Human Botulism in France, 1875–2016
Abstract
:1. Introduction
2. Foodborne Botulism
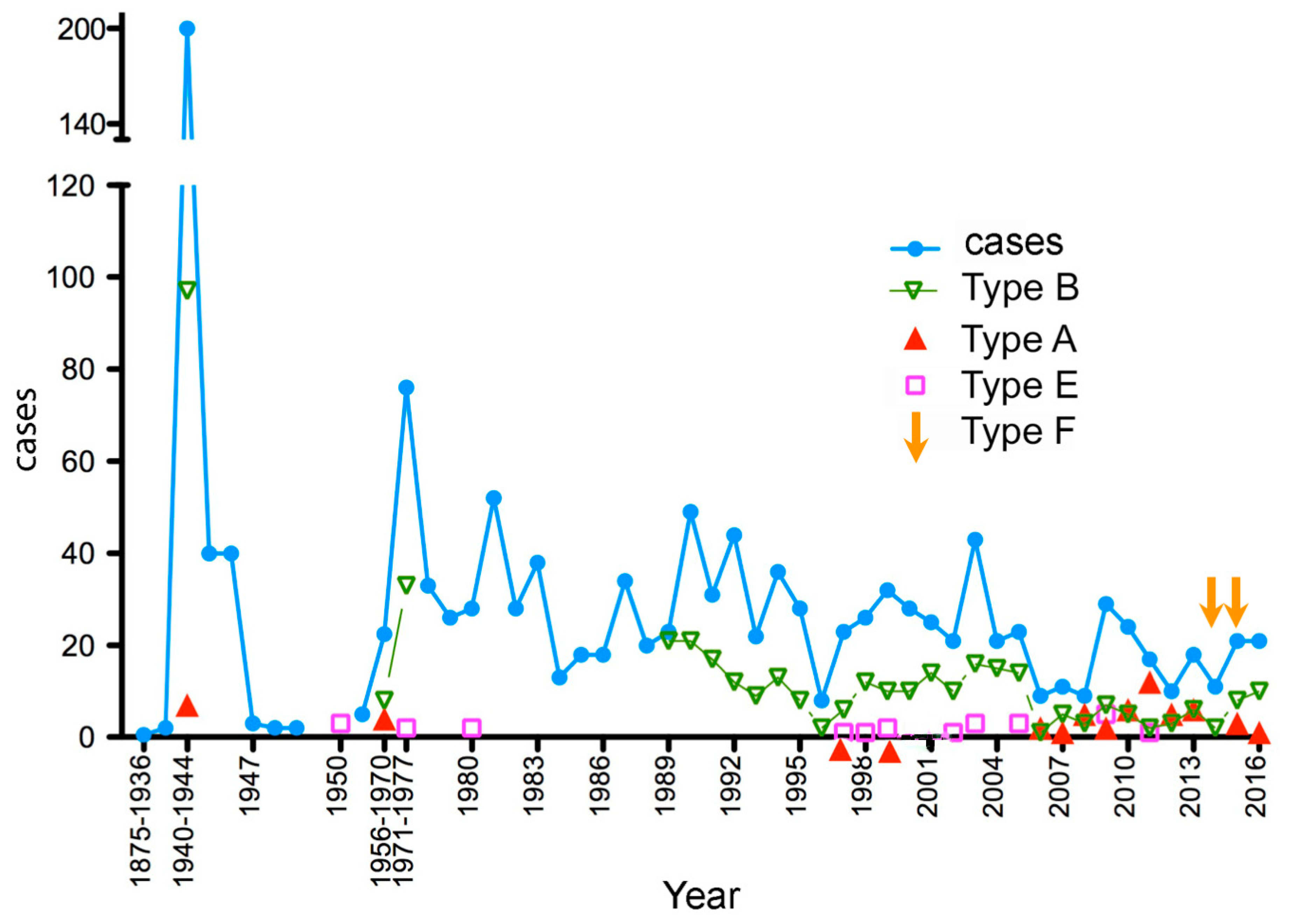
2.1. Period 1875–1944
2.2. Period 1945–1970
2.3. Period 1971–1986
2.4. Period 1987–2016
3. Infant Botulism and Botulism by Intestinal Colonization
4. Wound Botulism and Inhalational Botulism
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Peck, M.W.; Smith, T.J.; Anniballi, F.; Austin, J.W.; Bano, L.; Bradshaw, M.; Cuervo, P.; Cheng, L.W.; Derman, Y.; Dorner, B.G.; et al. Historical Perspectives and Guidelines for Botulinum Neurotoxin Subtype Nomenclature. Toxins 2017, 9, 38. [Google Scholar] [CrossRef]
- Zhang, S.; Masuyer, G.; Zhang, J.; Shen, Y.; Lundin, D.; Henriksson, L.; Miyashita, S.I.; Martinez-Carranza, M.; Dong, M.; Stenmark, P. Identification and characterization of a novel botulinum neurotoxin. Nat. Commun. 2017, 8, 14130. [Google Scholar] [CrossRef] [PubMed]
- Zornetta, I.; Azarnia Tehran, D.; Arrigoni, G.; Anniballi, F.; Bano, L.; Leka, O.; Zanotti, G.; Binz, T.; Montecucco, C. The first non Clostridial botulinum-like toxin cleaves VAMP within the juxtamembrane domain. Sci. Rep. 2016, 6, 30257. [Google Scholar] [CrossRef] [PubMed]
- Brunt, J.; Carter, A.T.; Stringer, S.C.; Peck, M.W. Identification of a novel botulinum neurotoxin gene cluster in Enterococcus. FEBS Lett. 2018, 592, 310–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Lebreton, F.; Mansfield, M.J.; Miyashita, S.I.; Zhang, J.; Schwartzman, J.A.; Tao, L.; Masuyer, G.; Martinez-Carranza, M.; Stenmark, P.; et al. Identification of a Botulinum Neurotoxin-like Toxin in a Commensal Strain of Enterococcus faecium. Cell Host Microbe 2018, 23, 169–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansfield, M.J.; Wentz, T.G.; Zhang, S.; Lee, E.J.; Dong, M.; Sharma, S.K.; Doxey, A.C. Bioinformatic discovery of a toxin family in Chryseobacterium piperi with sequence similarity to botulinum neurotoxins. Sci. Rep. 2019, 9, 1634. [Google Scholar] [CrossRef] [PubMed]
- Wentz, T.G.; Muruvanda, T.; Lomonaco, S.; Thirunavukkarasu, N.; Hoffmann, M.; Allard, M.W.; Hodge, D.R.; Pillai, S.P.; Hammack, T.S.; Brown, E.W.; et al. Closed Genome Sequence of Chryseobacterium piperi Strain CTM(T)/ATCC BAA-1782, a Gram-Negative Bacterium with Clostridial Neurotoxin-Like Coding Sequences. Genome Announc. 2017, 5, e01296-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, M.; Stenmark, P. The Structure and Classification of Botulinum Toxins. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–23. [Google Scholar] [CrossRef]
- Rasetti-Escargueil, C.; Lemichez, E.; Popoff, M.R. Public Health Risk Associated with Botulism as Foodborne Zoonoses. Toxins 2019, 12, 17. [Google Scholar] [CrossRef] [Green Version]
- Franz, D.R.; Pitt, L.M.; Clayton, M.A.; Hanes, M.A.; Rose, K.J. Efficacy of prophylactic and therapeutic administration of antitoxin for inhalation botulism. In Botulinum and Tetanus Neurotoxins; DasGupta, B.R., Ed.; Plenum Press: New York, NY, USA, 1993; pp. 473–476. [Google Scholar]
- Popoff, M.R. Botulinum toxins, Diversity, Mode of Action, Epidemiology of Botulism in France. In Botulinum Toxin; Nikolay, S., Ed.; IntechOpen: London, UK, 2018; pp. 1–28. [Google Scholar] [CrossRef] [Green Version]
- Popoff, M.R.; Mazuet, C.; Poulain, B. Botulism and Tetanus. In The Prokaryotes: Human Microbiology, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 5, pp. 247–290. [Google Scholar]
- Sobel, J. Botulism. Clin. Infect. Dis. 2005, 41, 1167–1173. [Google Scholar] [CrossRef]
- Erbguth, F.J.; Naumann, M. On the first systematic descriptions of botulism and botulinum toxin by Justinus Kerner (1786–1862). J. Hist. Neurosci. 2000, 9, 218–220. [Google Scholar] [CrossRef]
- Torrens, J.K. Clostridium botulinum was named because of association with “sausage poisoning”. BMJ 1998, 316, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Ermengem, E. Classics in infectious diseases. A new anaerobic bacillus and its relation to botulism. Rev. Infect. Dis. 1979, 1, 701–719, Originally published as “Van Ermengem, E. Ueber einen neuen anaeroben Bacillus und seine Beziehungen zum Botulismus”. Zeitschrift fur Hygiene und Infektionskrankheiten 1897, 26, 1–56. [Google Scholar] [CrossRef] [PubMed]
- Du Mesnil, O. Empoisonnement par de la viande de conserve. Ann. Santé Publique 1875, 43, 472–478. [Google Scholar]
- Garcia, R.; Adrian, J. Nicolas Appert: Inventor and manufacturer. Food Rev. Int. 2009, 25, 115–125. [Google Scholar] [CrossRef]
- Meyer, K.F. The status of botulism as a world health problem. Bull. World Health Organ. 1956, 15, 281–298. [Google Scholar]
- Legroux, R.; Levaditi, J.C.; Jéramec, C. Le botulisme en France pendant l’occupation. Presse Med. 1947, 57, 109–110. [Google Scholar]
- Marie, P.L. Un cas de botulisme. Bull. Soc. Med. Hôp. Paris 1920, 37, 1471–1475. [Google Scholar]
- De Saint-Martin. Les manifestations oculaires de botulisme. Bull. Soc. Med. Hôp. Paris 1920, 37, 52–55. [Google Scholar]
- Dreyfus, G.; Ravina, A.; Weill, J.; Orinstein, E.; Wimphen, A. Deux cas de botulisme consécutifs à l’ingestion d’épinards en conserve chez une fillette et son père diabétique. Bull. Soc. Med. Hôp. Paris 1936, 60, 891–896. [Google Scholar]
- Legroux, R.; Jeramec, C.; Levaditi, J.C. Statistique du botulisme de l’occupation 1940–1944. Bull. Acad. Med. 1945, 129, 643–645. [Google Scholar]
- Carlier, J.P.; Espié, E.; Popoff, M.R. Le botulisme en France, 2003–2006. Bull. Epidemiol. Hebd. 2007, 31, 281–284. [Google Scholar]
- Carlier, J.P.; Henry, C.; Lorin, V.; Popoff, M.R. Le botulisme en France a la fin du deuxième millénaire (1998–2000). Bull. Epidemiol. Hebd. 2001, 9, 37–39. [Google Scholar]
- Haeghebaert, S.; Carlier, J.P.; Popoff, M.R. Caractéristiques épidémiologiques du botulisme humain en France, 2001 et 2002. Bul. Epidemiol. Hebd. 2003, 29, 129–130. [Google Scholar]
- Haeghebaert, S.; Popoff, M.R.; Carlier, J.P.; Pavillon, G.; Delarocque-Astagneau, E. Caractéristiques épidémiologiques du botulisme humain en France, 1991–2000. Bul. Epidémiol. Hebd. 2002, 14, 57–59. [Google Scholar]
- Mazuet, C.; Silva, J.D.N.; Legeay, C.; Sautereau, J.; Popoff, M.R. Le botulisme humain en France, 2013–2016. Bull. Epidémiol. Hebd. 2018, 3, 46–54. [Google Scholar]
- Mazuet, C.; Bouvet, P.; King, L.A.; Popoff, M.R. Le botulisme humain en France, 2007–2009. Bull. Epidémiol. Hebd. 2011, 6, 49–53. [Google Scholar]
- Mazuet, C.; King, L.A.; Bouvet, P.; Legeay, C.; Sautereau, J.; Popoff, M.R. Le botulisme humain en France, 2010–2012. Bull. Epidémiol. Hebd. 2014, 6, 106–114. [Google Scholar]
- Sebald, M. Le botulisme humain en France: 1970–1995: Les données du Centre de Référence sur les Anaérobies. Rev. Epidemiol. Santé Publique 1996, 44, S47. [Google Scholar]
- Sebald, M.; Billon, J.; Cassaigne, R.; Rosset, R.; Poumeyrol, G. Le botulisme en France. Incidence, mortalité, aliments responsables avec étude des foyers dus à un aliment qui n’est pas de préparation familiale. Med. Nutr. 1980, 16, 262–268. [Google Scholar]
- Weinberg, M.; Nativelle, R.; Prévot, A.R. Les Microbes Anaérobies; Masson et Cie: Paris, France, 1937; p. 1186. [Google Scholar]
- Verge, J. Le Botulisme. Rec. Med. Vet. 1951, 127, 767–828. [Google Scholar]
- Prevot, A.R.; Huet, M. Existence in France of human botulism due to fish and to Clostridium botulinum E. Bull. Acad. Natl. Med. 1951, 135, 432–435. [Google Scholar] [PubMed]
- Prévot, A.R.; Loiseau, J.; Thévenrad, A. Nouveau cas de botulisme humain d’origine pisciaire. Résultat du traitement par l’anatoxine E. Bull. Acad. Med. 1952, 136, 663–664. [Google Scholar]
- Sebald, M. On botulism in France from 1956 to 1970. Bull. Acad. Natl. Med. 1970, 154, 703–707. [Google Scholar]
- Lamagnere, J.P.; Maupas, P.; Breteau, M.; Laugier, J.; Lamisse, F.; Gautier, J.; Desbuquois, G. Botulism. Sem. Hop. 1973, 49, 1077–1085. [Google Scholar]
- Sebald, M.; Saimot, G. Toxémie botulique. Intérêt de sa mise en évidence dans le diagnostic du botulisme humain de type B. Ann. Microbiol. (Paris) 1973, 124, 61–69. [Google Scholar]
- Olivares, R.; Hubert, B. Le botulisme en 1985 et 1986. Bull. Epidémiol. Hebd. 1987, 29, 113–115. [Google Scholar]
- Carré, H.; Gledel, J.; Poumeyrol, M.; Sebald, M.; Thomas, G.; Veit, P. Enquète sur un foyer de botulisme. Nécessité du respect des bonnes pratiques professionnelles. Med. Nutr. 1987, 23, 391–397. [Google Scholar]
- Sebald, M.; Jouglard, J.; Gilles, G. B botulism in man due to cheese (author’s transl). Ann. Microbiol. (Paris) 1974, 125A, 349–357. [Google Scholar]
- Blettery, B.; Soichot, P.; Virot, C.; Lorcerie, B.; Hillon, P. Type E botulism. Two recent cases (author’s transl). Nouv. Presse Med. 1982, 11, 1131–1133. [Google Scholar]
- Dine, G.; Soibinet, C.; Croix, J.C. Second French case of botulism caused by fresh water fish. Nouv. Presse Med. 1981, 10, 339. [Google Scholar]
- Maupas, P.; Lamagnere, J.P.; Lamisse, F.; Laugier, J. Botulisme de type C. Intérêt de la recherche de toxémie. Med. Mal. Infect. 1976, 6, 207–210. [Google Scholar] [CrossRef]
- Poumeyrol, M.; Billon, J.; Delille, F.; Haas, C.; Marmonier, A.; Sebald, M. Intoxication botulique mortelle due à une souche de Clostridium botulinum de type AB. Med. Mal. Infect. 1983, 13, 750–754. [Google Scholar] [CrossRef]
- Roblot, P.; Roblot, F.; Fauchère, J.L.; Devilleger, A.; Maréchaud, R.; Breux, J.P.; Grollier, G.; Becq-Giraudon, B. Retrospective study of 108 cases of botulism in Poitiers France. J. Med. Microbiol. 1994, 40, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Fourrier, A.; Delmer, M.; Wattel, F.; Furon, D.; Mouton, Y. Cas récents de botulisme dans le nord de la France. A propos de 26 observations (1962–1971). Rev. Med. 1972, 40, 2615–2628. [Google Scholar]
- Henry, C.; Moulin, M.; Richard, G.; Dor, F. 12 cases of ambulatory botulism in Charollais and in Blanzy mining-basin. Bull. Soc. Ophtalmol. Fr. 1970, 70, 296–301. [Google Scholar]
- Laroche, L.; Ollat, H.; Mignot, B.; Claux, J.M.; Saraux, H. Ocular manifestations of botulism. Apropos of 3 cases. Bull. Soc. Ophtalmol. Fr. 1984, 84, 555–558. [Google Scholar]
- Quenum, B.; Hubert, B.; Sebald, M. Le botulisme en 1987 et en 1988. Bull. Epidemiol. Hebd. 1989, 27, 91. [Google Scholar]
- Pelletier, A.; Hubert, B.; Sebald, M. Le botulisme en 1989 et en 1990 en France. Bull. Epidemiol. Hebd. 1991, 27, 91. [Google Scholar]
- Marchetti, P.; Lepoutre, A. Le botulisme en 1991 et en 1992 en France. Bull. Epidemiol. Hebd. 1993, 4, 15. [Google Scholar]
- Abgueguen, P.; Delbos, V.; Chennebault, J.M.; Fanello, S.; Brenet, O.; Alquier, P.; Granry, J.C.; Pichard, E. Nine cases of foodborne botulism type B in France and literature review. Eur. J. Clin. Microbiol. Infect. Dis. 2003, 22, 749–752. [Google Scholar] [CrossRef]
- Boyer, A.; Salah, A. Le botulisme en France: Épidémiologie et clinique. Ann. Med. Interne 2002, 153, 300–310. [Google Scholar]
- Salomon, J.; Delarocque-Astagneau, E.; Popoff, M.R.; Carlier, J.P. Le botulisme en France en 1997. Bull. Epidemiol. Hebd. 1998, 46, 201. [Google Scholar]
- Boyer, A.; Girault, C.; Bauer, F.; Korach, J.M.; Salomon, J.; Moirot, E.; Leroy, J.; Bonmarchand, G. Two cases of foodborne botulism type E and review of epidemiology in France. Eur. J. Clin. Microbiol. Infect. Dis. 2001, 20, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Espié, E.; Vaillant, V.; de Valk, H.; Popoff, M.R. France recalls internationally distributed halal meat products from the plant implicated as the source of a type B botulism outbreak. Eurosurveill. Wkly. 2003, 7, 030918. [Google Scholar]
- King, L.A. Two severe cases of botulism associated with industrially produced chicken enchiladas, France, August 2008. Eurosurveillance 2008, 13, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, L.A.; Niskanen, T.; Junnikkala, M.; Moilanen, E.; Lindstrôm, M.; Korkeala, H.; Korhonen, T.; Popoff, M.; Mazuet, C.; Callon, H.; et al. Botulism and hot-smoked whitefish: A family cluster of type E botulism in France, September 2009. Eurosurveillance 2009, 14, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Mazuet, C.; Sautereau, J.; Legeay, C.; Bouchier, C.; Bouvet, P.; Popoff, M.R. An Atypical Outbreak of Food-Borne Botulism Due to Clostridium botulinum Types B and E from Ham. J. Clin. Microbiol. 2015, 53, 722–726. [Google Scholar] [CrossRef] [Green Version]
- Pingeon, J.; Vanbockstael, C.; Popoff, M.; King, L.; Deschamps, B.; Pradel, G.; Dupont, H.; Spanjaard, A.; Houdard, A.; Mazuet, C.; et al. Two outbreaks of botulism associated with consumption of green olive paste, France, September 2011. Eurosurveillance 2011, 16, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Oriot, C.; D’Aranda, E.; Castanier, M.; Glaizal, M.; Galy, C.; Faivre, A.; Poisnel, E.; Truong, T.K.; Mercury, P.; Hayek-Lanthois, M.; et al. One collective case of type A foodborne botulism in Corsica. Clin. Toxicol. (Phila) 2011, 49, 752–754. [Google Scholar] [CrossRef]
- Caparros, L.; Bourget, S.; Hida, H. Trois cas de botulisme alimentaire en France. J. Pharm. Clin. 2016, 35, 164–168. [Google Scholar]
- Langlois, M.E.; Blanc-Lasserre, K.; Reynaud, C.; Bouteloup, M.; Blanc, Q. Clostridium baratii botulism. Presse Med. 2017, 46, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Mazuet, C.; Legeay, C.; Sautereau, J.; Bouchier, C.; Criscuolo, A.; Bouvet, P.; Trehard, H.; Da Silva, J.N.; Popoff, M.R. Characterization of Clostridium baratii Type F Strains Responsible for an Outbreak of Botulism Linked to Beef Meat Consumption in France. PLOS Curr. Outbreaks 2017. [Google Scholar] [CrossRef]
- Mazuet, C.; Sautereau, J.; Legeay, C.; Bouchier, C.; Bouvet, P.; Jourdan da Silva, N.; Castor, C.; Popoff, M.R. Characterization of the first Clostridium baratii strain responsible for an outbreak of botulism type F in France. Clin. Microbiol. Case Rep. 2015, 1, 1–4. [Google Scholar]
- Trehard, H.; Poujol, I.; Mazuet, C.; Blanc, Q.; Gillet, Y.; Rossignol, F.; Popoff, M.R.; Jourdan Da Silva, N. A cluster of three cases of botulism due to Clostridium baratii type F, France, August 2015. Euro Surveill 2016, 21, 2–5. [Google Scholar] [CrossRef]
- Mazuet, C.; Yoon, E.J.; Boyer, S.; Pignier, S.; Blanc, T.; Doehring, I.; Meziane-Cherif, D.; Dumant-Forest, C.; Sautereau, J.; Legeay, C.; et al. A penicillin- and metronidazole-resistant Clostridium botulinum strain responsible for an infant botulism case. Clin. Microbiol. Infect. 2016, 22, 644.e7–644.e12. [Google Scholar] [CrossRef] [Green Version]
- Bernardor, J.; Neveu, J.; Haas, H.; Pitelet, G.; Popoff, M.R.; Mazuet, C.; Berard, E.; Boulay, C.; Chabrol, B. Infant botulism: Two case reports and electroneuromyogram findings. Arch. Pediatr. 2018. [Google Scholar] [CrossRef]
- Hoarau, G.; Pelloux, I.; Gayot, A.; Wroblewski, I.; Popoff, M.R.; Mazuet, C.; Maurin, M.; Croize, J. Two cases of type A infant botulism in Grenoble, France: No honey for infants. Eur. J. Pediatr. 2012, 171, 589–591. [Google Scholar] [CrossRef]
- King, L.A.; Popoff, M.R.; Mazuet, C.; Espié, E.; Vaillant, V.; de Valk, H. Infant botulism in France, 1991–2009. Arch. Pediatr. 2010, 17, 1288–1292. [Google Scholar] [CrossRef]
- Paricio, C.; Bey, K.J.; Teyssier, G.; Ughetto, A.; Ros, A.; Rayet, I.; Lavocat, M.P. Botulism in a neonate. Arch. Pediatr. 2006, 13, 146–148. [Google Scholar] [CrossRef]
- Patural, H.; Goffaux, P.; Paricio, C.; Emeriaud, G.; Teyssier, G.; Barthelemy, J.C.; Pichot, V.; Roche, F. Infant botulism intoxication and autonomic nervous system dysfunction. Anaerobe 2009, 15, 197–200. [Google Scholar] [CrossRef]
- Fenicia, L.; Franciosa, G.; Pourshaban, M.; Aureli, P. Intestinal toxemia botulism in two young people, caused by Clostridium butyricum Type E. Clin. Infect. Dis. 1999, 29, 381–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roblot, F.; Popoff, M.; Carlier, J.P.; Godet, C.; Abbadie, P.; Matthis, S.; Eisendorn, A.; Le Moal, G.; Becq-Giraudon, B.; Roblot, P. Botulism in patients who inhale cocaine: The first cases in France. Clin. Infect. Dis. 2006, 43, e51–e52. [Google Scholar] [CrossRef] [PubMed]
- Anniballi, F.; Auricchio, B.; Fiore, A.; Lonati, D.; Locatelli, C.A.; Lista, F.; Fillo, S.; Mandarino, G.; De Medici, D. Botulism in Italy, 1986 to 2015. Eurosurveillance 2017, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotta, M.A.; Whitehead, T.R.; Zeltwanger, R.L. Isolation, characterization and comparison of bacteria from swine faeces and manure storage pits. Environ. Microbiol. 2003, 5, 737–745. [Google Scholar] [CrossRef] [Green Version]
- Dahlenborg, M.; Borch, E.; Radstrom, P. Prevalence of Clostridium botulinum types B, E and F in faecal samples from Swedish cattle. Int. J. Food Microbiol. 2003, 82, 105–110. [Google Scholar] [CrossRef]
- Klarmann, D. The detection of Clostridium botulinum in fecal samples of cattle and swine and in the raw material and animal meal of different animal body rendering plants. Berl. Munch. Tierarztl. Wochenschr. 1989, 102, 84–86. [Google Scholar]
- Myllykoski, J.; Nevas, M.; Lindstrôm, M.; Korkeala, H. The detection and prevalence of Clostridium botulinum in pig intestinal samples. Int. J. Food Microbiol. 2006, 110, 172–177. [Google Scholar] [CrossRef]
- Peck, M.W. Clostridium botulinum and the safety of minimally heated, chilled foods: An emerging issue? J. Appl. Microbiol. 2006, 101, 556–570. [Google Scholar] [CrossRef]
- Weisemann, J.; Krez, N.; Fiebig, U.; Worbs, S.; Skiba, M.; Endermann, T.; Dorner, M.B.; Bergstrom, T.; Munoz, A.; Zegers, I.; et al. Generation and Characterization of Six Recombinant Botulinum Neurotoxins as Reference Material to Serve in an International Proficiency Test. Toxins 2015, 7, 5035–5054. [Google Scholar] [CrossRef] [Green Version]
- Peck, M.W. Biology and genomic analysis of Clostridium botulinum. Adv. Microb. Physiol. 2009, 55, 183–265, 320. [Google Scholar]
- Bouvet, P.; Ruimy, R.; Bouchier, C.; Faucher, N.; Mazuet, C.; Popoff, M.R. An Atypical Clostridium Strain Related to the Clostridium botulinum Group III Strain Isolated from a Human Blood Culture. J. Clin. Microbiol. 2014, 52, 339–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention. National Botulism Surveillance. Available online: https://www.cdc.gov/botulism/surveillance.html (accessed on 11 April 2020).
| Clinical Sign/Symptom | Number of Cases/Total Cases | % of Cases |
|---|---|---|
| Gastrointestinal disorders | ||
| Dry mouth | 70/133 | 52.6 |
| Vomiting | 64/134 | 47.8 |
| Constipation | 62/135 | 45.9 |
| Diarrhea | 32/139 | 23.0 |
| Nausea | 22/133 | 16.5 |
| Abdominal pain | 20/130 | 15.4 |
| Oculomotor disorders | ||
| Diplopia | 83/146 | 56.8 |
| Blurred vision | 63/139 | 45.3 |
| Mydriasis | 55/146 | 37.7 |
| Ptosis | 30/137 | 21.9 |
| Paralysis | ||
| Dysphagia | 91/134 | 67.9 |
| Food choking | 54/129 | 41.9 |
| Diaphragmatic paralysis | 36/130 | 27.7 |
| Limb paralysis | 33/126 | 26.2 |
| Dysarthria | 20/122 | 16.4 |
| Outbreaks Where Food Was Identified | ||||||||
|---|---|---|---|---|---|---|---|---|
| Year | Outbreaks | Cases | Deaths | Type (Outbreaks) | Home-Made Ham or Ham from Small Scale Producers | Other Pork Meat Preparations Home-Made or from Small Scale Producers | Other Food (Outbreaks) | References |
| 1987 | 20 | 34 | 1 | B (29) | 18 | 5 | [52] | |
| 1988 | 15 | 20 | 0 | E (1) | 1 (ham from Portugal) | |||
| 1989 | 18 | 23 | 0 | B (43) A (1) | 27 | 8 | [53] | |
| 1990 | 28 | 49 | 0 | home-made canned asparagus (1) | ||||
| 1991 | 19 | 31 | 0 | B (29) | 13 confirmed 3 suspected | 3 suspected | home-made beans (1) | [28,54] |
| 1992 | 15 | 44 | 0 | |||||
| 1993 | 12 | 22 | 1 | B (9) | 28 confirmed or suspected | 15 (suspected not confirmed) | [28] | |
| 1994 | 14 | 36 | 0 | B (13) | ||||
| 1995 | 10 | 28 | 1 | B (8) | ||||
| 1996 | 5 | 8 | 0 | B (2) | ||||
| 1997 | 14 | 23 | 1 | B (6) | 4 | [28,55,56,57] | ||
| A (2) | home-made beans (2) | |||||||
| E (2) | scallops (2) | |||||||
| 1998 | 17 | 26 | 0 | B (11) | 2 | [26,28,56,58] | ||
| E (1) | home-made marinated fish (1) | |||||||
| 1999 | 21 | 32 | 2 | B (13) | 2 | industrial chicken sausage (1) | ||
| A (2) | industrial fish soup (1) | |||||||
| AB (1) | industrial blood sausage? (1) | |||||||
| E (2) | marinated fish (1), restaurant? (1) | |||||||
| 2000 | 15 | 28 | 0 | B (10) | 1 | |||
| 2001 | 18 | 29 | 0 | B (14) | 4 | [27] | ||
| 2002 | 17 | 33 | 0 | B (11) | 4 | |||
| E (1) | chestnut jam (1) | |||||||
| 2003 | 22 | 43 | 0 | B (16) | 4 | home-made pork meat preparation (1) | industrial beef sausage (3) | [25,59] |
| E (3) | canned sardines? (1) | |||||||
| 2004 | 13 | 21 | 0 | B (12) | 2 | |||
| 2005 | 16 | 23 | 0 | B (14) | 5 | home-made pie (1) | boar meat preparation (1) | |
| 2006 | 5 | 9 | 0 | B (1) | ||||
| A (1) | home-made pie (1) | |||||||
| 2007 | 6 | 11 | 0 | B (5) | 2 | [30,60,61,62] | ||
| A (1) | ||||||||
| 2008 | 6 | 9 | 0 | B (3) | ||||
| A (2) | industrial enchiladas with chicken meat (1) subtype A7 home-made pumpkin jam (1) subtype A1(F) | |||||||
| 2009 | 12 | 27 | 0 | B (7) | 2 1 | boar meat preparation (1) | ||
| A (2) | ||||||||
| E (1) | vacuum-packed hot-smoked whitefish (1) | |||||||
| 2010 | 7 | 24 | 1 | B (5) | 4 | home-made canned asparagus (1) subtype B2 | [31,63,64] | |
| A (2) | home-made beans (1) subtype A2 | |||||||
| 2011 | 9 | 17 | 0 | B (2) | home-made canned spinach (1) subtype B2 | |||
| A (5) | commercial tapenades with olives and dried tomatoes (2) subtype A1(B) industrial fresh pasta carbonara (1) subtype A5 | |||||||
| E (1) | ||||||||
| 2012 | 8 | 10 | 0 | B (3) | 2 | industrial pie (1) | ||
| A (4) | home-made canned eggplant (1) subtype A1(B) | |||||||
| 2013 | 11 | 18 | 0 | B (6) | 2, subtype B4 | chorizo (Portugal) (1) | [29,65,66,67,68,69] | |
| A (4) | ||||||||
| 2014 | 4 | 11 | 0 | B (2) | 1 | |||
| F (1) | ||||||||
| 2015 | 14 | 21 | 1 | B (8) | 2, subtype B4 | pie (Poland) (1) subtype B4 chorizo (Portugal) (1) | ||
| A (1) | home-made pheasant pâté (1) subtype A1 | |||||||
| F (1) | industrial ground meat, Bolognese sauce (1) subtype F7 | |||||||
| 2016 | 13 | 21 | 1 | B (10) | 4, subtype B4 | |||
| A (1) | ||||||||
| Total | 402 | 731 | 9 | B (254) | B (136) | B (19 confirmed, 18 suspected) | home-made food (5) industrial food (4) | |
| A (28) | A (1) | home-made food (6) industrial food (5) | ||||||
| AB (2) | industrial food (1) | |||||||
| E (12) | E (2) | home-made food (2) industrial food (4) | ||||||
| F (2) | industrial food (1) | |||||||
| Year | Sex | Age | Toxin in Serum | Toxin in Stool | Toxin in Stool Titer MLD/g | C. botulinum in Stool |
|---|---|---|---|---|---|---|
| 2004 | F | 17 d | B | nd | nd | A1 |
| 2005 | F | 6 m | B | nd | nd | B5 |
| 2006 | F | 2 m | A | A | 80 | A2 |
| 2007 | F | 5 m | A | A | nd | A2 |
| 2008 | F | 4 m | B | B | 20 | culture and PCR detection negative |
| 2009 | F | 2.5 m | A | A | 1000 | A1(B) |
| 2009 | F | 18 m | neg | neg | B2 | |
| 2009 | F | 6 m | neg | A | 20 | A2 |
| 2011 | F | 2.5 m | A | A | 100,000 | A2 |
| 2011 | F | 12 m | B | B | 600 | B2 |
| 2012 | F | 4 m | neg | A | 7000 | A1(B) |
| 2013 | F | 1 m | neg | B | 12,000 | Bf2 |
| 2013 | F | 3 m | neg | A | 4800–300,000 1 | A2 β-lactam resistant |
| 2013 | M | 6 m | neg | B | 340 | Bf |
| 2013 | M | 6 m | A | A | 10,240 | A2 |
| 2015 | M | 2 m | neg | B | 6000 | B5 |
| 2016 | F | 6 m | neg | B | 2000 | B2 |
| BoNT/B (MLD/g) | Number of Ham Samples (Total 26) |
|---|---|
| 60,000 | 1 |
| 40,000 | 1 |
| 20,000 | 4 |
| 12,000 | 1 |
| 10,000 | 1 |
| 8000 | 1 |
| 5000 | 1 |
| 4000 | 1 |
| 3000 | 1 |
| 2000 | 1 |
| 1000 | 1 |
| 400 | 1 |
| 300 | 1 |
| 200 | 2 |
| 140 | 1 |
| 40 | 4 |
| 20 | 1 |
| 10 | 1 |
| 4 | 1 |
| Number of Cases/Outbreak | Number of Outbreaks |
|---|---|
| 6 | 5 |
| 5 | 2 |
| 4 | 4 |
| 3 | 21 |
| 2 | 33 |
| 1 | 170 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasetti-Escargueil, C.; Lemichez, E.; Popoff, M.R. Human Botulism in France, 1875–2016. Toxins 2020, 12, 338. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12050338
Rasetti-Escargueil C, Lemichez E, Popoff MR. Human Botulism in France, 1875–2016. Toxins. 2020; 12(5):338. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12050338
Chicago/Turabian StyleRasetti-Escargueil, Christine, Emmanuel Lemichez, and Michel R. Popoff. 2020. "Human Botulism in France, 1875–2016" Toxins 12, no. 5: 338. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12050338





