Does Botulinum Toxin Treatment Affect the Ultrasonographic Characteristics of Post-Stroke Spastic Equinus? A Retrospective Pilot Study
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Clinical Evaluation
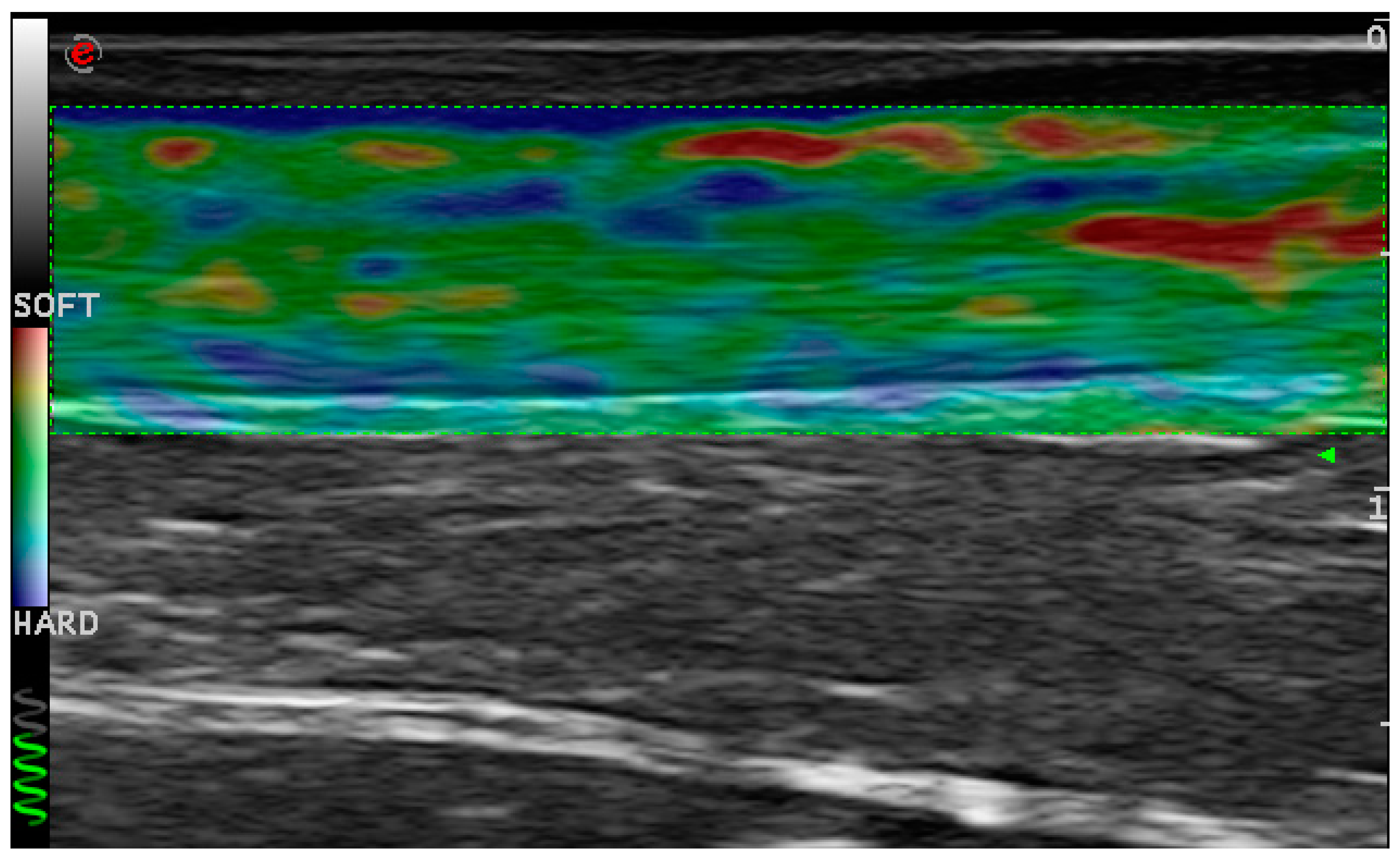
4.1.1. Ultrasonographic Evaluation
4.1.2. Clinical Evaluation
4.2. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simpson, D.M.; Hallett, M.; Ashman, E.J.; Comella, C.L.; Green, M.W.; Gronseth, G.S.; Armstrong, M.J.; Gloss, D.; Potrebic, S.; Jankovic, J.; et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the guideline development subcommittee of the American Academy of Neurology. Neurology 2016, 86, 1818–1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esquenazi, A.; Alfaro, A.; Ayyoub, Z.; Charles, D.; Dashtipour, K.; Graham, G.D.; McGuire, J.R.; Odderson, I.R.; Patel, A.T.; Simpson, D.M. OnabotulinumtoxinA for lower limb spasticity: Guidance from a Delphi panel approach. PM R 2017, 9, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Deltombe, T.; Wautier, D.; De Cloedt, P.; Fostier, M.; Gustin, T. Assessment and treatment of spastic equinovarus foot after stroke: Guidance from the Mont-Godinne interdisciplinary group. J. Rehabil. Med. 2017, 49, 461–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genêt, F.; Denormandie, P.; Keenan, M.A. Orthopaedic surgery for patients with central nervous system lesions: Concepts and techniques. Ann. Phys. Rehabil. Med. 2019, 62, 225–233. [Google Scholar] [CrossRef]
- Smania, N.; Picelli, A.; Munari, D.; Geroin, C.; Ianes, P.; Waldner, A.; Gandolfi, M. Rehabilitation procedures in the management of spasticity. Eur. J. Phys. Rehabil. Med. 2010, 46, 423–438. [Google Scholar] [PubMed]
- Picelli, A.; Santamato, A.; Chemello, E.; Cinone, N.; Cisari, C.; Gandolfi, M.; Ranieri, M.; Smania, N.; Baricich, A. Adjuvant treatments associated with botulinum toxin injection for managing spasticity: An overview of the literature. Ann. Phys. Rehabil. Med. 2019, 62, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Picelli, A.; Tamburin, S.; Cavazza, S.; Scampoli, C.; Manca, M.; Cosma, M.; Berto, G.; Vallies, G.; Roncari, L.; Melotti, C.; et al. Relationship between ultrasonographic, electromyographic, and clinical parameters in adult stroke patients with spastic equinus: An observational study. Arch. Phys. Med. Rehabil. 2014, 95, 1564–1570. [Google Scholar] [CrossRef] [PubMed]
- Picelli, A.; Bonetti, P.; Fontana, C.; Barausse, M.; Dambruoso, F.; Gajofatto, F.; Girardi, P.; Manca, M.; Gimigliano, R.; Smania, N. Is spastic muscle echo intensity related to the response to botulinum toxin type A in patients with stroke? A cohort study. Arch. Phys. Med. Rehabil. 2012, 93, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.M.; Jennekens, F.G.; Veldman, H. Botulinum toxin-induced myopathy in the rat. Brain 1995, 118, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.S.; Ertl-Wagner, B.; Britsch, S.; Schröder, J.M.; Nikolin, S.; Weis, J.; Müller-Felber, W.; Koerte, I.; Stehr, M.; Berweck, S.; et al. Muscle biopsy substantiates long-term MRI alterations one year after a single dose of botulinum toxin injected into the lateral gastrocnemius muscle of healthy volunteers. Mov. Disord. 2009, 24, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Tok, F.; Ozçakar, L.; Safaz, I.; Alaca, R. Effects of botulinum toxin-A on the muscle architecture of stroke patients: The first ultrasonographic study. J. Rehabil. Med. 2011, 43, 1016–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patrick, E.; Ada, L. The Tardieu Scale differentiates contracture from spasticity whereas the Ashworth Scale is confounded by it. Clin. Rehabil. 2006, 20, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Freire, B.; Dias, C.P.; Goulart, N.B.; de Castro, C.D.; Becker, J.; Gomes, I.; Vaz, M.A. Achilles tendon morphology, plantar flexors torque and passive ankle stiffness in spastic hemiparetic stroke survivors. Clin. Biomech. 2017, 41, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Ankle and foot spasticity patterns in chronic stroke survivors with abnormal gait. Toxins 2020, 12, 646. [Google Scholar] [CrossRef] [PubMed]
- Picelli, A.; La Marchina, E.; Gajofatto, F.; Pontillo, A.; Vangelista, A.; Filippini, R.; Baricich, A.; Cisari, C.; Smania, N. Sonographic and clinical effects of botulinum toxin type A combined with extracorporeal shock wave therapy on spastic muscles of children with cerebral palsy. Dev. Neurorehabil. 2017, 20, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Picelli, A.; Bonetti, P.; Fontana, C.; Barausse, M.; Dambruoso, F.; Gajofatto, F.; Tamburin, S.; Girardi, P.; Gimigliano, R.; Smania, N. Accuracy of botulinum toxin type A injection into the gastrocnemius muscle of adults with spastic equinus: Manual needle placement and electrical stimulation guidance compared using ultrasonography. J. Rehabil. Med. 2012, 44, 450–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picelli, A.; Chemello, E.; Verzini, E.; Ferrari, F.; Brugnera, A.; Gandolfi, M.; Saltuari, L.; Modenese, A.; Smania, N. Anatomical landmarks for tibial nerve motor branches in the management of spastic equinovarus foot after stroke: An ultrasonographic study. J. Rehabil. Med. 2019, 51, 380–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Patients’ Feature | |
|---|---|
| Age (years) mean (SD) | 65.6 (14.5) |
| Gender (n) male/female | 15/6 |
| Time since stroke onset (years) mean (SD) | 1.7 (0.9) |
| Stroke etiology (n) ischemic/hemorrhagic | 16/5 |
| Lesion location (n) cortica/subcortica/mixed | 4/6/11 |
| Outcomes | Before Treatment T0 | After Treatment T1 | T1 vs. T0 p Value (95% CI) |
|---|---|---|---|
| Gastrocnemius medialis echo intensity (1–4) median (IQR) | 2.0 (1.0; 3.0) | 2.0 (1.0; 2.5) | 0.705 (−0.32; 0.22) |
| Gastrocnemius lateralis echo intensity (1–4) median (IQR) | 2.0 (1.0; 3.0) | 2.0 (1.0; 2.5) | 0.739 (−0.35; 0.26) |
| Gastrocnemius medialis thickness (mm) mean (SD) | 14.7 (3.3) | 15.4 (3.0) | 0.225 (−0.33; 1.51) |
| Gastrocnemius lateralis thickness (mm) mean (SD) | 11.2 (3.1) | 11.1 (2.6) | 0.823 (−1.33; 0.99) |
| Gastrocnemius medialis pennation angle (°) mean (SD) | 20.8 (7.3) | 21.9 (7.2) | 0.227 (−0.56; 3.70) |
| Gastrocnemius lateralis pennation angle (°) mean (SD) | 17.4 (5.7) | 17.6 (4.9) | 0.709 (−2.53; 2.39) |
| Achilles tendon thickness (mm) mean (SD) | 5.1 (0.7) | 5.2 (0.6) | 0.872 (−0.23; 0.38) |
| Achilles tendon %HRD mean (SD) | 65.9 (12.2) | 60.6 (16.4) | 0.094 (−12.31; 2.72) |
| Ankle dorsiflexion PROM (°) mean (SD) | −6.4 (7.3) | −2.2 (5.6) | 0.002 (1.57; 7.01) * |
| Calf muscle spasticity (Ashworth scale, 0–4) median (IQR) | 2.0 (2.0; 3.0) | 2.0 (1.0; 3.0) | 0.008 (−0.87; −0.18) * |
| Calf muscle spasticity (Tardieu grade, 0–4) median (IQR) | 2.0 (1.5; 2.0) | 2.0 (1.0; 2.0) | 0.008 (−0.55; −0.11) * |
| Calf muscle spasticity (Tardieu angle, °) mean (SD) | 16.9 (5.4) | 10.2 (4.3) | <0.001 (−8.87; −4.47) * |
| Study Variables | Muscle Echogenicity | Muscle Thickness | Pennation Angle | Achilles Tendon Thickness | Achilles Tendon %HRD | |||
|---|---|---|---|---|---|---|---|---|
| GM | GL | GM | GL | GM | GL | |||
| Age | 0.006 | −0.062 | 0.128 | −0.062 | −0.173 | −0.509 * | 0.122 | 0.463 * |
| Time since onset | 0.458 * | 0.594 * | −0.046 | −0.089 | −0.083 | −0.241 | −0.127 | −0.339 |
| Ankle dorsiflexion PROM | 0.076 | −0.111 | 0.011 | 0.227 | −0.185 | −0.137 | −0.016 | −0.171 |
| Calf muscle spasticity | ||||||||
| Ashworth scale | −0.017 | 0.284 | −0.047 | 0.031 | −0.035 | 0.236 | 0.134 | 0.441 * |
| Tardieu grade | 0.238 | 0.176 | −0.387 | −0.251 | −0.093 | 0.181 | −0.139 | −0.048 |
| Tardieu angle | −0.067 | 0.177 | −0.087 | 0.241 | −0.110 | −0.051 | −0.226 | −0.073 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picelli, A.; Filippetti, M.; Melotti, C.; Guerrazzi, F.; Modenese, A.; Smania, N. Does Botulinum Toxin Treatment Affect the Ultrasonographic Characteristics of Post-Stroke Spastic Equinus? A Retrospective Pilot Study. Toxins 2020, 12, 797. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12120797
Picelli A, Filippetti M, Melotti C, Guerrazzi F, Modenese A, Smania N. Does Botulinum Toxin Treatment Affect the Ultrasonographic Characteristics of Post-Stroke Spastic Equinus? A Retrospective Pilot Study. Toxins. 2020; 12(12):797. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12120797
Chicago/Turabian StylePicelli, Alessandro, Mirko Filippetti, Camilla Melotti, Flavio Guerrazzi, Angela Modenese, and Nicola Smania. 2020. "Does Botulinum Toxin Treatment Affect the Ultrasonographic Characteristics of Post-Stroke Spastic Equinus? A Retrospective Pilot Study" Toxins 12, no. 12: 797. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12120797







