Orally Administered Fumonisins Affect Porcine Red Cell Membrane Sodium Pump Activity and Lipid Profile without Apparent Oxidative Damage
Abstract
:1. Introduction
2. Results
2.1. Body Weight, Organ Masses
2.1.1. Inter-Group Differences and Dose Response
2.1.2. Sodium Pump Activity Correlations
2.2. Red Cell Membrane Sodium Pump Activity and Dose Response
Inter-Group Differences and Dose Response
2.3. Red Cell Fatty Acid Profile
2.3.1. Inter-Group Differences and Dose Response
2.3.2. Sodium Pump Activity Correlations
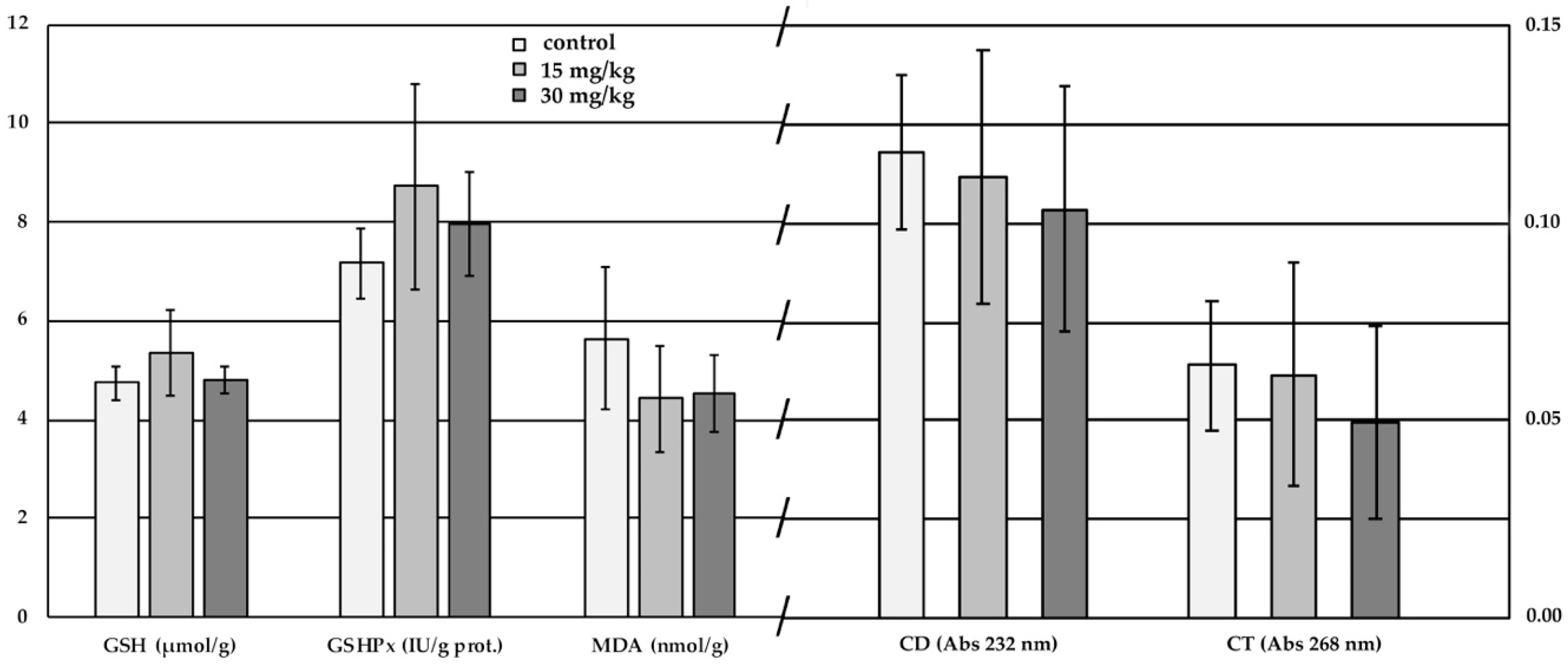
2.4. Red Cell Antioxidant Status and Lipid Peroxidation
3. Discussion
3.1. Body Weight, Organ Masses
Inter-Group Differences and Dose Response
3.2. Red Cell Membrane Sodium Pump Activity and Dose Response
3.3. Red Cell Fatty Acid Profile
3.3.1. Inter-Group Differences, Dose Response and Sodium Pump Activity Correlations
3.3.2. Sodium Pump Activity Correlations
3.4. Red Cell Antioxidant Status and Lipid Peroxidation
4. Conclusions
5. Materials and Methods
5.1. Experimental Design and Animals
5.2. Feed Mycotoxin Contamination
5.3. Blood Sampling, Erythrocythe “Ghost” Preparation
5.4. Determination of the RBC Na+/K+ ATPase Activity
5.5. Determination of the RBC Membrane Fatty Acid Composition
5.6. Red Cell Antioxidant Status and Lipid Peroxidation
5.7. Statistical Analysis
5.8. Ethical Issues
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rheeder, J.P.; Marasas, W.F.O.; Vismer, H.F. Production of fumonisin analogs by Fusarium species. Appl. Environ. Microbiol. 2002, 68, 2101–2105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Fan, L.; Yin, S.; Chen, H.; Hu, H. Molecular mechanisms of fumonisin B1-induced toxicities and its applications in the mechanism-based interventions. Toxicon 2019, 167, 1–5. [Google Scholar] [CrossRef] [PubMed]
- BIOMIN Holding GmbH, Getzersdorf, Austria. Available online: https://www.biomin.net/science-hub/world-mycotoxin-survey-impact-2020/ (accessed on 19 April 2020).
- Haschek, W.M.; Gumprecht, L.A.; Smith, G.; Tumbleson, M.E.; Constable, P.D. Fumonisin toxicosis in swine: An overview of porcine pulmonary edema and current perspectives. Environ. Health Perspect. 2001, 109, 251–257. [Google Scholar] [PubMed]
- Gumprecht, L.A.; Beasley, V.R.; Weigel, R.M.; Parker, H.M.; Tumbleson, M.E.; Bacon, C.W.; Meredith, F.I.; Haschek, W.M. Development of fumonisin-induced hepatotoxicity and pulmonary edema in orally dosed swine: Morphological and biochemical alterations. Toxicol. Pathol. 1998, 26, 777–788. [Google Scholar] [CrossRef]
- Stockmann-Juvala, H.; Savolainen, K. A review of the toxic effects and mechanisms of action of fumonisin B 1. Hum. Exp. Toxicol. 2008, 27, 799–809. [Google Scholar] [CrossRef]
- Loiseau, N.; Polizzi, A.; Dupuy, A.; Therville, N.; Rakotonirainy, M.; Loy, J.; Viadere, J.L.; Cossalter, A.M.; Bailly, J.D.; Puel, O.; et al. New insights into the organ-specific adverse effects of fumonisin B1: Comparison between lung and liver. Arch. Toxicol. 2015, 89, 1619–1629. [Google Scholar] [CrossRef]
- Wang, E.; Norred, W.P.; Bacon, C.W.; Riley, R.T.; Merrill, A.H. Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. J. Biol. Chem. 1991, 266, 14486–14490. [Google Scholar]
- Szabó, A.; Szabó-Fodor, J.; Fébel, H.; Romvári, R.; Kovács, M. Individual and combined haematotoxic effects of fumonisin B1 and T-2 mycotoxins in rabbits. Food Chem. Toxicol. 2014, 72, 257–264. [Google Scholar] [CrossRef]
- Haschek, W.M.; Motelin, G.; Ness, D.K.; Harlin, K.S.; Hall, W.F.; Vesonder, R.F.; Peterson, R.E.; Beasley, V.R. Characterization of fumonisin toxicity in orally and intravenously dosed swine. Mycopathologia 1992, 117, 83–96. [Google Scholar] [CrossRef]
- Mays, R.W.; Siemers, K.A.; Fritz, B.A.; Lowe, A.W.; Van Meer, G.; Nelson, W.J. Hierarchy of mechanisms involved in generating Na/K-ATPase polarity in MDCK epithelial cells. J. Cell Biol. 1995, 130, 1105–1115. [Google Scholar] [CrossRef] [Green Version]
- Terciolo, C.; Bracarense, A.P.; Souto, P.C.M.C.; Cossalter, A.M.; Dopavogui, L.; Loiseau, N.; Oliveira, C.A.F.; Pinton, P.; Oswald, I.P. Fumonisins at doses below EU regulatory limits induce histological alterations in piglets. Toxins 2019, 11, 548. [Google Scholar] [CrossRef] [Green Version]
- Ali, O.; Szabó-Fodor, J.; Fébel, H.; Mézes, M.; Balogh, K.; Glávits, R.; Kovács, M.; Zantomasi, A.; Szabó, A. Porcine hepatic response to fumonisin b1 in a short exposure period: Fatty acid profile and clinical investigations. Toxins 2019, 11, 655. [Google Scholar] [CrossRef] [Green Version]
- Szabó, A.; Szabó-Fodor, J.; Kachlek, M.; Mézes, M.; Balogh, K.; Glávits, R.; Ali, O.; Zeebone, Y.Y.; Kovács, M. Dose and exposure time-dependent renal and hepatic effects of intraperitoneally administered fumonisin B1 in rats. Toxins 2018, 10, 465. [Google Scholar]
- Szabó, A.; Fébel, H.; Ali, O.; Kovács, M. Fumonisin B1 induced compositional modifications of the renal and hepatic membrane lipids in rats–Dose and exposure time dependence. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2019, 36, 1722–1739. [Google Scholar] [CrossRef] [PubMed]
- Szabó, A.; Szabó-Fodor, J.; Fébel, H.; Mézes, M.; Repa, I.; Kovács, M. Acute hepatic effects of low-dose fumonisin B1 in rats. Acta Vet. Hung. 2016, 64, 436–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Song, G.; Lim, W. Effects of mycotoxin-contaminated feed on farm animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, H.; Li, X.; Yuan, Q.; Su, J.; Yang, L.; Ning, L.; Lei, H. Fumonisin B1 damages the barrier functions of porcine intestinal epithelial cells in vitro. J. Biochem. Mol. Toxicol. 2019, 33, e22397. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.T.; Torres, O.; Matute, J.; Gregory, S.G.; Ashley-Koch, A.E.; Showker, J.L.; Mitchell, T.; Voss, K.A.; Maddox, J.R.; Gelineau-van Waes, J.B. Evidence for fumonisin inhibition of ceramide synthase in humans consuming maize-based foods and living in high exposure communities in Guatemala. Mol. Nutr. Food Res. 2015, 59, 2209–2224. [Google Scholar] [CrossRef] [Green Version]
- Riley, R.T.; Voss, K.A.; Yoo, H.S.; Gelderblom, W.C.A.; Merrill Jnr, A.H. Mechanism of fumonisin toxicity and carcinogenesis. J. Food Prot. 1994, 57, 638–645. [Google Scholar] [CrossRef]
- Zitomer, N.C.; Mitchell, T.; Voss, K.A.; Bondy, G.S.; Pruett, S.T.; Garnier-Amblard, E.C.; Liebeskind, L.S.; Park, H.; Wang, E.; Sulllards, M.C.; et al. Ceramide synthase inhibition by fumonisin B1 causes accumulation of 1-deoxysphinganine. A novel category of bioactive 1-deoxysphingoid bases and 1-deoxydihydroceramides biosynthesized by mammalian cell lines and animals. J. Biol. Chem. 2009, 284, 4786–4795. [Google Scholar] [CrossRef] [Green Version]
- Cabral, L.M.P.; Wengert, M.; Almeida, F.G.; Caruso-Neves, C.; Vieyra, A.; Einicker-Lamas, M. Ceramide-activated protein kinases A and C zeta inhibit kidney proximal tubule cell Na+-ATPase. Arch. Biochem. Biophys. 2010, 498, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Kreydiyyeh, S.I.; Dakroub, Z. Ceramide and its metabolites modulate time-dependently the activity of the Na+/K+ ATPase in HepG2 cells. Int. J. Biochem. Cell Biol. 2014, 53, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Hughes, F.M.; Bortner, C.D.; Purdy, G.D.; Cidlowski, J.A. Intracellular K+ suppresses the activation of apoptosis in lymphocytes. J. Biol. Chem. 1997, 272, 30567–30576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, B.J.; Hulbert, A.J.; Storlien, L.H.; Else, P.L. Membrane lipids and sodium pumps of cattle and crocodiles: An experimental test of the membrane pacemaker theory of metabolism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R633–R641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Z.; Guo, H.; Wang, Y. Na+/K+-ATPase-mediated signal transduction and Na+/K+-ATPase regulation. Fundam. Clin. Pharmacol. 2008, 22, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Colina, C.; Cervino, V.; Benaim, G. Ceramide and sphingosine have an antagonistic effect on the plasma-membrane Ca2+-ATPase from human erythrocytes. Biochem. J. 2002, 362, 247–251. [Google Scholar] [CrossRef]
- Carafoli, E. Biogenesis: Plasma membrane calcium ATPase: 15 years of work on the purified enzyme 1. FASEB J. 1994, 8, 993–1002. [Google Scholar] [CrossRef]
- Nobel, C.S.I.; Aronson, J.K.; Van Den Dobbelsteen, D.J.; Slater, A.F.G. Inhibition of Na+/K+-ATPase may be one mechanism contributing to potassium efflux and cell shrinkage in CD95-induced apoptosis. Apoptosis 2000, 5, 153–163. [Google Scholar] [CrossRef]
- Arrebola, F.; Zabiti, S.; Cañizares, F.J.; Cubero, M.A.; Crespo, P.V.; Fernández-Segura, E. Changes in intracellular sodium, chlorine, and potassium concentrations in staurosporine-induced apoptosis. J. Cell. Physiol. 2005, 204, 500–507. [Google Scholar] [CrossRef]
- Martínez-Mas, J.V.; Peinado-Onsurbe, J.; Ruiz-Montasell, B.; Felipe, A.; Casado, F.J.; Pastor-Anglada, M. Na+,K+-ATPase expression during the early phase of liver growth after partial hepatectomy. FEBS Lett. 1995, 362, 85–88. [Google Scholar] [CrossRef] [Green Version]
- Therien, A.G.; Blostein, R. Mechanisms of sodium pump regulation. Am. J. Physiol.-Cell Physiol. 2000, 279, C541–C566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Else, P.L.; Wu, B.J.; Storlien, L.H.; Hulbert, A.J. Molecular activity of Na+,K+-ATPase relates to the packing of membrane lipids. In Annals of the New York Academy of Sciences; New York Academy of Sciences: New York, NY, USA, 2003; Volume 986, pp. 525–526. [Google Scholar]
- Dwight, J.F.S.J.; Mendes Ribeiro, A.C.; Hendry, B.M. Membrane incorporation of non-esterified fatty acids and effects on the sodium pump of human erythrocytes. Clin. Sci. 1992, 82, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Dwight, J.F.S.J.; Hendry, B.M. Effects of membrane incorporation of short-chain phospholipids on sodium pump function in human erythrocytes. Clin. Chim. Acta 1995, 243, 73–85. [Google Scholar] [CrossRef]
- Christie, W.W. A simple procedure for rapid transmethylation of glycerolipids and cholesteryl esters. J. Lipid Res. 1982, 23, 1072–1075. [Google Scholar]
- Yin, J.J.; Smith, M.J.; Eppley, R.M.; Page, S.W.; Sphon, J.A. Effects of fumonisin B1 on lipid peroxidation in membranes. Biochim. Biophys. Acta-Biomembr. 1998, 1371, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Fodor, J.; Balogh, K.; Weber, M.; Miklós, M.; Kametler, L.; Pósa, R.; Mamet, R.; Bauer, J.; Horn, P.; Kovács, F.; et al. Absorption, distribution and elimination of fumonisin B(1) metabolites in weaned piglets. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2008, 25, 88–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemens, M.R.; Waller, H.D. Lipid peroxidation in erythrocytes. Chem. Phys. Lipids 1987, 45, 251–268. [Google Scholar] [CrossRef]
- Fodor, J.; Kametier, L.; Kovács, M. Practical aspects of fumonisin production under laboratory conditions. Mycotoxin Res. 2006, 22, 211–216. [Google Scholar] [CrossRef]
- Bartók, T.; Tölgyesi, L.; Szekeres, A.; Varga, M.; Bartha, R.; Szécsi, Á.; Bartók, M.; Mesterházy, Á. Detection and characterization of twenty-eight isomers of fumonisin B1 (FB1) mycotoxin in a solid rice culture infected with Fusarium verticillioides by reversed-phase high-performance liquid chromatography/electrospray ionization time-of-flight and ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 35–42. [Google Scholar]
- Shanmugasundaram, K.R.; Padmavathi, C.; Acharya, S.; Vidhyalakshmi, N.; Vijayan, V.K. Exercise-induced cholesterol depletion and Na+,K+-ATPase activities in human red cell membrane. Exp. Physiol. 1992, 77, 933–936. [Google Scholar] [CrossRef] [Green Version]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Djemli-Shipkolye, A.; Raccah, D.; Pieroni, G.; Vague, P.; Coste, T.C.; Gerbi, A. Differential effect of ω3 PUFA supplementations on Na,K-ATPase and Mg-ATPase activities: Possible role of the membrane ω6/ω3 ratio. J. Membr. Biol. 2003, 191, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Bedin, M.; Helena, C.; Estrella, G.; Ponzi, D.; Duarte, D.V.; Dutra-Filho, C.S.; Wyse, A.T.S.; Wajner, M.; Wannmacher, C.M.D. Reduced Na+,K+-ATPase activity in erythrocyte membranes from patients with phenylketonuria. Pediatr. Res. 2001, 50, 56–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurst, R.O. The determination of nucleotide phosphorus with stannous chloride-hydrazine sulphate reagent. Can. J. Biochem. Physiol. 1964, 42, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Bełtowski, J.; Wójcicka, G. Spectrophotometric method for the determination of renal ouabain-sensitive H+,K+-ATPase activity. Acta Biochim. Pol. 2002, 49, 515–527. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Botsoglou, N.A.; Fletouris, D.J.; Papageorgiou, G.E.; Vassilopoulos, V.N.; Mantis, A.J.; Trakatellis, A.G. Rapid, sensitive and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food and feedstuff samples. J. Agric. Food Chem. 1994, 42, 1931–1937. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis (28.054), 14th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1984. [Google Scholar]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Lawrence, R.A.; Burk, R.F. Species, Tissue and Subcellular Distribution of Non Se-Dependent Glutathione Peroxidase Activity. J. Nutr. 1978, 108, 211–215. [Google Scholar] [CrossRef]
- SPSS. SPSS for Windows ver. 20; SPSS: Chicago, IL, USA, 2012. [Google Scholar]

| Group | Control | 15 mg/kg | 30 mg/kg | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatic Traits | Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | |||
| BW initial (g) | 12,980 | ± | 1720 | 13,800 | ± | 1200 | 13,800 | ± | 1340 | |||
| BW final (g) | 21,467 | ± | 1735 | a | 23,067 | ± | 1454 | ab | 23,367 | ± | 629 | b |
| cumulative feed intake (g) | 19,759 | ± | 2102 | 20,450 | ± | 1352 | 20,382 | ± | 1352 | |||
| lung (g) | 227.2 | ± | 45.0 | 237.1 | ± | 43.0 | 253.0 | ± | 70.0 | |||
| liver (g) | 527.4 | ± | 42.1 | 587.2 | ± | 56.9 | 563.0 | ± | 96.2 | |||
| kidney (g) | 84.1 | ± | 2.53 | 86.2 | ± | 6.83 | 79.4 | ± | 10.6 | |||
| pancreas (g) | 50.0 | ± | 6.39 | 57.1 | ± | 7.62 | 56.5 | ± | 9.81 | |||
| lung (% of BW) | 1.06 | ± | 0.20 | 1.03 | ± | 0.15 | 1.09 | ± | 0.30 | |||
| liver (% of BW) | 2.48 | ± | 0.38 | 2.55 | ± | 0.23 | 2.42 | ± | 0.45 | |||
| kindey (% of BW) | 0.39 | ± | 0.03 | b | 0.37 | ± | 0.04 | ab | 0.34 | ± | 0.05 | a |
| pancreas (% of BW) | 0.23 | ± | 0.04 | 0.25 | ± | 0.04 | 0.24 | ± | 0.04 | |||
| Group | Control | 15 mg/kg | 30 mg/kg | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Red Cell Membrane FA Profile | Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | |||
| C12:0 | 0.04 | ± | 0.01 | b | 0.02 | ± | 0.01 | a | 0.02 | ± | 0.00 | ab |
| C14:0 | 0.52 | ± | 0.04 | 0.50 | ± | 0.03 | 0.52 | ± | 0.05 | |||
| C15:0 | 0.10 | ± | 0.05 | 0.14 | ± | 0.08 | 0.09 | ± | 0.02 | |||
| C16:0 | 43.6 | ± | 0.97 | ab | 42.6 | ± | 1.69 | a | 44.5 | ± | 1.33 | b |
| C16:1n7 | 0.08 | ± | 0.02 | 0.11 | ± | 0.04 | 0.09 | ± | 0.04 | |||
| C17:0 | 0.40 | ± | 0.12 | 0.59 | ± | 0.25 | 0.38 | ± | 0.05 | |||
| C18:0 | 48.7 | ± | 1.52 | 46.7 | ± | 2.86 | 48.8 | ± | 1.63 | |||
| C18:1n9c | 3.90 | ± | 1.64 | ab | 6.15 | ± | 2.68 | b | 2.99 | ± | 0.72 | a |
| C18:1n7 | 0.23 | ± | 0.10 | 0.33 | ± | 0.18 | 0.17 | ± | 0.03 | |||
| C18:2n6 | 0.96 | ± | 0.70 | 1.54 | ± | 1.29 | 0.37 | ± | 0.11 | |||
| C18:3n3 | 0.02 | ± | 0.00 | 0.02 | ± | 0.01 | 0.03 | ± | 0.00 | |||
| C20:0 | 0.45 | ± | 0.03 | 0.43 | ± | 0.05 | 0.43 | ± | 0.03 | |||
| C20:1n9 | 0.02 | ± | 0.00 | 0.02 | ± | 0.01 | 0.02 | ± | 0.00 | |||
| C20:2n6 | 0.02 | ± | 0.01 | 0.02 | ± | 0.01 | 0.01 | ± | 0.01 | |||
| C20:3n6 | 0.02 | ± | 0.01 | ab | 0.03 | ± | 0.02 | b | 0.01 | ± | 0.00 | a |
| C21:0 | 0.03 | ± | 0.02 | 0.03 | ± | 0.02 | 0.04 | ± | 0.02 | |||
| C20:4n6 | 0.25 | ± | 0.17 | ab | 0.46 | ± | 0.33 | b | 0.10 | ± | 0.04 | a |
| C22:0 | 0.07 | ± | 0.01 | 0.06 | ± | 0.01 | 0.06 | ± | 0.00 | |||
| C22:6n3 | 0.15 | ± | 0.05 | 0.18 | ± | 0.04 | 0.15 | ± | 0.06 | |||
| C24:1n9 | 0.08 | ± | 0.08 | 0.09 | ± | 0.08 | 0.08 | ± | 0.07 | |||
| Σ saturated | 93.1 | ± | 0.91 | ab | 91.0 | ± | 4.32 | a | 96.0 | ± | 0.94 | b |
| Σ unsaturated | 6.92 | ± | 0.91 | 8.95 | ± | 4.32 | 3.96 | ± | 0.94 | |||
| Σ MUFA | 5.32 | ± | 0.63 | ab | 6.70 | ± | 2.92 | b | 3.34 | ± | 0.78 | a |
| Σ PUFA | 1.40 | ± | 0.87 | 2.25 | ± | 1.68 | 0.63 | ± | 0.17 | |||
| Σ n3 | 0.15 | ± | 0.05 | 0.19 | ± | 0.05 | 0.16 | ± | 0.06 | |||
| Σ n6 | 1.25 | ± | 0.88 | 2.06 | ± | 1.64 | 0.49 | ± | 0.16 | |||
| Σ n6/Σ n3 | 9.38 | ± | 7.30 | 9.70 | ± | 6.59 | 3.77 | ± | 1.49 | |||
| Σ odd chain FA | 0.50 | ± | 0.17 | 0.72 | ± | 0.33 | 0.47 | ± | 0.06 | |||
| Compound | Sig. | Pearson Corr. Coeff. |
|---|---|---|
| C18:2 n6 | 0.017 | −0.671 |
| C20:2 n6 | 0.001 | −0.821 |
| C20:3 n6 | 0.022 | −0.65 |
| C20:4 n6 | 0.023 | −0.648 |
| Σ PUFA | 0.012 | −0.697 |
| Σ n6 | 0.014 | −0.683 |
| Crude protein (%) | 17.50 |
| Crude fat (%) | 3.30 |
| Crude fiber (%) | 3.70 |
| Crude ash (%) | 5.00 |
| Lysine (g/kg) | 1.11 |
| Methionine (g/kg) | 0.37 |
| Ca (g/kg) | 0.65 |
| P (g/kg) | 0.50 |
| Na (g/kg) | 0.18 |
| DE (MJ/kg) | 14.70 |
| ME (MJ/kg) | 14.10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, A.; Ali, O.; Lóki, K.; Balogh, K.; Mézes, M.; Bartók, T.; Horváth, L.; Kovács, M. Orally Administered Fumonisins Affect Porcine Red Cell Membrane Sodium Pump Activity and Lipid Profile without Apparent Oxidative Damage. Toxins 2020, 12, 318. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12050318
Szabó A, Ali O, Lóki K, Balogh K, Mézes M, Bartók T, Horváth L, Kovács M. Orally Administered Fumonisins Affect Porcine Red Cell Membrane Sodium Pump Activity and Lipid Profile without Apparent Oxidative Damage. Toxins. 2020; 12(5):318. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12050318
Chicago/Turabian StyleSzabó, András, Omeralfaroug Ali, Katalin Lóki, Krisztián Balogh, Miklós Mézes, Tibor Bartók, Levente Horváth, and Melinda Kovács. 2020. "Orally Administered Fumonisins Affect Porcine Red Cell Membrane Sodium Pump Activity and Lipid Profile without Apparent Oxidative Damage" Toxins 12, no. 5: 318. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12050318





