Su, L.; Zhou, K.; Yu, L. Characterization of cold-pressed onion, parsley, cardamom, mullein, roasted pumpkin, and milk thistle seed oils. J. Am. Oil Chem. Soc. 2006, 83, 847–854. [Google Scholar] [CrossRef]
- Parry, J.; Su, L.; Luther, M.; Zhou, K.; Yurawecz, M.P.; Whittaker, A.P.; Yu, L. Fatty Acid Composition and Antioxidant Properties of Cold-Pressed Marionberry, Boysenberry, Red Raspberry, and Blueberry Seed Oils. J. Agric. Food Chem. 2005, 53, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.; Godfrey, A.K.; Hughes, J.F.; Goodheart, M.L.; Mitchell, R.N.; Page, D.C. Conservation, acquisition, and functional impact of sex-biased gene expression in mammals. Science 2019, 365, eaaw7317. [Google Scholar] [CrossRef]
- Chibane, L.B.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2018, 99, 1457–1474. [Google Scholar] [CrossRef] [Green Version]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Fangmeier, M.; Lehn, D.N.; Maciel, M.J.; De Souza, C.F.V. Encapsulation of Bioactive Ingredients by Extrusion with Vibrating Technology: Advantages and Challenges. Food Bioprocess Technol. 2019, 12, 1472–1486. [Google Scholar] [CrossRef]
- Abdel-Razek, A.G.; Badr, A.N.; El-Messery, T.M.; El-Said, M.M.; Hussein, A.M.S. Micro-nano encapsulation of black seed oil ameliorate its characteristics and its mycotoxin inhibition. Biosci. Res. 2018, 15, 2591–2601. [Google Scholar]
- Badr, A.N.; Shehata, M.G.; Abdel-Raze, A.G. Antioxidant Activities and Potential Impacts to Reduce Aflatoxins Utilizing Jojoba and Jatropha Oils and Extracts. Int. J. Pharmacol. 2017, 13, 1103–1114. [Google Scholar] [CrossRef]
- Shahat, M.S.; Badr, A.N.; Hegaziy, A.I.; Ramzy, S.; Samie, M.A. Reducing the Histopathological and Biochemical Toxicity of Aflatoxins Contaminated Soybean Using Ozone Treatment. Annu. Res. Rev. Biol. 2017, 15, 1–10. [Google Scholar] [CrossRef]
- Yu, J. Genetics and biochemistry of mycotoxin synthesis. J. Fungal Biotechnol. Agric. Food Environ. Appl. 2003, 21, 343–361. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Kong, W.; Zhao, G.; Yang, M. Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chem. 2017, 220, 1–8. [Google Scholar] [CrossRef]
- Riihinen, K.; Jaakola, L.; Kärenlampi, S.; Hohtola, A. Organ-specific distribution of phenolic compounds in bilberry (Vaccinium myrtillus) and ‘northblue’ blueberry (Vaccinium corymbosum x V. angustifolium). Food Chem. 2008, 110, 156–160. [Google Scholar] [CrossRef]
- Kim, J.H.; Yu, J.; Mahoney, N.; Chan, K.L.; Molyneux, R.J.; Varga, J.; Bhatnagar, D.; Cleveland, T.E.; Nierman, W.C.; Campbell, B.C. Elucidation of the functional genomics of antioxidant-based inhibition of aflatoxin biosynthesis. Int. J. Food Microbiol. 2008, 122, 49–60. [Google Scholar] [CrossRef]
- Vamvakas, S.-S.; Chroni, M.; Genneos, F.; Gizeli, S. Vaccinium myrtillus L. dry leaf aqueous extracts suppress aflatoxins biosynthesis by Aspergillus flavus. Food Biosci. 2020, 100790. [Google Scholar] [CrossRef]
- Reverberi, M.; Zjalic, S.; Ricelli, A.; Fabbri, A.A.; Fanelli, C. Oxidant/antioxidant balance in Aspergillus parasiticus affects aflatoxin biosynthesis. Mycotoxin Res. 2006, 22, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Reverberi, M.; Ricelli, A.; Zjalic, S.; Fabbri, A.A.; Fanelli, C. Natural functions of mycotoxins and control of their biosynthesis in fungi. Appl. Microbiol. Biotechnol. 2010, 87, 899–911. [Google Scholar] [CrossRef]
- Kim, J.H.; Campbell, B.C.; Yu, J.; Mahoney, N.; Chan, K.L.; Molyneux, R.J.; Bhatnagar, D.; Cleveland, T.E. Examination of fungal stress response genes using Saccharomyces cerevisiae as a model system: Targeting genes affecting aflatoxin biosynthesis by Aspergillus flavus Link. Appl. Microbiol. Biotechnol. 2004, 67, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Georgianna, D.R.; Payne, G.A. Genetic regulation of aflatoxin biosynthesis: From gene to genome. Fungal Genet. Biol. 2009, 46, 113–125. [Google Scholar] [CrossRef]
- Loi, M.; Paciolla, C.; Logrieco, A.F.; Mulè, G. Plant Bioactive Compounds in Pre- and Postharvest Management for Aflatoxins Reduction. Front. Microbiol. 2020, 11, 243. [Google Scholar] [CrossRef]
- Loi, M.; Fanelli, F.; Liuzzi, V.C.; Logrieco, A.F.; Mulè, G. Mycotoxin Biotransformation by Native and Commercial Enzymes: Present and Future Perspectives. Toxins 2017, 9, 111. [Google Scholar] [CrossRef]
- Lyagin, I.; Efremenko, E. Enzymes for Detoxification of Various Mycotoxins: Origins and Mechanisms of Catalytic Action. Molecules 2019, 24, 2362. [Google Scholar] [CrossRef] [Green Version]
- Velazhahan, R.; Vijayanandraj, S.; Vijayasamundeeswari, A.; Paranidharan, V.; Samiyappan, R.; Iwamoto, T.; Friebe, B.; Muthukrishnan, S. Detoxification of aflatoxins by seed extracts of the medicinal plant, Trachyspermum ammi (L.) Sprague ex Turrill—Structural analysis and biological toxicity of degradation product of aflatoxin G1. Food Control. 2010, 21, 719–725. [Google Scholar] [CrossRef]
- Badr, A.N.; Naeem, M.A. Protective efficacy using Cape-golden berry against pre-carcinogenic aflatoxins induced in rats. Toxicol. Rep. 2019, 6, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Chen, J.; Gao, R.; Liao, X.; Cai, Y. Adaptive Responses to Oxidative Stress in the Filamentous Fungal Shiraia bambusicola. Molecules 2016, 21, 1118. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; McNeil, B.; Harvey, L.M. Adaptive response to oxidative stress in the filamentous fungus Aspergillus niger B1-D. Free. Radic. Biol. Med. 2008, 44, 394–402. [Google Scholar] [CrossRef] [Green Version]
- Montibus, M.; Pinson-Gadais, L.; Richard-Forget, F.; Barreau, C.; Ponts, N. Coupling of transcriptional response to oxidative stress and secondary metabolism regulation in filamentous fungi. Crit. Rev. Microbiol. 2013, 41, 295–308. [Google Scholar] [CrossRef]
- Kenne, G.J.; Gummadidala, P.M.; Omebeyinje, M.H.; Mondal, A.M.; Bett, D.K.; McFadden, S.; Bromfield, S.; Banaszek, N.; Velez-Martinez, M.; Mitra, C.; et al. Activation of Aflatoxin Biosynthesis Alleviates Total ROS in Aspergillus parasiticus. Toxins 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Adrio, J.L.; Demain, A.L. Fungal biotechnology. Int. Microbiol. 2003, 6, 191–199. [Google Scholar] [CrossRef]
- Bayram, Ö.; Braus, G.H. Coordination of secondarymetabolism and development in fungi: The velvet family of regulatory proteins. FEMS Microbiol. Rev. 2012, 36, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Bennett, J.W.; Lee, L.S. Mycotoxins—Their Biosynthesis in Fungi: Aflatoxins and other Bisfuranoids. J. Food Prot. 1979, 42, 805–809. [Google Scholar] [CrossRef]
- Bhatnagar, D.; Cary, J.W.; Ehrlich, K.; Yu, J.; Cleveland, T.E. Understanding the genetics of regulation of aflatoxin production and Aspergillus flavus development. Mycopathologia 2006, 162, 155–166. [Google Scholar] [CrossRef]
- Bhatnagar, D.; Ehrlich, K.C.; Cleveland, T.E. Oxidation-reduction reactions in the biosynthesis of secondary metabolites. Handb. Appl. Mycol. 1991, 5, 255–286. [Google Scholar]
- Cleveland, T.E.; Bhatnagar, D. Molecular strategies for reducing aflatoxin levels in crops before harvest. In Molecular Approaches to Improving Food Quality and Safety; Springer: Berlin/Heidelberg, Germany, 1992; pp. 205–228. [Google Scholar]
- Parry, J.; Yu, L. Fatty Acid Content and Antioxidant Properties of Cold-pressed Black Raspberry Seed Oil and Meal. J. Food Sci. 2006, 69, FCT189–FCT193. [Google Scholar] [CrossRef]
- Calvo, A.M.; Wilson, R.A.; Bok, J.W.; Keller, N.P. Relationship between Secondary Metabolism and Fungal Development. Microbiol. Mol. Biol. Rev. 2002, 66, 447–459. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, M.F.; Kroh, L.W.; Mörsel, J.-T. Radical Scavenging Activity of Black Cumin (Nigella sativa L.), Coriander (Coriandrum sativum L.), and Niger (Guizotia abyssinica Cass.) Crude Seed Oils and Oil Fractions. J. Agric. Food Chem. 2003, 51, 6961–6969. [Google Scholar] [CrossRef]
- Brighente, I.; Dias, M.; Verdi, L.; Pizzolatti, M. Antioxidant Activity and Total Phenolic Content of Some Brazilian Species. Pharm. Biol. 2007, 45, 156–161. [Google Scholar] [CrossRef]
- Balz, M.K.; Schulte, E.; Thier, H.-P. Simultaneous Determination of α-Tocopheryl Acetate, Tocopherols and Tocotrienols by HPLC with Fluorescence Detection in Foods. Fette Seifen Anstrichm. 1993, 95, 215–220. [Google Scholar] [CrossRef]
- Stuper-Szablewska, K.; Kurasiak-Popowska, D.; Nawracała, J.; Perkowski, J. Response of non-enzymatic antioxidative mechanisms to stress caused by infection with Fusarium fungi and chemical protection in different wheat genotypes. Chem. Ecol. 2017, 33, 949–962. [Google Scholar] [CrossRef]
- Kurasiak-Popowska, D.; Ryńska, B.; Kurasiak-Popowska, D. Analysis of Distribution of Selected Bioactive Compounds in Camelina sativa from Seeds to Pomace and Oil. Agronomy 2019, 9, 168. [Google Scholar] [CrossRef] [Green Version]
- Ishtiaque, S.; Khan, N.; Siddiqui, M.A.; Siddiqi, R.; Naz, S. Antioxidant Potential of the Extracts, Fractions and Oils Derived from Oilseeds. Antioxidants 2013, 2, 246–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, G.-C.; Duh, P.-D. Antioxidant activity of methanolic extracts of peanut hulls from various cultivars. J. Am. Oil Chem. Soc. 1995, 72, 1065–1067. [Google Scholar] [CrossRef]
- Bussmann, R.; Malca-García, G.; Glenn, A.; Sharon, D.; Chait, G.; Díaz, D.; Pourmand, K.; Jonat, B.; Somogy, S.; Guardado, G.; et al. Minimum inhibitory concentrations of medicinal plants used in Northern Peru as antibacterial remedies. J. Ethnopharmacol. 2010, 132, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Shehata, M.G.; Badr, A.N.; El Sohaimy, S.A.; Asker, D.; Awad, T.S. Characterization of antifungal metabolites produced by novel lactic acid bacterium and their potential application as food biopreservatives. Ann. Agric. Sci. 2019, 64, 71–78. [Google Scholar] [CrossRef]
- Badr, A.N.; Logrieco, A.F.; Amra, H.A.; Hussien, T. Ochratoxin A Occurrence on Egyptian Wheat During Seasons (2009–2014). Asian J. Sci. Res. 2017, 10, 178–185. [Google Scholar] [CrossRef]
- Whitehead, R.H.; Robinson, P.S. Establishment of conditionally immortalized epithelial cell lines from the intestinal tissue of adult normal and transgenic mice. Am. J. Physiol. Liver Physiol. 2009, 296, G455–G460. [Google Scholar] [CrossRef]
- Gerlier, D.; Thomasset, N. Use of MTT colorimetric assay to measure cell activation. J. Immunol. Methods 1986, 94, 57–63. [Google Scholar] [CrossRef]

| Biochemical Parameter | Pomegranate Oil |
|---|---|
| Total phenolic (mg GAE/g) | 39.06 ± 1.47 |
| Total flavonoid (mg CE/g) | 12.4 ± 0.96 |
| Tocochromanols (μg/g) | |
| α-tocopherol | 16 ± 2 |
| β-tocopherol | 0.4 ± 0.42 |
| δ-tocopherol | 414.6 ± 2.31 |
| γ-tocopherol | 13.7 ± 1.53 |
| α-tocotrienol | 1.6 ± 0.15 |
| β-tocotrienol | 0.67 ± 0.12 |
| δ-tocotrienol | 2.03 ± 0.21 |
| γ-tocotrienol | 462.7 ± 3.05 |
| Total | 911.7 ± 9.79 |
| Carotenoids (µg/g) | |
| Lutein | 188.3 ± 1.79 |
| Zeaxanthin | 16.47 ± 0.21 |
| β-Carotene | 0.54 ± 0.078 |
| Total | 205.31 ± 2.08 |
| Sterols (mg/100g) | |
| Campesterol | 55.95 ± 2.55 |
| Stigmasterol | 0.11 ± 0.029 |
| β-sitosterol | 1573.47 ± 3.47 |
| Brassicasterol | 0.023 ± 0.009 |
| Campestanol | 0.077 ± 0.021 |
| Delta-5 Avenasterol | 9.93 ± 0.125 |
| Delta-7 Stigmastenol | 0.273 ± 0.012 |
| Total | 1639.36 ± 6.22 |
| Phenolic Compounds mg/100g | Average |
|---|---|
| Apigenin | 26.53 ± 0.35 |
| Catechin | 396.63 ± 0.45 |
| Kaempferol | 85.5 ± 0.3 |
| Luteolin | 73.97 ± 0.15 |
| Naringenin | 27.07 ± 0.35 |
| Quercetin | 135.83 ± 0.31 |
| Rutin | 148.33 ± 0.25 |
| Vitexin | 31.3 ± 0.26 |
| 4-hydroxybenzoic | 72.4 ± 0.17 |
| Caffeic | 115.37 ± 0.30 |
| Chlorogenic | 175.57 ± 0.31 |
| Ferulic | 0.013 ± 0.005 |
| Gallic | 784.13 ± 0.25 |
| p-Cumaric | 12.67 ± 0.23 |
| Protocatechuic | 99.33 ± 0.15 |
| Sinapic | ND |
| Syringic | 0.017 ± 0.011 |
| t-Cinnamic | 13.4 ± 0.3 |
| Vanillic | 6.37 ± 0.21 |
| Vanillin | 42.43 ± 0.31 |
| Synonym | Fatty Acid | % |
|---|---|---|
| C12:0 | Lauric acid | ND |
| C14:0 | Meristic Acid | 0.02 |
| C16:0 | Palmitic Acid | 2.57 |
| C16:1 | Palmitoleic Acid | 0.1 |
| C17:0 | Margaric Acid | 0.14 |
| C18:0 | Stearic Acid | 2.07 |
| C18:1 | Oleic Acid | 4.33 |
| C18:1 trans | Vaccenic Acid | 0.64 |
| C18:2 | Linoleic Acid | 5.24 |
| C18:3 | Linolenic Acid | 0.18 |
| C18:3 n5 | Punicic Acid | 81.29 |
| C20:0 | Arachidic Acid | 0.46 |
| C20:1 | Gondoic Acid | 0.56 |
| C20:1 | Gadoleic Acid | ND |
| C20:5 | Eicosapentaenoic acid | 0.8 |
| C22:0 | Behenic Acid | 0.13 |
| C22:1 | Erucic Acid | 1.05 |
| C24:0 | Lignoceric acid | 0.39 |
| C24:1 | Nervonic Acid | 0.03 |
| ∑ SFA | Sum of saturated fatty acids | 5.78 |
| ∑ MUFA | Sum of monounsaturated fatty acids | 6.71 |
| ∑ PUFA | Sum of polyunsaturated fatty acids | 87.51 |
| MUFA/PUFA | Fractionation | 0.077 |
| SFA/PUFA | Fractionation | 0.061 |
| PGO (mg/mL) | MPG (mg/mL) | NPGO (mg/mL) | Azithromycin (mg/mL) | Note | |
|---|---|---|---|---|---|
| Escherichia coli ATCC 11229 | 220 | 120 | 70 | 0.015 | (G-) bacteria |
| Salmonella typhi ATCC 14028 | 130 | 80 | 50 | 0.016 | (G-) bacteria |
| Shigella sonni ATCC 29930 | 160 | 90 | 60 | 0.016 | (G-) bacteria |
| Staphylococcus aureus ATCC 33591 | 180 | 100 | 50 | 0.012 | (G+) bacteria |
| Listeria monocytogenes ATCC19111 | 150 | 90 | 50 | 0.012 | (G+) bacteria |
| Bacillus cereus ATCC11778 | 150 | 90 | 50 | 0.012 | (G+) bacteria |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badr, A.N.; Ali, H.S.; Abdel-Razek, A.G.; Shehata, M.G.; Albaridi, N.A. Bioactive Components of Pomegranate Oil and Their Influence on Mycotoxin Secretion. Toxins 2020, 12, 748. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12120748
Badr AN, Ali HS, Abdel-Razek AG, Shehata MG, Albaridi NA. Bioactive Components of Pomegranate Oil and Their Influence on Mycotoxin Secretion. Toxins. 2020; 12(12):748. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12120748
Chicago/Turabian StyleBadr, Ahmed Noah, Hatem Salama Ali, Adel Gabr Abdel-Razek, Mohamed Gamal Shehata, and Najla A. Albaridi. 2020. "Bioactive Components of Pomegranate Oil and Their Influence on Mycotoxin Secretion" Toxins 12, no. 12: 748. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12120748