Nematicidal Activity of Stevia rebaudiana (Bertoni) Assisted by Phytochemical Analysis
Abstract
:1. Introduction
2. Results
2.1. Soil Amending with S. rebaudiana Leaves Powder (LP) and Wood Powder (WP) to Treat against M. incognita and Subsequent Biofertilization in Tomato Plants: A Dose-Response
2.2. Paralysis Effect of Leaves Water Extract (LWE) and Wood Water Extract (WWE) on the Plant Parasitic Nematode M. incognita and M. javanica Second Stage Juveniles (J2)
2.3. Chemical Composition Analyses of LWE and EO; S. rebaudiana Glycosides and Terpenes Content
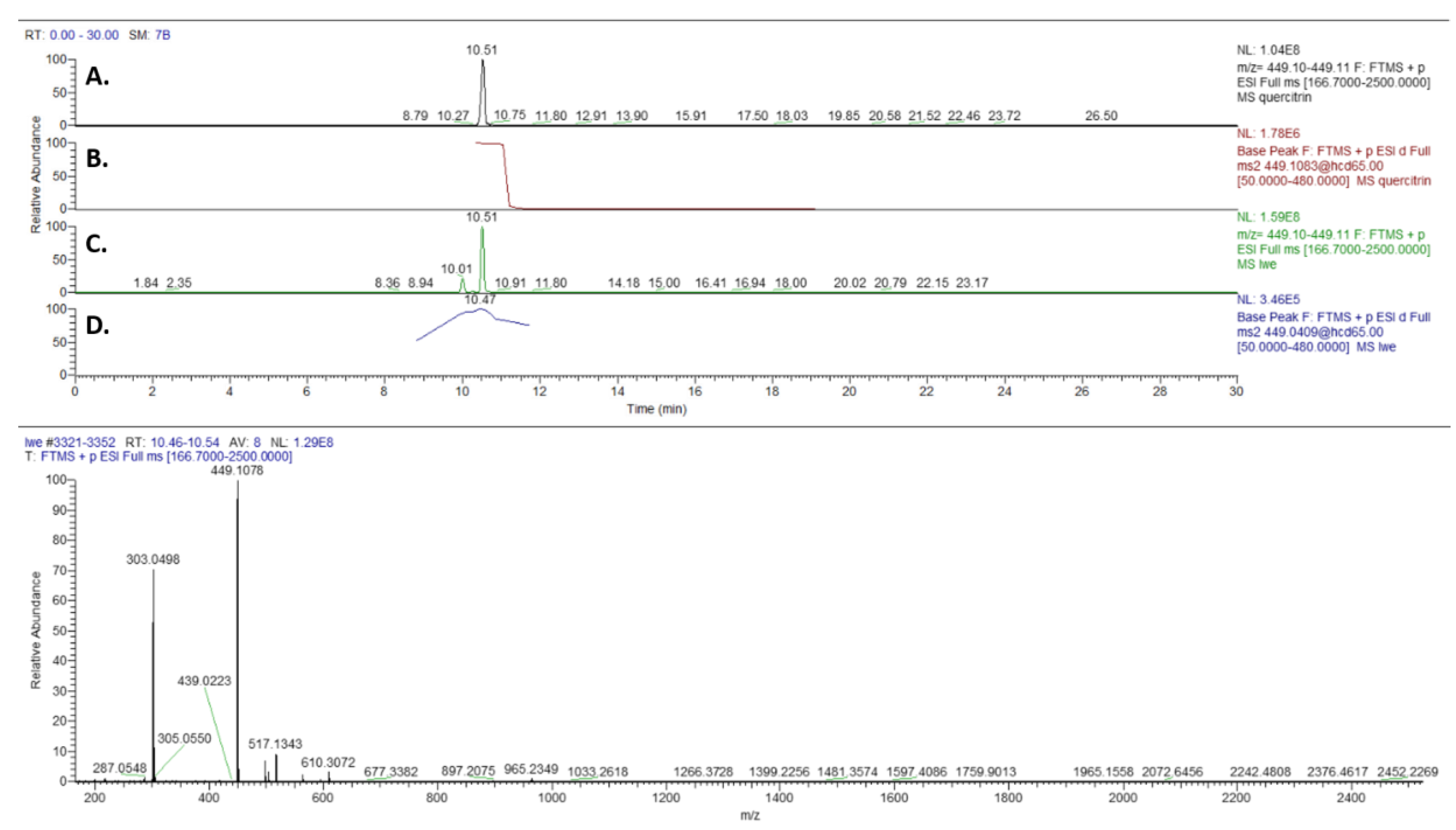
2.3.1. High-Performance Liquid Chromatography-Electrospray Photo Diode Array Mass Spectrometry Analyses of LWE and Constituent Glycosides
2.3.2. Gas Chromatography-Mass Spectrometry Analyses of EO and Constituent Terpenes
2.4. Other Compounds Constituing Nematicidal Leaves Water Extract (LWE) and Wood Water Extract (WWE) Identified by Means of Ultra High Performance Liquid Chromatography Coupled to Orbitrap High Resolution Mass Spectrometry (UHPLC-HRMS)
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Material, Nematodes Populations and Reagents
5.2. Soil Amending with S. rebaudiana Leaves Powder (LP) and Wood Powder (WP) to Treat against M. incognita and Subsequent Biofertilization in Tomato Plants: A Dose-Response
5.3. Essential Oil (EO) and Water Extracts (LWE & WWE) Production
5.4. Paralysis Effect of Leaves Water Extract (LWE) and Wood Water Extract (WWE) on the Plant Parasitic Nematode M. incognita and M. javanica Second Stage Juveniles (J2)
5.5. High-Performance Liquid Chromatography - Photo Diode Array Electrospray Mass Spectrometry Analyses of LWE
5.6. Gas Chromatography-Mass Spectrometry Analyses of EO
Analytical Method Validation-Quantitation of Components
5.7. Ultra High Performance Liquid Chromatpgraphy—Coupled to Orbitrap High Resolution Mass Spectrometry Analysis of Leaves Water Extract (LWE) and Wood Water Extract (WWE)
5.8. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LP | Culture residues in the form of leaves powder |
| WP | culture residues in the form of wood powder |
| LWE | leaves water extract |
| WWE | wood water extract |
| EO | essential oil |
| GC/MS | Gas Chromatography/Mass Spectrometry |
| UHPLC-HRMS | Ultra High Performance Liquid Chromatography coupled to Orbitrap High Resolution Mass Spectrometry |
| HPLC-PDA-ESI/MS | High-Performance Liquid Chromatography-Photo Diode Array Electrospray Mass Spectrometry |
References
- Kinghorn, A.D.; Soejarto, D.D. Current status of stevioside as a sweetening agent for human use. In Progress in Medicinal and Economic Plant Research; Wagner, H., Hikino, H., Farnsworth, N.R., Eds.; Academic Press: London, UK, 1985; Volume 1, pp. 1–52. [Google Scholar]
- Soejarto, D.D.; Compadre, C.M.; Medon, P.J.; Kamath, S.K.; Kinghorn, A.D. Potential sweetening agents of plant origin. II. Field search for sweet-tasting Stevia species. Econ. Bot. 1983, 37, 71–79. [Google Scholar] [CrossRef]
- Soejarto, D.D. Botany of stevia and Stevia rebaudiana. In Stevia: The Genus Stevia, Medicinal and Aromatic Plants—Industrial Profiles Series; Kinghorn, A.D., Ed.; Taylor & Francis: London, UK, 2002; Volume 19, pp. 18–39. [Google Scholar]
- Soejarto, D.D. Ethnobotany of stevia and Stevia rebaudiana. In Stevia: The Genus Stevia, Medicinal and Aromatic Plants—Industrial Profiles Series; Kinghorn, A.D., Ed.; Taylor & Francis: London, UK, 2002; Volume 19, pp. 18–39. [Google Scholar]
- Soejarto, D.D.; Addo, E.M.; Kinghorn, A.D. Highly sweet compounds of plant origin: From ethnobotanical observations to wide utilization. J. Ethnopharmacol. 2019, 243, 112056. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, M.S. Le Kaá-êhé—Sa nature et ses proprieties. An. Científicos Parag. Sér. I 1905, 5, 1–14. [Google Scholar]
- Bertoni, M.S. La Stevia rebaudiana Bertoni. An. Científicos Parag. Sér. II 1918, 2, 129–134. [Google Scholar]
- Singh, D.P.; Kumari, M.; Prakash, H.G.; Rao, G.P.; Solomon, S. Phytochemical and Pharmacological Importance of Stevia: A Calorie-Free Natural Sweetener. Sugar Tech. 2019, 21, 227–234. [Google Scholar] [CrossRef]
- Kurek, J.M.; Krejpcio, Z. The functional and health-promoting properties of Stevia rebaudiana Bertoni and its glycosides with special focus on the antidiabetic potential—A review. J. Funct. Foods 2019, 61, 103465. [Google Scholar] [CrossRef]
- Mejia, E.; Pearlman, M. Natural alternative sweeteners and diabetes management. Curr. Diabetes Rep. 2019, 19, 142. [Google Scholar] [CrossRef]
- Salehi, B.; López, M.D.; Martínez-López, S.; Victoriano, M.; Sharifi-Rad, J.; Martorell, M.; Rodrigues, C.; Martins, N. Stevia rebaudiana Bertoni bioactive effects: From in vivo to clinical trials towards future therapeutic approaches. Phytoth. Res. 2019, 33, 2904–2917. [Google Scholar] [CrossRef]
- Pawar, R.S.; Krynitsky, A.J.; Rader, J.L. Sweeteners from plants—With emphasis on Stevia rebaudiana (Bertoni) and Siraitia grosvernorii (Swingle). Anal. Bioanal. Chem. 2013, 405, 4397–4407. [Google Scholar] [CrossRef]
- Turko, Y.A.; Korobko, N.V.; Shokun, V.V.; Chernyak, E.N.; Vyalkov, A.I.; Stepankina, O.N.; Kerimzhanova, B.F.; Baltaev, U.A. GC—MS research. I. Essential oil from Stevia rebaudiana. Chem. Nat. Compd. 2007, 43, 744–745. [Google Scholar] [CrossRef]
- Hossain, M.A.; Siddique, A.B.; Rahman, S.M.M.; Hossain, M.A. Chemical composition of the essential oils of Stevia rebaudiana Bertoni leaves. Asian. J. Tradit. Med. 2015, 5, 56–61. [Google Scholar]
- Ramírez, P.G.; Ramírez, D.G.; Mejía, E.Z.; Ocampo, S.A.; Díaz, C.N.; Rojas Martínez, R.I. Extracts of Stevia rebaudiana against Fusarium oxysporum associated with tomato cultivation. Sci. Hortic. 2020, 259, 108683. [Google Scholar] [CrossRef]
- Shukla, S.; Mehta, A.; Bajpai, V.K. Phytochemical screening and anthelmintic and antifungal activities of leaf extracts of Stevia rebaudiana. J. Biol. Act. Prod. Nat. 2013, 3, 56–63. [Google Scholar]
- European Commission. Council directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market. Off. J. Eur. Communities L 1991, 230, 1–32. [Google Scholar]
- European Commission. Regulation (EC) No 1107/2009 of the European 515 Parliament and of the Council of 21 October 2009 concerning the placing of plant 516 protection products on the market and repealing Council Directives 79/117/EEC 517 and 91/414/EEC. Off. J. Eur. Union 2009, 309, 1–50. [Google Scholar]
- Hernández-Carlos, B.; Gamboa-Angulo, M. Insecticidal and nematicidal contributions of Mexican flora in the search for safer biopesticides. Molecules 2019, 24, 897. [Google Scholar] [CrossRef] [Green Version]
- Adenubi, O.T.; Ahmed, A.S.; Fasina, F.O.; McGaw, L.J.; Eloff, J.N.; Naidoo, V. Pesticidal plants as a possible alternative to synthetic acaricides in tick control: A systematic review and meta-analysis. Ind. Crops Prod. 2018, 123, 779–806. [Google Scholar] [CrossRef]
- Ntalli, N.G.; Menkissoglu-Spiroudi, U. Pesticides of Botanical Origin: A Promising Tool in Plant Protection. Pesticides—Formulations, Effects, Fate; IntechOpen: London, UK, 2011; ISBN 978-953-7619-X-X. [Google Scholar]
- Verdejo-Lucas, S.; Talavera, M. Root-knot nematodes on zucchini (Cucurbita pepo subsp. pepo): Pathogenicity and management. Crop Prot. 2019, 126, 104943. [Google Scholar] [CrossRef]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.; Gaur, H.S.; Helder, J.; Jones, M.G.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- Ciulu, M.; Quirantes-Piné, R.; Spano, N.; Sanna, G.; Borrás-Linares, I.; Segura-Carretero, A. Evaluation of new extraction approaches to obtain phenolic compound-rich extracts from Stevia rebaudiana Bertoni leaves. Ind. Crops Prod. 2017, 108, 106–112. [Google Scholar] [CrossRef]
- Karaköse, H.; Müller, A.; Kuhnert, N. Profiling and Quantification of Phenolics in Stevia rebaudiana Leaves. J. Agric. Food Chem. 2015, 63, 9188–9198. [Google Scholar] [CrossRef]
- Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives; Document 32008L0098; European Parliament: Strasbourg, France, 2008.
- SANCO/10363/2012-rev.9 Working Document on the Procedure for Application of Basic Substances to be Approved in Compliance with Article 23 of Regulation (EC) No 1107/2009; European Commission Health & Consumers Directorate-General: Copenhagen, Denmark, 2009.
- Briedel, M.; Lavielle, R. Sur le principe sucré des feuilles de Kaá-êh-é (Stevia rebaudiana B). Acad. Sci. Paris C. R. 1931, 192, 1123–1125. [Google Scholar]
- Kohda, H.; Kasai, R.; Yamasaki, K.; Murakami, K.; Tanaka, O. New sweet diterpene glycosides from Stevia rebaudiana. Phytochemistry 1976, 15, 981–983. [Google Scholar] [CrossRef]
- Kasai, R.; Kaneda, N.; Tanaka, O.; Yamasaki, K.; Sakamoto, I.; Morimoto, K.; Okada, S.; Kitahata, S.; Furakawa, H. Sweet diterpene-glycosides of leaves of Stevia rebaudiana Bertoni—Synthesis and structure-sweetness relationships of rebaudiosides- A, -D, -E and their related glycosides. Nippon Kagaku Kaishi 1981, 5, 726–735. [Google Scholar] [CrossRef]
- Tanaka, O. Steviol-glycosides: New natural sweeteners. Trends Anal. Chem. 1982, 1, 246–248. [Google Scholar] [CrossRef]
- Kennelly, E.J. Sweet and non-sweet constituents of Stevia rebaudiana. In Stevia: The Genus Stevia, Medicinal and Aromatic Plants—Industrial Profiles Series; Kinghorn, A.D., Ed.; Taylor & Francis: London, UK, 2002; Volume 19, pp. 68–85. [Google Scholar]
- Ntalli, N.G.; Ferrari, F.; Giannakou, I.; Menkissoglu-Spiroudi, U. Phytochemistry and nematicidal activity of the essential oils from 8 greek lamiaceae aromatic plants and 13 terpene components. J. Agric. Food Chem. 2010, 58, 7856–7863. [Google Scholar] [CrossRef] [PubMed]
- Ntalli, N.G.; Ferrari, F.; Giannakou, I.; Menkissoglu-Spiroudi, U. Synergistic and antagonistic interactions of terpenes against Meloidogyne incognita and the nematicidal activity of essential oils from seven plants indigenous to Greece. Pest Man. Sci. 2011, 67, 341–351. [Google Scholar] [CrossRef]
- Bai, P.H.; Bai, C.Q.; Liu, Q.Z.; Du, S.S.; Liu, Z.L. Nematicidal activity of the essential oil of Rhododendron anthopogonoides aerial parts and its constituent compounds against Meloidogyne incognita. Z. Naturforsch. C J. Biosci. 2013, 68C, 307–312. [Google Scholar] [CrossRef]
- Duschatzky, C.B.; Martinez, A.M.; Almeida, N.V.; Bonivardo, S.L. Nematicidal Activity of the Essential Oils of Several Argentina Plants Against the Root-Knot Nematode. J. Essent. Oil Res. 2004, 16, 626–628. [Google Scholar] [CrossRef]
- Guo, L.; Wu, J.-Z.; Han, T.; Cao, T.; Rahman, K.; Qin, L.-P. Chemical Composition, Antifungal and Antitumor Properties of Ether Extracts of Scapania verrucosa Heeg. and its Endophytic Fungus Chaetomium fusiforme. Molecules 2008, 13, 2114–2125. [Google Scholar] [CrossRef] [Green Version]
- da Silva, S.E.L.; Minguzzi, S.; da Silva, R.C.L.; Matos, M.F.C.; Tofoli, D.; de Carvalho, J.E.; Ruiz, A.L.T.G.; da Costa, W.F.; Simionatto, E. Chemical Composition and Cytotoxic Activity of the Root Essential Oil from Jatropha ribifolia (Pohl) Baill (Euphorbiaceae). J. Braz. Chem. Soc. 2015, 26, 233–238. [Google Scholar]
- Arriola, N.D.A.; Chater, P.I.; Wilcox, M.; Lucini, L.; Rocchetti, G.; Dalmina, M.; Pearson, J.P.; Amboni, R.D.d.M.C. Encapsulation of stevia rebaudiana Bertoni aqueous crude extracts by ionic gelation—Effects of alginate blends and gelling solutions on the polyphenolic profile. Food Chem. 2019, 275, 123–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, S.; Behm, C.A.; Mathesius, U. Functions of Flavonoids in Plant–Nematode Interactions. Plants 2018, 7, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, C.; Wang, A.; Wang, X.; Li, J.; Liu, H.; Wang, M.; Wang, L.; Lai, D.; Zhou, L. A new proline-containing flavonol glycoside from Caragana leucophloea Pojark. Nat. Prod. Res. 2015, 29, 1811–1819. [Google Scholar] [CrossRef]
- Rios, M.Y.; León-Rivera, I.; Ríos-Gomez, R.; Córdova-Albores, L.C.; Aguilar-Guadarrama, A.B. Phytotoxic and nematicide evaluation of Croton ehrenbergii (Euphorbiaceae). Pest Man. Sci. 2019, 75, 2165. [Google Scholar] [CrossRef]
- Abdel Bar, F.M.; Ibrahim, D.S.; Gedara, S.R.; Abdel-Raziq, M.S.; Zaghloul, A.M. Nematicidal compounds from the leaves of Schinus terebinthifolius against root-knot nematode, Meloidogyne incognita infecting tomato. Nat. Prod. Sci. 2018, 24, 272–283. [Google Scholar] [CrossRef]
- Caboni, P.; Saba, M.; Tocco, G.; Casu, L.; Murgia, A.; Maxia, A.; Menkissoglu-Spiroudi, U.; Ntalli, N. Nematicidal activity of mint aqueous extracts against the root-knot nematode Meloidogyne incognita. J. Agric. Food Chem. 2013, 61, 9784–9788. [Google Scholar] [CrossRef]
- Zinoveva, S.V.; Vasyukova, N.I.; Il’inskaya, L.I.; Udalova, Z.V.; Ozeretskovskaya, O.L. Immunization of tomato plants against root-knot nematode Meloidogyne incognita with biogenic elicitors. Appl. Bioch. Microb. 1997, 33, 293–296. [Google Scholar]
- Hajji-Hedfi, L.; Larayedh, A.; Hammas, N.-C.; Regaieg, H.; Horrigue-Raouani, N. Biological activities and chemical composition of Pistacia lentiscus in controlling Fusarium wilt and root-knot nematode disease complex on tomato. Eur. J. Plant Path. 2019, 155, 281–291. [Google Scholar] [CrossRef]
- Machado, A.R.T.; Ferreira, S.R.; da Silva Medeiros, F.; Fujiwara, R.T.; de Souza Filho, J.D.; Pimenta, L.P.S. Nematicidal activity of Annona crassiflora leaf extract on Caenorhabditis elegans. Parasites Vectors 2015, 8, 113. [Google Scholar] [CrossRef] [Green Version]
- Hussey, R.S.; Barker, K.R. Comparison of methods of collecting inocula of Meloidogyne spp. including a new technique. Plant Dis. Rep. 1973, 57, 1025–1028. [Google Scholar]
- Pantelelis, I.; Karpouzas, D.G.; Menkissoglu-Spiroudi, U.; Tsiropoulos, N. Influence of soil physicochemical and biological properties on the degradation and adsorption of the nematicide fosthiazate. J. Agric. Food Chem. 2006, 54, 6783–6789. [Google Scholar] [CrossRef]
- Ntalli, N.; Parlapani, A.B.; Tzani, K.; Samara, M.; Boutsis, G.; Dimou, M.; Menkissoglu-Spiroudi, U.; Monokrousos, N. Thymus citriodorus (Schreb) botanical products as ecofriendly nematicides with bio-fertilizing properties. Plants 2020, 9, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, J.B.; Wilkerson, G.G.; Linker, H.M.; Wilcut, J.W.; Leidy, R.B.; Senseman, S.; Witt, W.W.; Barrett, M.; Vencill, W.K.; Shaw, D.R.; et al. A proposal to standardize soil/solution herbicide distribution coefficients. Weed Sci. 2000, 48, 75–88. [Google Scholar] [CrossRef]
- Byrd, D.W.; Krikpatrick, T.; Barker, K.R. An improved technique for cleaning and staining plant tissue for detection of nematodes. J. Nematol. 1983, 15, 142–143. [Google Scholar]
- Pacifico, S.; Piccolella, S.; Nocera, P.; Tranquillo, E.; Dal Poggetto, F.; Catauro, M. Steviol glycosides content in cultivated Stevia rebaudiana Bertoni: A new sweet expectation from the Campania region (Italy). J. Food Compos. Anal. 2017, 63, 111–120. [Google Scholar] [CrossRef]
- ICH. Validation of Analytical Procedures: Text and Methodology Q2 (R1). Available online: http://wwwichorg/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guidelinepdf (accessed on 5 November 2018).
- SANTE/11813/2017. Guidance Document on Analytical Quality Control and Validation Procedures for Pesticide Residues Analysis in Food and Feed. Available online: https://ec.europa.eu/food/sites/food/files/plant/docs/pesticides_mrl_guidelines_wrkdoc_2017-11813.pdf (accessed on 10 December 2018).
- Betz, J.M.; Brown, P.N.; Roman, M.C. Accuracy, precision, and reliability of chemical measurements in natural products research. Fitoterapia 2011, 82, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Puntener, W. Manual for field trials. In Plant Protection, 2nd ed.; Ciba Geigy Limited: Basle, Switzerland, 1981; p. 205. [Google Scholar]
- Seefeldt, S.S.; Jensen, J.E.; Fuerst, E.P. Log-logistic analysis of herbicide dose-response relationships. Weed Technol. 1995, 9, 218–227. [Google Scholar] [CrossRef]




| EC50 (g kg−1) (Abbott: ♀ g−1 Root) | Std. Error | CI95% |
|---|---|---|
| LP | ||
| <1 | - | - |
| WP | ||
| 3.13 | 0.564 | 1.96–4.29 |
| Exposure | EC50 (mg mL−1) | Std. Error | CI95% | |
|---|---|---|---|---|
| LWE | ||||
| M. incognita | 1 h | 2.86 | 0.39 | 2.04–3.68 |
| 24 h | 2.30 | 0.29 | 1.70–2.90 | |
| 48 h | 1.36 | 0.17 | 1.00–1.73 | |
| M. javanica | 1 h | 3.11 | 0.33 | 2.43–3.80 |
| 24 h | 0.41 | 0.04 | 0.32–0.49 | |
| 48 h | 0.68 | 0.09 | 0.49–0.88 | |
| WWE | ||||
| M. incognita | 1 h | >5.4 | - | - |
| 24 h | 0.30 | 0.02 | 0.25–0.34 | |
| 48 h | 0.95 | 0.07 | 0.79–1.10 | |
| M. javanica | 1 h | >5.4 | - | - |
| 24 h | 0.42 | 0.04 | 0.34–0.51 | |
| 48 h | 0.51 | 0.05 | 0.39–0.63 | |
| S. rebaudiana Leaves | S. rebaudiana Stems | |
|---|---|---|
| Constituent | Concentration (mg g−1) * | |
| Rebaudioside A | 21.468 ± 0.181 | 3.937 ± 0.045 |
| Rebaudioside C | 9.679 ± 0.220 | 0.643 ± 0.037 |
| Dulcoside A | 1.076 ± 0.047 | 0.076 ± 0.003 |
| Stevioside | 19.729 ± 0.135 | 3.625 ± 0.064 |
| Analyte | Retention Time (min) | RI * | Relative Amount (%) |
|---|---|---|---|
| α-Terpineol | 14.56 | 1189 (1189) | 0.47 ± 0.08 |
| α-Bourbonene | 19.97 | 1384 (1384) | 0.15 ± 0.04 |
| β-Maaliene | 20.64 | 1405 (1415) | 0.09 ± 0.04 |
| Caryophyllene | 20.92 | 1419 (1417) | 0.32 ± 0.06 |
| Aromadendrene | 21.32 | 1440 (1439) | 0.67 ± 0.08 |
| epi-β-Caryophyllene | 21.86 | 1466 (1465) | 1.02 ± 0.11 |
| β-Guaiene | 22.00 | 1490 (1490) | 0.77 ± 0.09 |
| β-Ionone | 22.62 | 1491 (1490) | 3.11 ± 0.21 |
| Eremophilene | 22.97 | 1499 (1502) | 1.02 ± 0.15 |
| γ-Cadinene | 23.42 | 1513 (1511) | 1.24 ± 0.09 |
| (-)-β-Cadinene | 23.63 | 1518 (1518) | 1.33 ± 0.23 |
| Cadala-1(10),3,8-triene | 24.14 | 1555 (1562) | 0.36 ± 0.05 |
| Nerolidol | 24.60 | 1564 (1565) | 2.83 ± 0.33 |
| (-)-Spathulenol | 25.08 | 1577 (1578) | 22.81 ± 1.49 |
| Caryophyllene oxide | 25.19 | 1581 (1582) | 20.18 ± 1.15 |
| Isoaromadendrene epoxide | 25.81 | 1589 (1594) | 4.24 ± 0.41 |
| t-Cadinol | 26.55 | 1640 (1639) | 5.88 ± 0.52 |
| α-Cadinol | 26.87 | 1653 (1650) | 3.82 ± 0.27 |
| 6-Isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydro-napthalen-2-ol | 27.30 | 1690 (1690) | 3.10 ± 0.28 |
| Ent-Germacra-4(15),5,10,(14)-trien-1β-ol | 27.66 | 1695 (1694.5) | 0.73 ± 0.10 |
| Unidentified | 32.97 | - | 1.92 ± 0.15 |
| Unidentified | 33.24 | - | 3.03 ± 0.30 |
| Unidentified | 33.60 | - | 1.63 ± 0.29 |
| Manool oxide | 34.3 | 1992 (1989) | 17.19 ± 1.02 |
| Epimanoyl oxide | 34.76 | 2011 (2010) | 1.74 ± 0.27 |
| Oxomanoyl oxide | 38.66 | 2207 (2208) | 0.35 ± 0.10 |
| m/z | tR 1 (min) | Molecular Formula | Adduct | Dppm 2 | Identification/Annotation | WWE | LWE |
|---|---|---|---|---|---|---|---|
| 355.1023 | 8.09 | C16H18O9 | [M+H]+ | −0.56 | Chlorogenic acid * | + | + |
| 611.1611 | 9.60 | C27H30O16 | [M+H]+ | −1.15 | Rutin * | + | + |
| 449.1078 | 10.52 | C21H20O11 | [M+H]+ | 0.22 | Quercitrin * | + | + |
| 271.0602 | 13.06 | C15H10O5 | [M+H]+ | 0 | Apigenin * | - | + |
| 353.0879 | 8.07 | C16H18O9 | [M-H]- | 3.40 | Neochlorogenic acid | + | + |
| 447.0935 | 10.52 | C21H20O11 | [M-H]- | 2.91 | Astragalin | + | + |
| 433.1132 | 11.12 | C21H20O10 | [M+H]+ | 0.69 | Afzelin | + | + |
| 303.0500 | 10.53 | C15H10O7 | [M+H]+ | 0.33 | Quercetin | + | + |
| 305.2478 | 18.44 | C20H32O2 | [M+H]+ | 0.98 | Arachidonic acid | + | + |
| 193.0709 | 1.80 | C7H12O6 | [M+H]+ | 1.04 | Quinic acid | + | + |
| 191.0190 | 2.38 | C6H8O7 | [M-H]- | 2.09 | Citric acid | + | + |
| 611.1611 | 9.60 | C27H30O16 | [M+H]+ | 0.65 | Luteolin-3’,7-Diglucoside | + | + |
| 303.0497 | 10.51 | C15H10O7 | [M+H]+ | −0.66 | Morin | - | + |
| 433.1129 | 10.65 | C21H20O10 | [M+H]+ | 0 | Apigetrin | + | + |
| 595.1660 | 9.95 | C27H30O15 | [M+H]+ | 0.50 | Keracyanin | - | + |
| 435.0925 | 10.28 | C20H18O11 | [M+H]+ | 0.69 | Avicularin | + | + |
| 375.1077 | 15.26 | C19H18O8 | [M+H]+ | 0.80 | 5,2’-Dihydroxy-6,7,8,6’-tetramethoxyflavone | - | + |
| 465.1031 | 9.85 | C21H20O12 | [M+H]+ | 0.86 | Quercetin-3β-D-glucoside | + | + |
| 741.2245 | 9.45 | C33H40O19 | [M+H]+ | 1.08 | Robinin | - | + |
| 517.1344 | 10.43 | C25H24O12 | [M+H]+ | 0.58 | 4,5-Dicaffeoylquinic acid | + | + |
| 341.0866 | 7.39 | C15H16O9 | [M+H]+ | −0.29 | Esculin | - | + |
| 359.1490 | 9.69 | C20H22O6 | [M+H]+ | 0.28 | Matairesinol | - | + |
| 285.2214 | 15.89 | C20H28O | [M+H]+ | 0.35 | (9cis)-Retinal | - | + |
| 175.1190 | 1.69 | C6H14N4O2 | [M+H]+ | 0 | DL-Arginine | + | + |
| 277.1393 | 1.69 | C11H20N2O6 | [M+H]+ | −0.36 | L-Saccharopine | - | + |
| 182.0813 | 2.38 | C9H11NO3 | [M+H]+ | 0.55 | L-Tyrosine | + | + |
| 303.2319 | 15.89 | C20H30O2 | [M+H]+ | 0 | Eicosapentaenoic acid | - | + |
| 295.2269 | 19.53 | C18H30O3 | [M+H]+ | 0.34 | 9-Oxo-10(E),12(E)-octadecadienoic acid | - | + |
| 321.2425 | 15.88 | C20H32O3 | [M+H]+ | 0.31 | (3S)-5-[(4aR,8aS)-2,5,5,8a-Tetramethyl-3-oxo-4a,6,7,8-tetrahydro-4H-naphthalen-1-yl]-3-methylpentanoic acid | - | + |
| 268.1041 | 2.37 | C10H13N5O4 | [M+H]+ | 0.37 | Adenosine | + | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntalli, N.; Kasiotis, K.M.; Baira, E.; Stamatis, C.L.; Machera, K. Nematicidal Activity of Stevia rebaudiana (Bertoni) Assisted by Phytochemical Analysis. Toxins 2020, 12, 319. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12050319
Ntalli N, Kasiotis KM, Baira E, Stamatis CL, Machera K. Nematicidal Activity of Stevia rebaudiana (Bertoni) Assisted by Phytochemical Analysis. Toxins. 2020; 12(5):319. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12050319
Chicago/Turabian StyleNtalli, Nikoletta, Konstantinos M. Kasiotis, Eirini Baira, Christos L. Stamatis, and Kyriaki Machera. 2020. "Nematicidal Activity of Stevia rebaudiana (Bertoni) Assisted by Phytochemical Analysis" Toxins 12, no. 5: 319. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12050319





