Evidence for the Range Expansion of Ciguatera in French Polynesia: A Revisit of the 2009 Mass-Poisoning Outbreak in Rapa Island (Australes Archipelago)
Abstract
:1. Introduction
2. Results
2.1. Epidemiology of the 2009 Outbreak in Rapa Island
2.2. Abundance, Distribution, and Toxicity of Gambierdiscus Populations
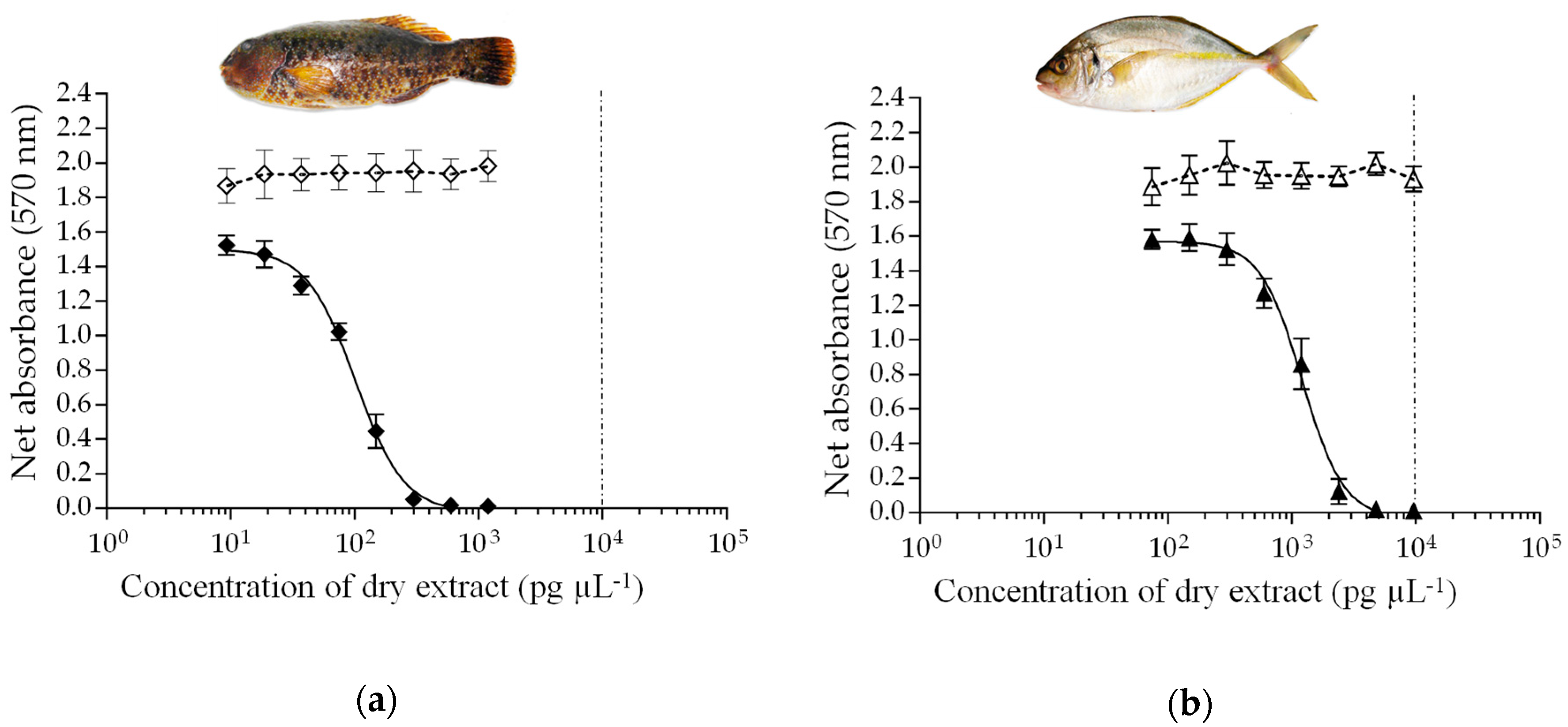
2.3. rRBA, CBA-N2a and LC-MS/MS Toxicity Data in Fish
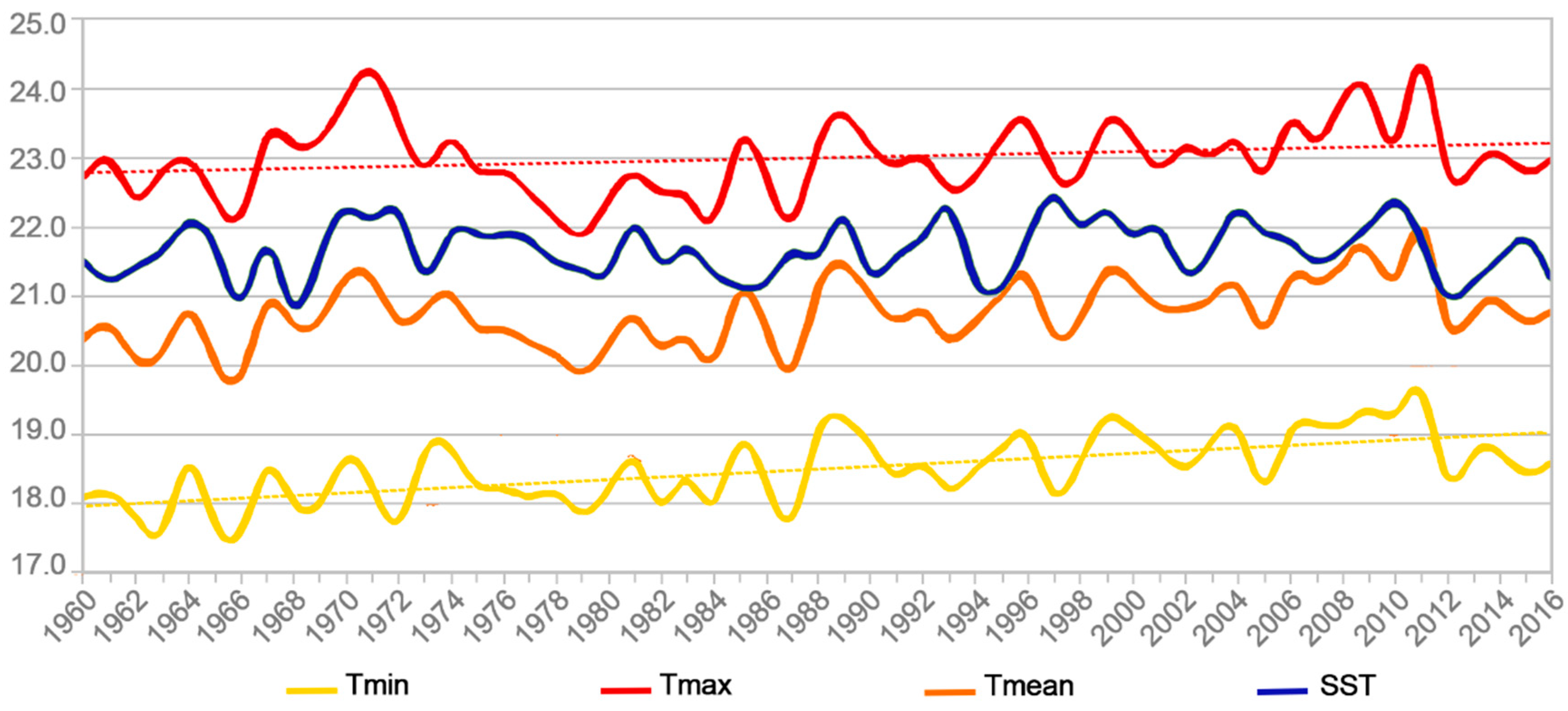
2.4. Temperature Trends in Rapa Island
3. Discussion
4. Conclusions
5. Materials and Methods
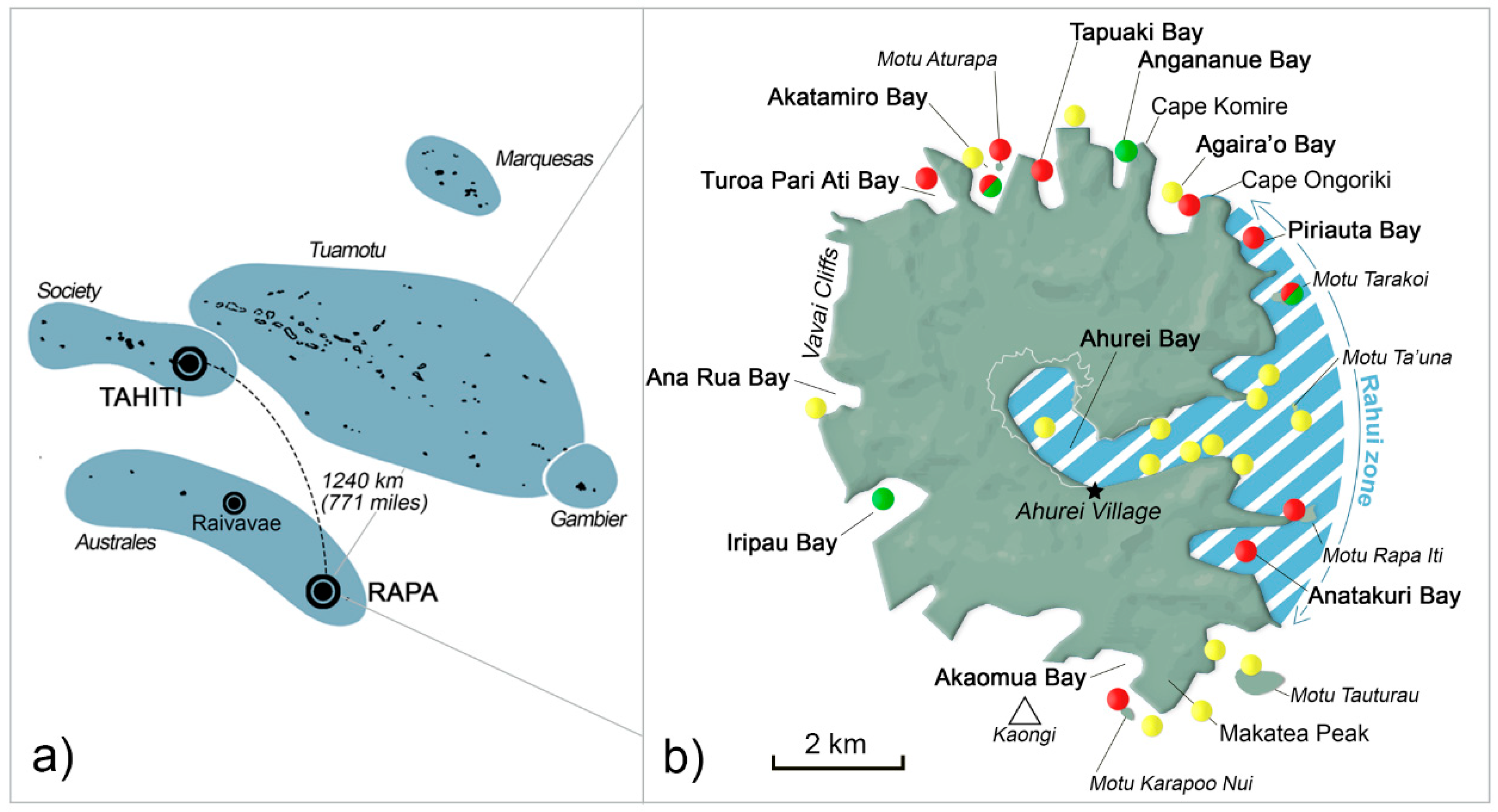
5.1. Study Site
5.2. Biological Samples
5.2.1. Macroalgal Samples
5.2.2. Fish Samples
5.3. Epidemiological Data
5.4. Toxin Extraction
5.4.1. Micro-Algal Samples
5.4.2. Fish Samples
5.5. Toxicological Analyses
5.5.1. Radioactive Receptor Binding Assay (rRBA)
5.5.2. Neuroblastoma Cell-Based Assay (CBA-N2a)
5.5.3. Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS)
5.6. Time-Series Temperature Data
5.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Dolah, F.M. Marine algal toxins: Origins, health effects, and their increased occurrence. Environ. Health Perspect. 2000, 108 (Suppl. 1), 133–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toda, M.; Uneyama, C.; Toyofuku, H.; Morikawa, K. Trends of food poisonings caused by natural toxins in Japan. J. Food Hyg. Soc. Jpn. 2012, 53, 105–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansdell, V. Seafood Poisoning. In Travel Medicine, 4th ed.; Keystone, J.S., Kozarsky, P.E., Connor, B.A., Nothdurft, H.D., Mendelson, M., Leder, K., Eds.; Elsevier: London, UK, 2019; pp. 449–456. [Google Scholar] [CrossRef]
- Chinain, M.; Gatti, C.M.I.; Darius, H.T.; Quod, J.P.; Tester, P.A. Ciguatera poisonings: A global review of occurrences and trends. Harmful Algae 2020, 101873. [Google Scholar] [CrossRef]
- Daneshian, M.; Botana, L.M.; Dechraoui Bottein, M.-Y.; Buckland, G.; Campàs, M.; Dennison, N.; Dickey, R.W.; Diogène, J.; Fessard, V.; Hartung, T.; et al. A roadmap for hazard monitoring and risk assessment of marine biotoxins on the basis of chemical and biological test systems. Altern. Anim. Exp. ALTEX 2013, 30, 487–545. [Google Scholar] [CrossRef] [Green Version]
- Parsons, M.L.; Aligizaki, K.; Dechraoui Bottein, M.-Y.; Fraga, S.; Morton, S.L.; Penna, A.; Rhodes, L. Gambierdiscus and Ostreopsis: Reassessment of the state of knowledge of their taxonomy, geography, ecophysiology, and toxicology. Harmful Algae 2012, 14, 107–129. [Google Scholar] [CrossRef]
- Litaker, R.W.; Holland, W.C.; Hardison, D.R.; Pisapia, F.; Hess, P.; Kibler, S.R.; Tester, P.A. Ciguatoxicity of Gambierdiscus and Fukuyoa species from the Caribbean and Gulf of Mexico. PLoS ONE 2017, 12, e0185776. [Google Scholar] [CrossRef]
- Chinain, M.; Gatti, C.M.; Roué, M.; Darius, H.T. Ciguatera-causing dinoflagellates in the genera Gambierdiscus and Fukuyoa: Distribution, ecophysiology and toxicology. In Dinoflagellates: Morphology, Life History and Ecological Significance; Subba Rao, D.V., Ed.; The Nova Science Publishers, Inc.: New York, NY, USA, 2020; pp. 405–457. [Google Scholar]
- Earle, K.V. Pathological effects of two West Indian echinoderms. Trans. R. Soc. Trop. Med. Hyg. 1940, 33, 447–452. [Google Scholar] [CrossRef]
- Rongo, T.; van Woesik, R. Ciguatera poisoning in Rarotonga, Southern Cook Islands. Harmful Algae 2011, 10, 345–355. [Google Scholar] [CrossRef]
- Mak, Y.L.; Wai, T.-C.; Murphy, M.B.; Chan, W.H.; Wu, J.J.; Lam, J.C.W.; Chan, L.L.; Lam, P.K.S. Pacific ciguatoxins in food web components of coral reef systems in the Republic of Kiribati. Environ. Sci. Technol. 2013, 47, 14070–14079. [Google Scholar] [CrossRef]
- Roué, M.; Darius, H.T.; Picot, S.; Ung, A.; Viallon, J.; Gaertner-Mazouni, N.; Sibat, M.; Amzil, Z.; Chinain, M. Evidence of the bioaccumulation of ciguatoxins in giant clams (Tridacna maxima) exposed to Gambierdiscus spp. cells. Harmful Algae 2016, 57, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Darius, H.T.; Roué, M.; Sibat, M.; Viallon, J.; Gatti, C.M.; Vandersea, M.W.; Tester, P.A.; Litaker, R.W.; Amzil, Z.; Hess, P.; et al. Tectus niloticus (Tegulidae, Gastropod) as a novel vector of Ciguatera Poisoning: Detection of Pacific ciguatoxins in toxic samples from Nuku Hiva Island (French Polynesia). Toxins 2018, 10, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darius, H.T.; Roué, M.; Sibat, M.; Viallon, J.; Gatti, C.M.i.; Vandersea, M.W.; Tester, P.A.; Litaker, R.W.; Amzil, Z.; Hess, P.; et al. Toxicological investigations on the sea urchin Tripneustes gratilla (Toxopneustidae, Echinoid) from Anaho Bay (Nuku Hiva, French Polynesia): Evidence for the presence of Pacific ciguatoxins. Mar. Drugs 2018, 16, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehane, L.; Lewis, R.J. Ciguatera: Recent advances but the risk remains. Int. J. Food Microbiol. 2000, 61, 91–125. [Google Scholar] [CrossRef]
- Ikehara, T.; Kuniyoshi, K.; Oshiro, N.; Yasumoto, T. Biooxidation of ciguatoxins leads to species-specific toxin profiles. Toxins 2017, 9, 205. [Google Scholar] [CrossRef] [Green Version]
- Chateau-Degat, M.L.; Dewailly, E.; Cerf, N.; Nguyen, N.L.; Huin-Blondey, M.O.; Hubert, B.; Laudon, F.; Chansin, R. Temporal trends and epidemiological aspects of ciguatera in French Polynesia: A 10-year analysis. Trop. Med. Int. Health 2007, 12, 485–492. [Google Scholar] [CrossRef]
- Stempf, E. Incidence des Formes Chroniques de la Ciguatera au Sein de la Population Réunionnaise de 2000 à 2014; Université de Bordeaux Segalen: Bordeaux, France, 2015; p. 58. [Google Scholar]
- Friedman, M.A.; Fernandez, M.; Backer, L.C.; Dickey, R.W.; Bernstein, J.; Schrank, K.; Kibler, S.; Stephan, W.; Gribble, M.O.; Bienfang, P.; et al. An updated review of Ciguatera Fish Poisoning: Clinical, epidemiological, environmental, and public health management. Mar. Drugs 2017, 15, 72. [Google Scholar] [CrossRef]
- Gatti, C.M.; Lonati, D.; Darius, H.T.; Zancan, A.; Roué, M.; Schicchi, A.; Locatelli, C.A.; Chinain, M. Tectus niloticus (Tegulidae, Gastropod) as a novel vector of Ciguatera Poisoning: Clinical characterization and follow-up of a mass poisoning event in Nuku Hiva Island (French Polynesia). Toxins 2018, 10, 102. [Google Scholar] [CrossRef] [Green Version]
- Bienfang, P.; Oben, B.; DeFelice, S.; Moeller, P.; Huncik, K.; Oben, P.; Toonen, R.; Daly-Engel, T.; Bowen, B. Ciguatera: The detection of neurotoxins in carnivorous reef fish from the coast of Cameroon, West Africa. Afr. J. Mar. Sci. 2008, 30, 533–540. [Google Scholar] [CrossRef]
- Rongo, T.; van Woesik, R. Socioeconomic consequences of ciguatera poisoning in Rarotonga, Southern Cook Islands. Harmful Algae 2012, 20, 92–100. [Google Scholar] [CrossRef]
- Morin, E.; Gatti, C.; Bambridge, T.; Chinain, M. Ciguatera fish poisoning: Incidence, health costs and risk perception on Moorea Island (Society Archipelago, French Polynesia). Harmful Algae 2016, 60, 1–10. [Google Scholar] [CrossRef]
- Hardison, D.R.; Holland, W.C.; Darius, H.T.; Chinain, M.; Tester, P.A.; Shea, D.; Bogdanoff, A.K.; Morris, J.A., Jr.; Flores Quintana, H.A.; Loeffler, C.R.; et al. Investigation of ciguatoxins in invasive lionfish from the greater Caribbean region: Implications for fishery development. PLoS ONE 2018, 13, e0198358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tester, P.A.; Feldman, R.L.; Nau, A.W.; Kibler, S.R.; Litaker, W.R. Ciguatera fish poisoning and sea surface temperatures in the Caribbean Sea and the West Indies. Toxicon 2010, 56, 698–710. [Google Scholar] [CrossRef]
- Skinner, M.P.; Lewis, R.J.; Morton, S. Ecology of the ciguatera causing dinoflagellates from the Northern Great Barrier Reef: Changes in community distribution and coastal eutrophication. Mar. Pollut. Bull. 2013, 77, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Perez-Arellano, J.L.; Luzardo, O.P.; Perez Brito, A.; Hernandez Cabrera, M.; Zumbado, M.; Carranza, C.; Angel-Moreno, A.; Dickey, R.W.; Boada, L.D. Ciguatera fish poisoning, Canary Islands. Emerg. Infect. Dis. 2005, 11, 1981–1982. [Google Scholar] [CrossRef] [Green Version]
- Boada, L.D.; Zumbado, M.; Luzardo, O.P.; Almeida-González, M.; Plakas, S.M.; Granade, H.R.; Abraham, A.; Jester, E.L.E.; Dickey, R.W. Ciguatera fish poisoning on the West Africa Coast: An emerging risk in the Canary Islands (Spain). Toxicon 2010, 56, 1516–1519. [Google Scholar] [CrossRef]
- Otero, P.; Pérez, S.; Alfonso, A.; Vale, C.; Rodriguez, P.; Gouveia, N.N.; Gouveia, N.; Delgado, J.; Vale, P.; Hirama, M.; et al. First toxin profile of ciguateric fish in Madeira Arquipelago (Europe). Anal. Chem. 2010, 82, 6032–6039. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, D.; Matute, P.; Garcia, A.; Garcia, P.; Abadía, N. Outbreak of ciguatera food poisoning by consumption of amberjack (Seriola spp.) in the Canary Islands, may 2012. Euro Surveill. 2012, 17, 20188. [Google Scholar]
- Caillaud, A.; Eixarch, H.; de la Iglesia, P.; Rodriguez, M.; Dominguez, L.; Andree, K.B.; Diogène, J. Towards the standardisation of the neuroblastoma (neuro-2a) cell-based assay for ciguatoxin-like toxicity detection in fish: Application to fish caught in the Canary Islands. Food Addit. Contam. Part A 2012, 29, 1000–1010. [Google Scholar] [CrossRef]
- Bravo, J.; Suarez, F.C.; Ramirez, A.S.; Acosta, F. Ciguatera, an emerging human poisoning in Europe. J. Aquac. Mar. Biol. 2015, 3, 00053. [Google Scholar] [CrossRef]
- Estevez, P.; Castro, D.; Manuel Leao, J.; Yasumoto, T.; Dickey, R.; Gago-Martinez, A. Implementation of liquid chromatography tandem mass spectrometry for the analysis of ciguatera fish poisoning in contaminated fish samples from Atlantic coasts. Food Chem. 2019, 280, 8–14. [Google Scholar] [CrossRef]
- Estevez, P.; Castro, D.; Pequeño-Valtierra, A.; Leao, J.M.; Vilariño, O.; Diogène, J.; Gago-Martínez, A. An attempt to characterize the ciguatoxin profile in Seriola fasciata causing ciguatera fish poisoning in Macaronesia. Toxins 2019, 11, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Henao, J.A.; García-Álvarez, N.; Fernández, A.; Saavedra, P.; Silva Sergent, F.; Padilla, D.; Acosta-Hernández, B.; Martel Suárez, M.; Diogène, J.; Real, F. Predictive score and probability of CTX-like toxicity in fish samples from the official control of ciguatera in the Canary Islands. Sci. Total Environ. 2019, 673, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Henao, A.; García-Álvarez, N.; Silva Sergent, F.; Estévez, P.; Gago-Martínez, A.; Martín, F.; Ramos-Sosa, M.; Fernández, A.; Diogène, J.; Real, F. Presence of CTXs in moray eels and dusky groupers in the marine environment of the Canary Islands. Aquat. Toxicol. 2020, 105427. [Google Scholar] [CrossRef] [PubMed]
- Bentur, Y.; Spanier, E. Ciguatoxin-like substances in edible fish on the Eastern Mediterranean. Clin. Toxicol. 2007, 45, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Villareal, T.A.; Hanson, S.; Qualia, S.; Jester, E.L.E.; Granade, H.R.; Dickey, R.W. Petroleum production platforms as sites for the expansion of ciguatera in the northwestern Gulf of Mexico. Harmful Algae 2007, 6, 253–259. [Google Scholar] [CrossRef]
- Aligizaki, K.; Nikolaidis, G. Morphological identification of two tropical dinoflagellates of the genera Gambierdiscus and Sinophysis in the Mediterranean Sea. J. Biol. Res. Thessalon. 2008, 9, 75–82. [Google Scholar]
- Fraga, S.; Rodriguez, F.; Caillaud, A.; Diogεne, J.; Raho, N.; Zapata, M. Gambierdiscus excentricus sp. nov. (Dinophyceae), a benthic toxic dinoflagellate from the Canary Islands (NE Atlantic Ocean). Harmful Algae 2011, 11, 10–22. [Google Scholar] [CrossRef] [Green Version]
- Tester, P.A.; Vandersea, M.W.; Buckel, C.A.; Kibler, S.R.; Holland, W.C.; Davenport, E.D.; Clark, R.D.; Edwards, K.F.; Taylor, J.C.; Vander Pluym, J.L.; et al. Gambierdiscus (Dinophyceae) species diversity in the Flower Garden Banks National Marine Sanctuary, Northern Gulf of Mexico, USA. Harmful Algae 2013, 29, 1–9. [Google Scholar] [CrossRef]
- Fraga, S.; Rodriguez, F. Genus Gambierdiscus in the Canary Islands (NE Atlantic Ocean) with description of Gambierdiscus silvae sp. nov., a new potentially toxic epiphytic benthic dinolflagellate. Protist 2014, 165, 839–853. [Google Scholar] [CrossRef]
- Rodríguez, F.; Fraga, S.; Ramilo, I.; Rial, P.; Figueroa, R.I.; Riobó, P.; Bravo, I. Canary Islands (NE Atlantic) as a biodiversity ‘hotspot’ of Gambierdiscus: Implications for future trends of ciguatera in the area. Harmful Algae 2017, 67, 131–143. [Google Scholar] [CrossRef]
- Pisapia, F.; Holland, W.C.; Hardison, D.R.; Litaker, R.W.; Fraga, S.; Nishimura, T.; Adachi, M.; Nguyen-Ngoc, L.; Séchet, V.; Amzil, Z.; et al. Toxicity screening of 13 Gambierdiscus strains using neuro-2a and erythrocyte lysis bioassays. Harmful Algae 2017, 63, 173–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reverté, L.; Toldrà, A.; Andree, K.B.; Fraga, S.; Falco, G.; Campàs, M.; Diogène, J. Assessment of cytotoxicity in ten strains of Gambierdiscus australes from Macaronesian Islands by neuro-2a cell-based assays. J. Appl. Phycol. 2018, 30, 2447–2461. [Google Scholar] [CrossRef]
- Rossignoli, A.E.; Tudó, A.; Bravo, I.; Díaz, P.A.; Diogène, J.; Riobó, P. Toxicity characterisation of Gambierdiscus species from the Canary Islands. Toxins 2020, 12, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tudó, À.; Toldrà, A.; Rey, M.; Todolí, I.; Andree, K.B.; Fernández-Tejedor, M.; Campàs, M.; Sureda, F.X.; Diogène, J. Gambierdiscus and Fukuyoa as potential indicators of ciguatera risk in the Balearic Islands. Harmful Algae 2020, 99, 101913. [Google Scholar] [CrossRef] [PubMed]
- Tudó, À.; Gaiani, G.; Rey Varela, M.; Tsumuraya, T.; Andree, K.B.; Fernández-Tejedor, M.; Campàs, M.; Diogène, J. Further advance of Gambierdiscus species in the Canary Islands, with the first report of Gambierdiscus belizeanus. Toxins 2020, 12, 692. [Google Scholar] [CrossRef] [PubMed]
- Ruff, T.A. Ciguatera in the Pacific: A link with military activities. Lancet 1989, 333, 201–205. [Google Scholar] [CrossRef]
- Chinain, M.; Darius, H.T.; Ung, A.; Tchou Fouc, M.; Revel, T.; Cruchet, P.; Pauillac, S.; Laurent, D. Ciguatera risk management in French Polynesia: The case study of Raivavae Island (Australes Archipelago). Toxicon 2010, 56, 674–690. [Google Scholar] [CrossRef] [PubMed]
- Rongo, T.; van Woesik, R. The effects of natural disturbances, reef state, and herbivorous fish densities on ciguatera poisoning in Rarotonga, southern Cook Islands. Toxicon 2013, 64, 87–95. [Google Scholar] [CrossRef]
- Rongo, T.; Bush, M.; Van Woesik, R. Did ciguatera prompt the late Holocene Polynesian voyages of discovery? J. Biogeogr. 2009, 36, 1423–1432. [Google Scholar] [CrossRef]
- Llewellyn, L.E. Revisiting the association between sea surface temperature and the epidemiology of fish poisoning in the South Pacific: Reassessing the link between ciguatera and climate change. Toxicon 2010, 56, 691–697. [Google Scholar] [CrossRef]
- Tester, P.A.; Berdalet, E.; Litaker, R.W. Climate change and benthic harmful microalgae. Harmful Algae 2020, 91, 101655. [Google Scholar] [CrossRef] [PubMed]
- Kibler, S.R.; Tester, P.A.; Kunlec, K.E.; Moored, S.K.; Litaker, W.R. Effects of ocean warming on growth and distribution of dinoflagellates associated with ciguatera fish poisoning in the Caribbean. Ecol. Model. 2015, 316, 194–210. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Gatti, C.M.i.; Garrido Gamarro, E.; Suzuki, A.; Teah, H.Y. Modeling the time-lag effect of sea surface temperatures on ciguatera poisoning in the South Pacific: Implications for surveillance and response. Toxicon 2020, 182, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Hales, S.; Weinstein, P.; Woodward, A. Ciguatera (Fish Poisoning), El Nino, and Pacific sea surface temperatures. Ecosyst. Health 1999, 5, 20–25. [Google Scholar] [CrossRef]
- Chateau-Degat, M.-L.; Chinain, M.; Cerf, N.; Gingras, S.; Hubert, B.; Dewailly, E. Seawater temperature, Gambierdiscus spp. variability and incidence of ciguatera poisoning in French Polynesia. Harmful Algae 2005, 4, 1053–1062. [Google Scholar] [CrossRef]
- Gingold, D.B.; Strickland, M.J.; Hess, J.J. Ciguatera fish poisoning and climate change: Analysis of National Poison Center Data in the United States, 2001–2011. Environ. Health Perspect. 2014, 122, 580–586. [Google Scholar] [CrossRef]
- Bagnis, R.; Bennet, J.; Barsinas, M.; Chebret, M.; Jacquet, G.; Lechat, I.; Mitermite, Y.; Perolat, P.H.; Rongeras, S. Epidemiology of ciguatera in French Polynesia from 1960 to 1984. In Proceedings of the Fifth International Coral Reef Congress, Tahiti, French Polynesia, 27 May–1 June 1985; Gabrie, C., Salvat, B., Eds.; Antenne Museum-EPHE: Moorea, French Polynesia, 1985; pp. 475–482. [Google Scholar]
- Chateau-Degat, M.-L.; Chinain, M.; Darius, H.T.; Dewailly, E.; Mallet, H.-P. Epidemiological survey of ciguatera in French Polynesia. Bull. Epidemiol. Hebd. 2009, 48/50, 522–525. [Google Scholar]
- Skinner, M.P.; Brewer, T.D.; Johnstone, R.; Fleming, L.E.; Lewis, R.J. Ciguatera fish poisoning in the Pacific Islands (1998 to 2008). PLoS Negl. Trop. Dis. 2011, 5, e1416. [Google Scholar] [CrossRef]
- Available online: https://www.ciguatera.pf (accessed on 27 October 2020).
- Chinain, M.; Darius, H.T.; Gatti, C.M.; Roué, M. Update on ciguatera research in French Polynesia. SPC Fish. Newsl. 2016, 150, 42–51. [Google Scholar]
- Laurent, V.; Maamaatuaiahutapu, K. Atlas Climatologique de la Polynésie Française, 2nd ed.; Météo France: Tahiti, French Polynesia, 2019; 228p. [Google Scholar]
- Viallon, J.; Chinain, M.; Darius, H.T. Revisiting the neuroblastoma cell-based assay (CBA-N2a) for the improved detection of marine toxins active on voltage gated sodium channels (VGSCs). Toxins 2020, 12, 281. [Google Scholar] [CrossRef]
- Sibat, M.; Herrenknecht, C.; Darius, H.T.; Roué, M.; Chinain, M.; Hess, P. Detection of Pacific ciguatoxins using liquid chromatography coupled to either low or high resolution mass spectrometry (LC-MS/MS). J. Chromatogr. A 2018, 1571, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Z. Neurotoxins from marine dinoflagellates: A brief review. Mar. Drugs 2008, 6, 349–371. [Google Scholar] [CrossRef] [PubMed]
- Vetter, I.; Touska, F.; Hess, A.; Hinsbey, R.; Sattler, S.; Lampert, A.; Sergejeva, M.; Sharov, A.; Collins, L.S.; Eberhardt, M.; et al. Ciguatoxins activate specific cold pain pathways to elicit burning pain from cooling. EMBO J. 2012, 31, 3795–3808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cameron, J.; Capra, M.F. The basis of the paradoxical disturbance of temperature perception in ciguatera poisoning. J. Toxicol. Clin. Toxicol. 1993, 31, 571–579. [Google Scholar] [CrossRef]
- Pearn, J. Neurology of ciguatera. J. Neurol. Neurosurg. Psychiatry 2001, 70, 4–8. [Google Scholar] [CrossRef] [Green Version]
- Edwards, A.; Zammit, A.; Farrell, H. Four recent ciguatera fish poisoning incidents in New South Wales, Australia linked to imported fish. Commun. Dis. Intell. 2019, 43, 1–9. [Google Scholar] [CrossRef]
- Hamilton, B.; Whittle, N.; Shaw, G.; Eaglesham, G.; Moore, M.R.; Lewis, R.J. Human fatality associated with Pacific ciguatoxin contaminated fish. Toxicon 2010, 56, 668–673. [Google Scholar] [CrossRef]
- Chan, T.Y.K. Characteristic features and contributory factors in fatal ciguatera fish poisoning—Implications for prevention and public education. Am. J. Trop. Med. Hyg. 2016, 94, 704–709. [Google Scholar] [CrossRef] [Green Version]
- Pawlowiez, R.; Darius, H.T.; Cruchet, P.; Rossi, F.; Caillaud, A.; Laurent, D.; Chinain, M. Evaluation of seafood toxicity in the Australes archipelago (French Polynesia) using the neuroblastoma cell-based assay. Food Addit. Contam. Part A 2013, 30, 567–586. [Google Scholar] [CrossRef]
- Fukuyo, Y. Taxonomical study on benthic dinoflagellates collected in coral reefs. Bull. Jpn. Soc. Sci. Fish. 1981, 47, 967–978. [Google Scholar] [CrossRef] [Green Version]
- Bagnis, R.; Bennett, J.; Prieur, C.; Legrand, A.M. The dynamics of three toxic benthic dinoflagellates and the toxicity of ciguateric surgeonfish in French Polynesia. In Toxic Dinoflagellates; Anderson, D.M., White, A.N., Baden, D.G., Eds.; Elsevier: New York, NY, USA, 1985; pp. 177–182. [Google Scholar]
- Chomérat, N.; Bilien, G.; Derrien, A.; Henry, K.; Ung, A.; Viallon, J.; Darius, H.T.; Gatti, C.; Roué, M.; Hervé, F.; et al. Ostreopsis lenticularis Y. Fukuyo (Dinophyceae, Gonyaulacales) from French Polynesia (South Pacific Ocean): A revisit of its morphology, molecular phylogeny and toxicity. Harmful Algae 2019, 84, 95–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chomérat, N.; Bilien, G.; Couté, A.; Quod, J.-P. Reinvestigation of Ostreopsis mascarenensis Quod (Dinophyceae, Gonyaulacales) from Réunion Island (SW Indian Ocean): Molecular phylogeny and emended description. Phycologia 2020, 59, 1–14. [Google Scholar] [CrossRef]
- Alcala, A.C.; Alcala, L.C.; Garth, J.S.; Yasumura, D.; Yasumoto, T. Human fatality due to ingestion of the crab Demania reynaudii that contained a palytoxin-like toxin. Toxicon 1988, 26, 105–107. [Google Scholar] [CrossRef]
- Randall, J.E. Review of clupeotoxism, an often fatal illness from the consumption of clupeoid fishes. Pac. Sci. 2005, 59, 73–77. [Google Scholar] [CrossRef]
- Deeds, J.R.; Schwartz, M.D. Human risk associated with palytoxin exposure. Toxicon 2010, 56, 150–162. [Google Scholar] [CrossRef] [Green Version]
- Taniyama, S.; Arakawa, O.; Terada, M.; Nishio, S.; Takatani, T.; Mahmud, Y.; Noguchi, T. Ostreopsis sp., a possible origin of palytoxin (PTX) in parrotfish Scarus ovifrons. Toxicon 2003, 42, 29–33. [Google Scholar] [CrossRef]
- García-Portela, M.; Riobó, P.; Franco, J.M.; Bañuelos, R.M.; Rodríguez, F. Genetic and toxinological characterization of North Atlantic strains of the dinoflagellate Ostreopsis and allelopathic interactions with toxic and non-toxic species from the genera Prorocentrum, Coolia and Gambierdiscus. Harmful Algae 2016, 60, 57–69. [Google Scholar] [CrossRef] [Green Version]
- Sparrow, L.; Momigliano, P.; Russ, G.R.; Heimann, K. Effects of temperature, salinity and composition of the dinoflagellate assemblage on the growth of Gambierdiscus carpenteri isolated from the Great Barrier Reef. Harmful Algae 2017, 65, 52–60. [Google Scholar] [CrossRef]
- Rhodes, L.; Harwood, T.; Smith, K.; Argyle, P.; Munday, R. Production of ciguatoxin and maitotoxin by strains of Gambierdiscus australes, G. pacificus and G. polynesiensis (Dinophyceae) isolated from Rarotonga, Cook Islands. Harmful Algae 2014, 39, 185–190. [Google Scholar] [CrossRef]
- Longo, S.; Sibat, M.; Viallon, J.; Darius, H.T.; Hess, P.; Chinain, M. Intraspecific variability in the toxin production and toxin profiles of in vitro cultures of Gambierdiscus polynesiensis (Dinophyceae) from French Polynesia. Toxins 2019, 11, 735. [Google Scholar] [CrossRef] [Green Version]
- Chinain, M.; Germain, M.; Deparis, X.; Pauillac, S.; Legrand, A.M. Seasonal abundance and toxicity of the dinoflagellate Gambierdiscus spp. (Dinophyceae), the causative agent of ciguatera in Tahiti, French Polynesia. Mar. Biol. 1999, 135, 259–267. [Google Scholar] [CrossRef]
- Litaker, R.W.; Vandersea, M.W.; Faust, M.A.; Kibler, S.R.; Nau, A.W.; Holland, W.C.; Chinain, M.; Holmes, M.J.; Tester, P.A. Global distribution of ciguatera causing dinoflagellates in the genus Gambierdiscus. Toxicon 2010, 56, 711–730. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). Appendix 5—FDA and EPA Safety Levels in Regulations and Guidance. In Fish and Fishery Products Hazards and Control Guidance, 4th ed.; University of Florida: Gainesville, FL, USA, 2020; p. A5-1. [Google Scholar]
- European Food Safety Authority (EFSA). Scientific opinion on marine biotoxins in shellfish—Emerging toxins: Ciguatoxin group. EFSA J. 2010, 8, 1627. [Google Scholar] [CrossRef]
- Clausing, R.J.; Chinain, M.; Dechraoui Bottein, M.Y. Practical sampling guidance for determination of ciguatoxin in fish. In Guide for Designing and Implementing a Plan to Monitor Toxin-Producing Microalgae, 2nd ed.; Reguera, B., Alonso, R., Moreira, Á., Méndez, S., Dechraoui Bottein, M.-Y., Eds.; IOC Manuals & Guides: Paris, France; Vienna, Austria, 2016; Volume 59, pp. 51–61. [Google Scholar]
- Bagnis, R.; Bennet, J.; Barsinas, M.; Drollet, J.H.; Jacquet, G.; Legrand, A.M.; Cruchet, P.H.; Pascal, H. Correlation between ciguateric fish and damage to the reefs in the Gambier Islands (French Poynesia). In Proceedings of the Sixth International Coral Reef Symposium, Townsville, Australia, 8–12 August 1988; Choat, J.H., Barnes, D., Borowitzka, M.A., Coll, J.C., Davies, P.J., Flood, P., Hatcher, B.G., Hopley, D., Hutchings, P.A., Kinsey, G.R., et al., Eds.; 6th International Coral Reef Symposium Executive Committee: Townsville, Australia, 1988; pp. 195–200. [Google Scholar]
- Lewis, R.J.; Holmes, M.J. Origin and transfer of toxins involved in ciguatera. Comp. Biochem. Physiol. C 1993, 106, 615–628. [Google Scholar] [CrossRef]
- Pottier, I.; Vernoux, J.P.; Lewis, R.J. Ciguatera Fish Poisoning in the Caribbean Islands and Western Atlantic. In Reviews of Environmental Contamination and Toxicology: Continuation of Residue Reviews; Ware, G.W., Ed.; Springer: New York, NY, USA, 2001; pp. 99–141. [Google Scholar]
- Oshiro, N.; Yogi, K.; Asato, S.; Sasaki, T.; Tamanaha, K.; Hirama, M.; Yasumoto, T.; Inafuku, Y. Ciguatera incidence and fish toxicity in Okinawa, Japan. Toxicon 2010, 56, 656–661. [Google Scholar] [CrossRef]
- Chan, T.Y.K. Emergence and epidemiology of ciguatera in the coastal cities of Southern China. Mar. Drugs 2015, 13, 1175–1184. [Google Scholar] [CrossRef] [Green Version]
- Hossen, V.; Soliño, L.; Leroy, P.; David, E.; Velge, P.; Dragacci, S.; Krys, S.; Flores Quintana, H.; Diogène, J. Contribution to the risk characterization of ciguatoxins: LOAEL estimated from eight ciguatera fish poisoning events in Guadeloupe (French West Indies). Environ. Res. 2015, 143, 100–108. [Google Scholar] [CrossRef]
- Ha, D.V.; Uesugi, A.; Uchida, H.; Ky, P.X.; Minh, D.Q.; Watanabe, R.; Matsushima, R.; Oikawa, H.; Nagai, S.; Iwataki, M.; et al. Identification of causative ciguatoxins in red snappers Lutjanus bohar implicated in ciguatera fish poisonings in Vietnam. Toxins 2018, 10, 420. [Google Scholar] [CrossRef] [Green Version]
- Darius, H.T.; Ponton, D.; Revel, T.; Cruchet, P.; Ung, A.; Tchou Fouc, M.; Chinain, M. Ciguatera risk assessment in two toxic sites of French Polynesia using the receptor-binding assay. Toxicon 2007, 50, 612–626. [Google Scholar] [CrossRef]
- Yogi, K.; Oshiro, N.; Inafuku, Y.; Hirama, M.; Yasumoto, T. Detailed LC-MS/MS analysis of ciguatoxins revealing distinct regional and species characteristics in fish and causative alga from the Pacific. Anal. Chem. 2011, 83, 8886–8891. [Google Scholar] [CrossRef]
- Yogi, K.; Sakugawa, S.; Oshiro, N.; Ikehara, T.; Sugiyama, K.; Yasumoto, T. Determination of toxins involved in Ciguatera Fish Poisoning in the Pacific by LC/MS. J. AOAC Int. 2014, 97, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Bottein Dechraoui, M.Y.; Tiedeken, J.A.; Persad, R.; Wang, Z.; Granade, H.R.; Dickey, R.W.; Ramsdell, J.S. Use of two detection methods to discriminate ciguatoxins from brevetoxins: Application to great barracuda from Florida Keys. Toxicon 2005, 46, 261–270. [Google Scholar] [CrossRef]
- Hardison, D.R.; Holland, W.C.; McCall, J.R.; Bourdelais, A.J.; Baden, D.G.; Darius, H.T.; Chinain, M.; Tester, P.A.; Shea, D.; Flores Quintana, H.A.; et al. Fluorescent Receptor Binding Assay for detecting ciguatoxins in fish. PLoS ONE 2016, 11, e0153348. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Asencio, L.; Clausing, R.J.; Vandersea, M.; Chamero-Lago, D.; Gómez-Batista, M.; Hernández-Albernas, J.I.; Chomérat, N.; Rojas-Abrahantes, G.; Litaker, R.W.; Tester, P.; et al. Ciguatoxin occurrence in food-web components of a Cuban coral reef ecosystem: Risk-assessment implications. Toxins 2019, 11, 722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harwood, D.T.; Murray, S.; Boundy, M.J. Chapter Three—Sample preparation prior to marine toxin analysis. In Comprehensive Analytical Chemistry; Diogène, J., Campàs, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 78, pp. 89–136. [Google Scholar] [CrossRef]
- Roué, M.; Smith, K.F.; Sibat, M.; Viallon, J.; Henry, K.; Ung, A.; Biessy, L.; Hess, P.; Darius, H.T.; Chinain, M. Assessment of ciguatera and other phycotoxin-related risks in Anaho Bay (Nuku Hiva Island, French Polynesia): Molecular, toxicological, and chemical analyses of passive samplers. Toxins 2020, 12, 321. [Google Scholar] [CrossRef]
- Soliño, L.; Costa, P.R. Differential toxin profiles of ciguatoxins in marine organisms: Chemistry, fate and global distribution. Toxicon 2018, 150, 124–143. [Google Scholar] [CrossRef]
- Parsons, M.L.; Settlemier, C.J.; Bienfang, P.K. A simple model capable of simulating the population dynamics of Gambierdiscus, the benthic dinoflagellate responsible for ciguatera fish poisoning. Harmful Algae 2010, 10, 71–80. [Google Scholar] [CrossRef]
- Carlson, R.D.; Tindall, D.R. Distribution and periodicity of toxic dinoflagellates in the Virgin Islands. In Toxic Dinoflagellates; Anderson, D.M., White, A.W., Baden, D.G., Eds.; Elsevier: Amsterdam, The Netherlands, 1985; pp. 171–176. [Google Scholar]
- Loeffler, C.R.; Richlen, M.L.; Brandt, M.E.; Smith, T.B. Effects of grazing, nutrients, and depth on the ciguatera-causing dinoflagellate Gambierdiscus in the US Virgin Islands. Mar. Ecol. Prog. Ser. 2015, 531, 91–104. [Google Scholar] [CrossRef]
- Yasumoto, T.; Inoue, A.; Ochi, T.; Fujimoto, K.; Oshima, Y.; Fukuyo, Y.; Adachi, R.; Bagnis, R. Environmental studies on a toxic dinoflagellate responsible for ciguatera. Bull. Jpn. Soc. Sci. Fish. 1980, 46, 1397–1404. [Google Scholar] [CrossRef] [Green Version]
- Ichinotsubo, D.; Asahina, A.Y.; Titus, E.; Chun, S.; Hong, T.W.P.; Shirai, J.L.; Hokama, Y. Survey for ciguatera fish poisoning in west Hawaii. Mem. Qld. Mus. 1994, 34, 513–522. [Google Scholar]
- Parsons, M.L.; Preskitt, L.B. A survey of epiphytic dinoflagellates from the coastal waters of the island of Hawai’i. Harmful Algae 2007, 6, 658–669. [Google Scholar] [CrossRef]
- Institut de la Statistique de la Polynésie Française. Available online: https://www.ispf.pf/ (accessed on 27 October 2020).
- Tester, P.A.; Kibler, S.R.; Holland, W.C.; Usup, G.; Vandersea, M.W.; Leaw, C.P.; Teen, L.P.; Larsen, J.; Mohammad-Noor, N.; Faust, M.A.; et al. Sampling harmful benthic dinoflagellates: Comparison of artificial and natural substrate methods. Harmful Algae 2014, 39, 8–25. [Google Scholar] [CrossRef]
- Smith, K.F.; Biessy, L.; Argyle, P.A.; Trnski, T.; Halafihi, T.; Rhodes, L.L. Molecular identification of Gambierdiscus and Fukuyoa (Dinophyceae) from environmental samples. Mar. Drugs 2017, 15, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinain, M.; Darius, H.T.; Ung, A.; Cruchet, P.; Wang, Z.; Ponton, D.; Laurent, D.; Pauillac, S. Growth and toxin production in the ciguatera-causing dinoflagellate Gambierdiscus polynesiensis (Dinophyceae) in culture. Toxicon 2010, 56, 739–750. [Google Scholar] [CrossRef]
- Power, S.; Casey, T.; Folland, C.K.; Colman, A.; Mehta, V. Interdecadal modulation of the impact of ENSO on Australia. Clim. Dyn. 1999, 15, 319–323. [Google Scholar] [CrossRef]
- Huang, B.; Thorne, P.W.; Banzon, V.F.; Boyer, T.; Chepurin, G.; Lawrimore, J.H.; Menne, M.J.; Smith, T.M.; Vose, R.S.; Zhang, H. Extended reconstructed sea surface temperature, Version 5 (ERSSTv5): Upgrades, validations, and intercomparisons. J. Clim. 2017, 30, 8179–8205. [Google Scholar] [CrossRef]



| Year | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 1 | 2018 | 2019 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of CP events | 6 | 71 | 21 | 11 | 17 | 32 | 11 | 9 | 6 | - | 4 | 6 | |
| Number of reported CP cases | - | 7 | 87 | 27 | 13 | 17 | 33 | 11 | 15 | 7 | - | 6 | 7 |
| Number of unreported cases2 | - | 5 | 59 | 3 | 6 | 2 | 1 | - | 7 | 5 | - | 1 | 6 |
| Number of fatal cases | - | - | 2 3 | - | - | - | - | - | - | - | - | - | - |
| Incidence rates (IR)4 (reported cases/10,000 inhab.) | - | 145 | 1805 | 560 | 269 | 330 | 660 | 214 | 291 | 136 | - | 118 | 138 |
| Fish species involved (% of event) | |||||||||||||
| Seriola lalandi | - | 50 | 6 | 24 | 18 | - | 7 | - | 44 | 17 | - | 25 | 33 |
| Kyphosus cinerascens | - | 33 | 29 | 29 | 27 | 6 | 3 | - | - | 33 | - | - | 17 |
| Leptoscarus vaigiensis | - | - | 45 | 19 | 9 | 6 | 28 | 27 | - | - | - | - | 17 |
| Others: | - | 17 | 20 | 28 | 46 | 88 | 62 | 73 | 56 | 50 | - | 75 | 33 |
| Scombridae | 175 | 3 | |||||||||||
| Carangidae | 9 | 9 | 17 | 3 | 9 | 17 | 25 | ||||||
| Acanthuridae | 17 | 11 | |||||||||||
| Scaridae | 1 | 9 | 12 | 19 | 27 | 34 | 17 | 17 | |||||
| Serranidae | 3 | 9 | 12 | 34 | 18 | 11 | 50 | 16 | |||||
| miscellaneous | 13 | 10 | 28 | 30 | 6 | 19 | 16 | ||||||
| Fishing sites involved (% of event) | |||||||||||||
| Rāhui zone | - | 67 | 49 | 33 | 45 | 35 | 12 | 18 | 11 | 67 | - | 25 | 17 |
| Akatamiro Bay | - | - | 33 | - | - | 6 | 16 | 9 | - | - | - | - | 17 |
| Others: | - | - | 14 | 53 | 27 | 35 | 44 | 18 | 67 | - | - | 50 | 33 |
| Turoa Pari Ati Bay | 2 | 10 | |||||||||||
| Tapuaki Bay | 4 | 5 | |||||||||||
| Agaira’o Bay | 10 | ||||||||||||
| Kaongi | 5 | ||||||||||||
| Motu Tauturau | 10 | 11 | |||||||||||
| Akaomua Bay | 5 | 3 | |||||||||||
| Iripau Bay | 14 | 6 | 9 | 11 | |||||||||
| Ana Rua Bay | 5 | 9 | 9 | 25 | |||||||||
| Vavai Cliffs | 1 | 9 | 23 | 3 | 25 | ||||||||
| offshore | 7 | 9 | 9 | 12 | 12 | 45 | 33 | ||||||
| Unknown | - | 33 | 4 | 14 | 28 | 24 | 28 | 55 | 22 | 33 | 25 | 33 | |
| Year | 2009 | 2010 |
|---|---|---|
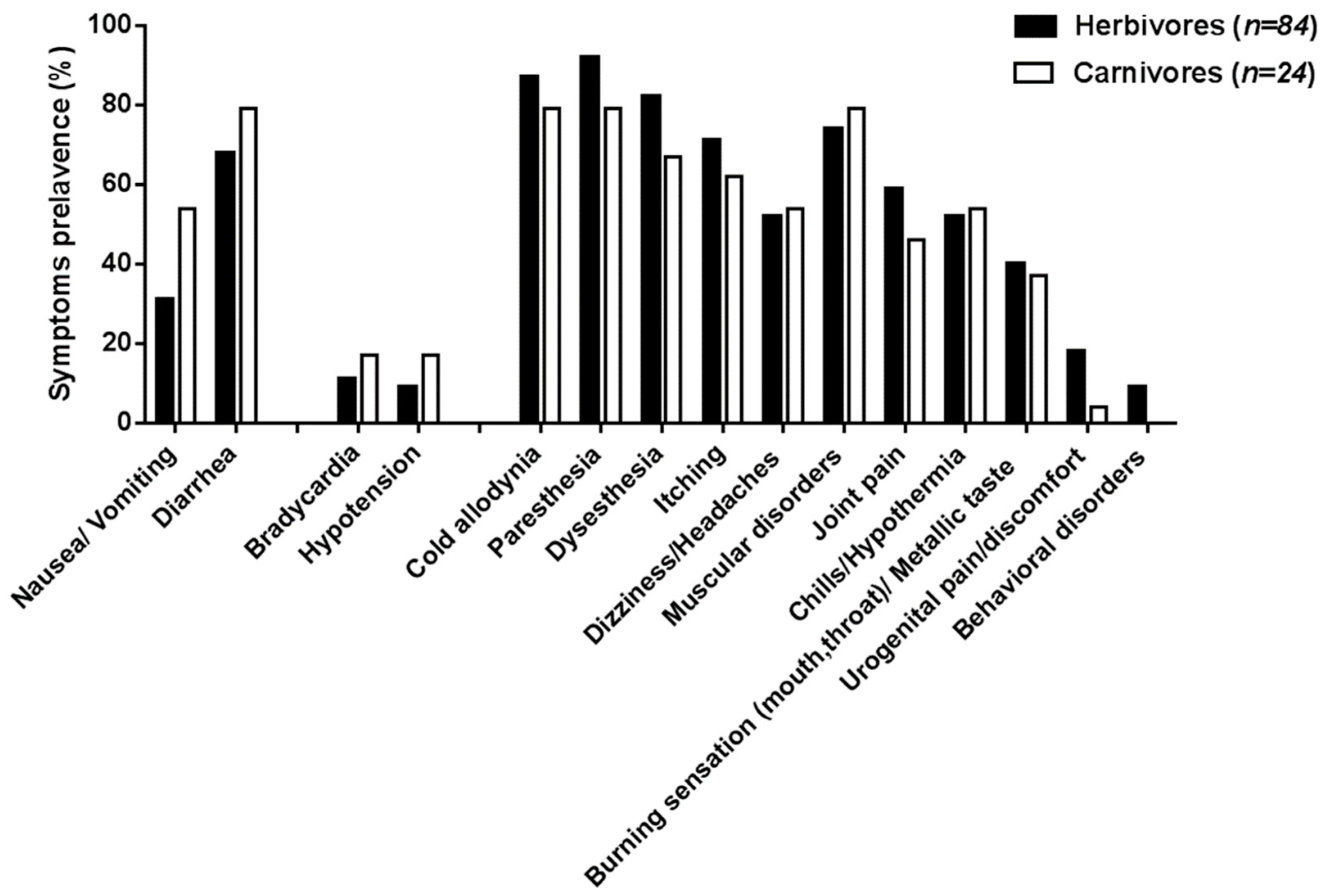
| Total number of CP cases reported | 87 | 27 |
| Digestive disorders | ||
| Nausea/vomiting | 28 (32%) | 11 (39%) |
| Diarrhea | 59 (68%) | 19 (68%) |
| Cardiovascular disorders 1 | ||
| Bradycardia | 10 (14%) 1 | 6 (22%) |
| Hypotension | 9 (12%) 1 | 3 (11%) |
| Neurological and systemic disorders | ||
| Cold allodynia | 80 (92%) | 15 (54%) |
| Paresthesia | 83 (95%) | 17 (61%) |
| Dysesthesia | 75 (86%) | 15 (54%) |
| Itching | 69 (79%) | 12 (43%) |
| Dizziness/headaches | 50 (57%) | 10 (36%) |
| Muscular disorders | 67 (77%) | 19 (68%) |
| Joint pains | 56 (64%) | 11 (39%) |
| Chills/hypothermia | 54 (62%) | 8 (29%) |
| Burning sensation (throat, mouth)/”metallic” taste | 43 (49%) | 4 (14%) |
| Urogenital burning/pain/discomfort | 15 (17%) | 4 (14%) |
| Behavioral disorders (agitation, disorientation) | 9 (10%) | 2 (7%) |
| Genus | Gambierdiscus spp. | Ostreopsis spp. | |||
|---|---|---|---|---|---|
| Sampling Sites | Macroalgal Host Species | Abundance (Cells) | CTX-Like Activity 2 | Macroalgal Host Species | Abundance (Cells) |
| Turoa Pari Ati Bay | Lobophora variegata | 6.1 × 106 | |||
| Akatamiro Bay | Lobophora variegata | 2250 | 13.5 | Lobophora variegata | 5.4 × 106 |
| Motu Aturapa | Dictyota bartayresiana | 13 × 106 | |||
| Cape Komire | Dictyota dichotoma | 10,300 | 0.5 | ||
| Cape Ongoriki | Sargassum sp. | 15.7 × 106 | |||
| Piriauta Bay 1 | Sargassum sp. | 19 × 106 | |||
| Motu Tarakoi 1 | Lobophora variegata | 5000 | 1.6 * | Sargassum sp. | 3.4 × 106 |
| Motu Rapa Iti 1 | Lobophora variegata | 15.8 × 106 | |||
| Anatakuri Bay 1 | Lobophora variegata | 2.2 × 106 | |||
| Motu Karapoo Nui | Lobophora variegata | 1.7 × 106 | |||
| Iripau Bay | Lobophora variegata | 10,350 | 3.5 | ||
| Sampling Site | ID # | Scientific Name | Diet | rRBA | CBA-N2a * | LC-MS/MS |
|---|---|---|---|---|---|---|
| Iripau Bay | 74 | Ctenochaetus striatus | H | 7.3 | 0.33 ± 0.05 | <LOQ |
| Akaomua Bay | 159 | Kyphosus cinerascens | H | <LOD | <LOD | <LOQ |
| Motu Ta’una | 143 | Kyphosus cinerascens | H | 1.4 | 0.52 ± 0.09 | <LOQ |
| Akaomua Bay | 161 | Chlorurus microrhinos | H | <LOD | 0.05 ± 0.01 | <LOD |
| Motu Rapa Iti | 209 | Chlorurus microrhinos | H | 7.8 | 0.31 ± 0.03 | <LOQ |
| Akaomua Bay | 163 | Leptoscarus vaigiensis | H | <LOD | <LOD | <LOD |
| Motu Rapa Iti | 214 | Leptoscarus vaigiensis | H | 5.0 | 4.75 ± 0.25 | 0.75 |
| Akaomua Bay | 167 | Epinephelus fasciatus | C | <LOD | <LOD | <LOD |
| Tapuaki Bay | 48 | Epinephelus fasciatus | C | 0.8 | 0.23 ± 0.02 | <LOD |
| Ahurei Bay | 229 | Epinephelus merra | C | 1.4 | 0.11 ± 0.02 | <LOQ |
| Anatakuri Bay | 198 | Monotaxis grandoculis | C | <LOD | 0.10 ± 0.01 | <LOD |
| Motu Rapa Iti | 211 | Pseudocaranx dentex | C | 1.0 | 1.18 ± 0.08 | <LOQ |
| Temperature Data Series | Kendall’s Tau | 2-Sided p Value | Alpha | Conclusion |
|---|---|---|---|---|
| Tmax | 0.202 (2.14) | 3.2 × 10−2 | 0.05 | HA |
| Tmin | 0.473 (5.07) | 3.58 × 10−7 | 0.05 | HA |
| Tmean | 0.347 (3.71) | 2.1 × 10−4 | 0.05 | HA |
| SST (ERSSTv5) | 0.067 (0.71) | 0.48 | 0.05 | H0 |
| Time Period (Scale) | GHRSST/ERSST | GHRSST/Tmax | GHRSST/Tmin | GHRSST/Tmean |
|---|---|---|---|---|
| 1998–2016 (month) | 0.91 | 0.95 | 0.92 | 0.94 |
| 1998–2016 (year) | 0.25 | 0.86 | 0.78 | 0.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinain, M.; Gatti, C.M.i.; Ung, A.; Cruchet, P.; Revel, T.; Viallon, J.; Sibat, M.; Varney, P.; Laurent, V.; Hess, P.; et al. Evidence for the Range Expansion of Ciguatera in French Polynesia: A Revisit of the 2009 Mass-Poisoning Outbreak in Rapa Island (Australes Archipelago). Toxins 2020, 12, 759. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12120759
Chinain M, Gatti CMi, Ung A, Cruchet P, Revel T, Viallon J, Sibat M, Varney P, Laurent V, Hess P, et al. Evidence for the Range Expansion of Ciguatera in French Polynesia: A Revisit of the 2009 Mass-Poisoning Outbreak in Rapa Island (Australes Archipelago). Toxins. 2020; 12(12):759. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12120759
Chicago/Turabian StyleChinain, Mireille, Clémence Mahana iti Gatti, André Ung, Philippe Cruchet, Taina Revel, Jérôme Viallon, Manoëlla Sibat, Patrick Varney, Victoire Laurent, Philipp Hess, and et al. 2020. "Evidence for the Range Expansion of Ciguatera in French Polynesia: A Revisit of the 2009 Mass-Poisoning Outbreak in Rapa Island (Australes Archipelago)" Toxins 12, no. 12: 759. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12120759










