Molecular Biology of Escherichia coli Shiga Toxins’ Effects on Mammalian Cells
Abstract
:1. Introduction
2. Variants and Molecular Structure of Shiga Toxins
3. Receptor Globotriaosylceramide (Gb3/CD77)
3.1. Structure, Synthesis, and Regulation of Cell Surface Expression
3.2. Cellular and Tissue Distribution
3.3. Interactions with Shiga Toxins
3.3.1. Binding Affinity and Kinetics
3.3.2. The Carbohydrate Moiety
3.3.3. The Lipid Moiety
3.3.4. Receptor-Binding Domains of the Toxins
3.3.5. Interactions with Physiological (Host) Ligands
4. Shiga Toxins’ Modes of Action
4.1. Internalization and Enzymatic Activity
4.1.1. Receptor-Mediated Endocytosis
4.1.2. Gb3/CD77-Independent Endocytosis
4.1.3. Intracellular Processing in the Target Cell
4.1.4. Translocation of the A Subunit into the Cytosol
4.1.5. Inhibition of Protein Biosynthesis by Ribosomal Inactivation

- Arg170 binds the ribose-phosphate backbone of 28S rRNA by forming ionic bonds. Tyrosine 77 and 114 and Trp203 stabilize this binding with their aromatic rings and adjust adenine residue 4324 of the rRNA.
- Tyr77 transfers a proton to a nitrogen atom in the adenine ring and weakens the bond between C1 of the ribose and N9 of the adenine residue.
- The protonated adenine dissociates, leaving behind a positively charged oxocarbonium ion in the ribose ring, stabilized by Glu167.
- Finally, a water molecule attacks the oxocarbonium ion, hydroxylating the ribose and restoring the proton donor Tyr77.
4.1.6. Nuclear Transport and Intra-Nuclear Effect
4.2. Induction of Eukaryotic Cell Death
4.2.1. Consequences of Protein Biosynthesis Inhibition
4.2.2. Direct Activation of the Apoptosis Program
4.2.3. Activation of Gb3/CD77-Dependent Signaling Pathways
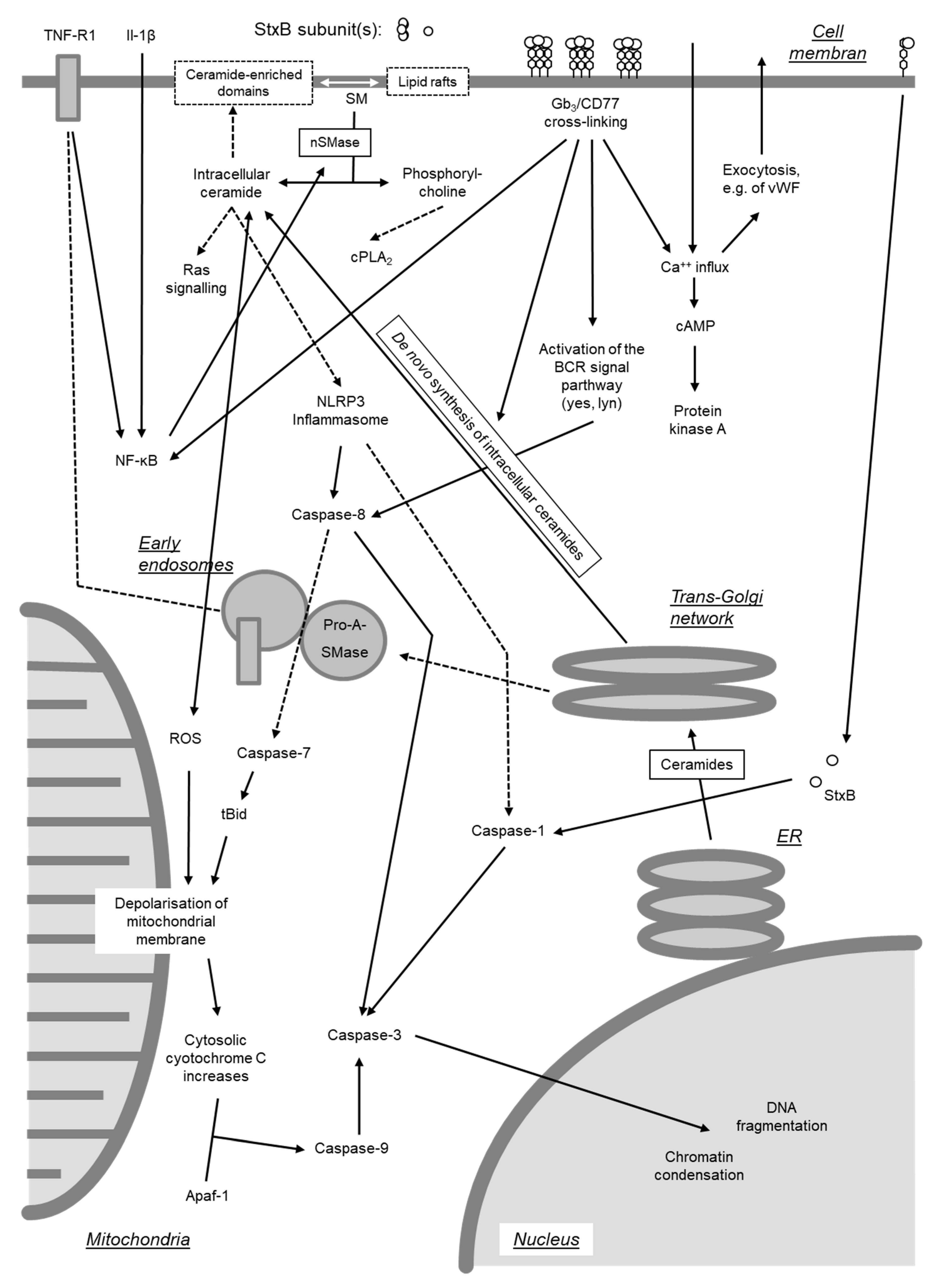
4.3. Ribotoxic Stress Response
4.4. Endoplasmic Reticulum Stress and Autophagy
4.5. Induction of Proteinaceous (Inflammatory) Mediators
| Cell Type/Line | Species | Toxin | Induction of | Reference | |
|---|---|---|---|---|---|
| mRNA 1 | Protein 1 | ||||
| Intestinal epithelial cells | |||||
| HCT-8 | Human | Stx1 | IL-8, GRO-α, GRO-β, GRO-γ, ENA-78 | IL-8, GRO-α | [328] |
| Stx1 + 2 | IL-8 | IL-8 | [309] | ||
| Caco-2 | Human | Stx1 + 2 | IL-8, MCP-1, MIP-1α, MIP-1β, TNF-α | IL-8 | [322] |
| Renal epithelial cells | |||||
| Glomerulum, primary | Human | Stx1 | IL-1, IL-6, TNF-α | IL-1, IL-6, TNF-α | [331] |
| Tubulus, primary | Human | Stx1 | IL-1, IL-6, TNF-α | IL-1, TNF-α | [332] |
| HK-2 (proximal tubulus) | Human | Stx1 | IL-1β, TNF-α | [317] | |
| Stx2 | IL-1β, IL-8, TNF-α, MIP-1α, MIP-1β | MIP-1α, MIP-1β | [317] | ||
| Endothelial cells | |||||
| Brain, primary | Human | Stx1 | IL-1β, IL-6, TNF-α | IL-6, IL-8 | [333] |
| Aortic, primary | Bovine | Stx1 (+2) | Preproendothelin-1 | Endothelin | [139] |
| Umbilical vein, primary | Human | Stx1 | IL-8, GRO-α, GRO-β, GRO-γ, TNF-α | IL-6, IL-8, GRO-α, MCP-1 | [334] |
| Stx2 | IL-8, GRO-α, GRO-β, GRO-γ, IL-6, IL-16, MCP-1, TNF-α | IL-6, IL-8, GM-CSF, GRO-α, MCP-1 | [334] | ||
| Fibrocytes/-blasts | |||||
| NIH3T3 | Mouse | StxB1 2 | IL-1β, TNF-α | IL-1β, TNF-α | [268] |
| Monocyte/Macrophage-like | |||||
| THP-1, differentiated | Human | Stx1 + 2 | TNF-α | TNF-α | [312] |
| THP-1, differentiated | Human | Stx1 | IL-1β, TNF-α | IL-1β | [320] |
| THP-1, differentiated | Human | Stx1 | IL-8, MIP-1α, MIP-1α, GRO-α, IL-1α, TNF-α | IL-8, MIP-1α, MIP-1β, GRO-α | [329] |
| THP-1, differentiated | Human | Stx1 + 2 | GRO, G-CSF, IL-1β, IL-8, TNF-α | [324] | |
| THP-1, differentiated | Human | Stx1 | TNF-α | [200] | |
| THP-1, un-differentiated | Human | Stx1 | IL-1β, TNF-α | [115] | |
| THP-1 | Human | Stx1 | TNF-α | TNF-α | [321] |
| Peripheral blood, primary | Human | Stx1 + 2 | GM-CSF, TNF-α | [313] | |
| Peripheral blood, primary | Human | Stx1 + 2 | IL-8, IL-1β, TNF-α | IL-8, IL-1β, TNF-α | [335,336] |
| Peripheral blood, primary | Human | Stx1 | IL-1β, TNF-α | [115] | |
| Peripheral blood, primary | Human | Stx1 | IL-1β, TNF-α, IL-6, G-CSF, IL-8, CCL2, CCL4 | [241] | |
| Peripheral blood, primary | Bovine | Stx1 | IL-4, IL-6, IL-10, IFN-γ, TNF-α, IL-8 and GRO-α | [160] | |
| Peripheral blood, primary, non-adherent | Human | Stx1 | IL-6 | IL-8, IL-1β, IL-6, TNF-α | [157] |
| Colonic mucosal macrophages, primary | Bovine | Stx1 | IL-8, GRO-α, MCP-1, RANTES, IL-10 | [133] | |
| Peritoneal exudate | Mouse | Stx2 | TNF-α | [337] | |
| Peritoneal exudate | Mouse | Stx1 + 2 | TNF-α | IL-1β, IL-6, TNF-α | [159] |
| Mesangial cells, primary | Human | Stx1 | MCP-1 | [338] | |
| Neutrophils | |||||
| Peripheral blood, primary | Human | Stx1 | IL-8, CCL4, G-CSF, TNF-α, IL-1β | [241] | |
| Lymphocytes | |||||
| Ileal intraepithelial | Bovine | Stx1 | IL-4 | [339] | |
4.6. Induction of Arachidonic Metabolite Synthesis
4.7. Interaction of Shiga Toxins with Soluble Factors
4.8. Transcellular Transport
5. Conclusive Summary
Funding
Acknowledgments
Conflicts of Interest
References
- Karmali, M.A. Infection by verocytotoxin-producing Escherichia coli. Clin. Microbiol. Rev. 1989, 2, 15–38. [Google Scholar] [CrossRef] [PubMed]
- Wieler, L.H.; Franke, S.; Menge, C.; Rose, M.; Bauerfeind, R.; Karch, H.; Baljer, G. The immune response in edema disease of weaned piglets measured with a recombinant B subunit of shiga-like toxin II. Dtsch. Tierarztl. Wochenschr. 1995, 102, 40–43. [Google Scholar] [PubMed]
- Bielaszewska, M.; Mellmann, A.; Zhang, W.; Kock, R.; Fruth, A.; Bauwens, A.; Peters, G.; Karch, H. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: A microbiological study. Lancet Infect. Dis. 2011, 11, 671–676. [Google Scholar] [CrossRef] [Green Version]
- Hamm, K.; Barth, S.A.; Stalb, S.; Geue, L.; Liebler-Tenorio, E.; Teifke, J.P.; Lange, E.; Tauscher, K.; Kotterba, G.; Bielaszewska, M.; et al. Experimental Infection of Calves with Escherichia coli O104:H4 outbreak strain. Sci. Rep. 2016, 6, 32812. [Google Scholar] [CrossRef] [Green Version]
- Stalb, S.; Barth, S.A.; Sobotta, K.; Liebler-Tenorio, E.; Geue, L.; Menge, C. Pro-inflammatory capacity of Escherichia coli O104:H4 outbreak strain during colonization of intestinal epithelial cells from human and cattle. Int. J. Med. Microbiol. 2018, 308, 899–911. [Google Scholar] [CrossRef]
- Cray, W.C., Jr.; Moon, H.W. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl. Environ. Microbiol. 1995, 61, 1586–1590. [Google Scholar] [CrossRef] [Green Version]
- Matthias, D. [Localization of beta-hemolytic E. coli in various organs of swine with edema disease]. Zentralbl. Bakteriol. [Orig] 1969, 212, 103–108. [Google Scholar]
- Lee, M.S.; Koo, S.; Jeong, D.G.; Tesh, V.L. Shiga Toxins as Multi-Functional Proteins: Induction of Host Cellular Stress Responses, Role in Pathogenesis and Therapeutic Applications. Toxins (Basel) 2016, 8, 77. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.S.; Tesh, V.L. Roles of Shiga Toxins in Immunopathology. Toxins (Basel) 2019, 11, 212. [Google Scholar] [CrossRef] [Green Version]
- Harel, Y.; Silva, M.; Giroir, B.; Weinberg, A.; Cleary, T.B.; Beutler, B. A reporter transgene indicates renal-specific induction of tumor necrosis factor (TNF) by shiga-like toxin. Possible involvement of TNF in hemolytic uremic syndrome. J. Clin. Investig. 1993, 92, 2110–2116. [Google Scholar] [CrossRef] [Green Version]
- Proulx, F.; Seidman, E.G.; Karpman, D. Pathogenesis of Shiga toxin-associated hemolytic uremic syndrome. Pediatr. Res. 2001, 50, 163–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, C.L.; Leibowitz, C.S.; Kurosawa, S.; Stearns-Kurosawa, D.J. Shiga toxins and the pathophysiology of hemolytic uremic syndrome in humans and animals. Toxins (Basel) 2012, 4, 1261–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Law, D. Adhesion and its role in the virulence of enteropathogenic Escherichia coli. Clin. Microbiol. Rev. 1994, 7, 152–173. [Google Scholar] [CrossRef] [PubMed]
- Barth, S.A.; Menge, C.; Eichhorn, I.; Semmler, T.; Wieler, L.H.; Pickard, D.; Belka, A.; Berens, C.; Geue, L. The Accessory Genome of Shiga Toxin-Producing Escherichia coli Defines a Persistent Colonization Type in Cattle. Appl. Environ. Microbiol. 2016, 82, 5455–5464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaper, J.B. The locus of enterocyte effacement pathogenicity island of Shiga toxin-producing Escherichia coli O157:H7 and other attaching and effacing E. coli. Jpn. J. Med. Sci. Biol. 1998, 51, S101–S107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro, R.; Ageorges, V.; Rojas-Lopez, M.; Schmidt, H.; Weiss, A.; Bertin, Y.; Forano, E.; Jubelin, G.; Henderson, I.R.; Livrelli, V.; et al. A secretome view of colonisation factors in Shiga toxin-encoding Escherichia coli (STEC): from enterohaemorrhagic E. coli (EHEC) to related enteropathotypes. FEMS Microbiol. Lett. 2016, 363. [Google Scholar] [CrossRef]
- Ritchie, M.; Partington, S.; Jessop, J.; Kelly, M.T. Comparison of a direct fecal Shiga-like toxin assay and sorbitol-MacConkey agar culture for laboratory diagnosis of enterohemorrhagic Escherichia coli infection. J. Clin. Microbiol. 1992, 30, 461–464. [Google Scholar] [CrossRef] [Green Version]
- Donohue-Rolfe, A.; Keusch, G.T. Shigella dysenteriae 1 cytotoxin: periplasmic protein releasable by polymyxin B and osmotic shock. Infect. Immun. 1983, 39, 270–274. [Google Scholar] [CrossRef] [Green Version]
- Bauwens, A.; Kunsmann, L.; Marejkova, M.; Zhang, W.; Karch, H.; Bielaszewska, M.; Mellmann, A. Intrahost milieu modulates production of outer membrane vesicles, vesicle-associated Shiga toxin 2a and cytotoxicity in Escherichia coli O157:H7 and O104:H4. Environ. Microbiol. Rep. 2017, 9, 626–634. [Google Scholar] [CrossRef]
- Pradhan, S.; Karve, S.S.; Weiss, A.A.; Hawkins, J.; Poling, H.M.; Helmrath, M.A.; Wells, J.M.; McCauley, H.A. Tissue Responses to Shiga Toxin in Human Intestinal Organoids. Cell Mol. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef] [Green Version]
- Schuller, S.; Frankel, G.; Phillips, A.D. Interaction of Shiga toxin from Escherichia coli with human intestinal epithelial cell lines and explants: Stx2 induces epithelial damage in organ culture. Cell Microbiol. 2004, 6, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.; Tyrrell, G.; Maloney, M.; Gyles, C.; Brunton, J.; Lingwood, C. Alteration of the glycolipid binding specificity of the pig edema toxin from globotetraosyl to globotriaosyl ceramide alters in vivo tissue targetting and results in a verotoxin 1-like disease in pigs. J. Exp. Med. 1993, 177, 1745–1753. [Google Scholar] [CrossRef] [Green Version]
- Hall, G.A.; Chanter, N.; Bland, A.P. Comparison in gnotobiotic pigs of lesions caused by verotoxigenic and non-verotoxigenic Escherichia coli. Vet. Pathol. 1988, 25, 205–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, J.; Oryshak, A.; Wenetsek, M.; Grabiec, J.; Handy, S. The colonic pathology of Escherichia coli O157:H7 infection. Am. J. Surg. Pathol. 1990, 14, 87–92. [Google Scholar] [CrossRef]
- Richardson, S.E.; Karmali, M.A.; Becker, L.E.; Smith, C.R. The histopathology of the hemolytic uremic syndrome associated with verocytotoxin-producing Escherichia coli infections. Hum. Pathol. 1988, 19, 1102–1108. [Google Scholar] [CrossRef]
- Philpott, D.J.; McKay, D.M.; Sherman, P.M.; Perdue, M.H. Infection of T84 cells with enteropathogenic Escherichia coli alters barrier and transport functions. Am. J. Physiol. 1996, 270, G634–G645. [Google Scholar] [CrossRef] [PubMed]
- Hurley, B.P.; Thorpe, C.M.; Acheson, D.W. Shiga toxin translocation across intestinal epithelial cells is enhanced by neutrophil transmigration. Infect. Immun. 2001, 69, 6148–6155. [Google Scholar] [CrossRef] [Green Version]
- Acheson, D.W.; Moore, R.; De Breucker, S.; Lincicome, L.; Jacewicz, M.; Skutelsky, E.; Keusch, G.T. Translocation of Shiga toxin across polarized intestinal cells in tissue culture. Infect. Immun. 1996, 64, 3294–3300. [Google Scholar] [CrossRef] [Green Version]
- Hurley, B.P.; Jacewicz, M.; Thorpe, C.M.; Lincicome, L.L.; King, A.J.; Keusch, G.T.; Acheson, D.W. Shiga toxins 1 and 2 translocate differently across polarized intestinal epithelial cells. Infect. Immun. 1999, 67, 6670–6677. [Google Scholar] [CrossRef] [Green Version]
- Philpott, D.J.; Ackerley, C.A.; Kiliaan, A.J.; Karmali, M.A.; Perdue, M.H.; Sherman, P.M. Translocation of verotoxin-1 across T84 monolayers: mechanism of bacterial toxin penetration of epithelium. Am. J. Physiol. 1997, 273, G1349–G1358. [Google Scholar] [CrossRef]
- Bitzan, M.; Richardson, S.; Huang, C.; Boyd, B.; Petric, M.; Karmali, M.A. Evidence that verotoxins (Shiga-like toxins) from Escherichia coli bind to P blood group antigens of human erythrocytes in vitro. Infect. Immun. 1994, 62, 3337–3347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- te Loo, D.M.; Monnens, L.A.; van Der Velden, T.J.; Vermeer, M.A.; Preyers, F.; Demacker, P.N.; van Den Heuvel, L.P.; van Hinsbergh, V.W. Binding and transfer of verocytotoxin by polymorphonuclear leukocytes in hemolytic uremic syndrome. Blood 2000, 95, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A.L.; Sartz, L.; Nelsson, A.; Bekassy, Z.D.; Karpman, D. Shiga toxin and lipopolysaccharide induce platelet-leukocyte aggregates and tissue factor release, a thrombotic mechanism in hemolytic uremic syndrome. PLoS ONE 2009, 4, e6990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stahl, A.L.; Arvidsson, I.; Johansson, K.E.; Chromek, M.; Rebetz, J.; Loos, S.; Kristoffersson, A.C.; Bekassy, Z.D.; Morgelin, M.; Karpman, D. A novel mechanism of bacterial toxin transfer within host blood cell-derived microvesicles. PLoS Pathog. 2015, 11, e1004619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koster, F.; Levin, J.; Walker, L.; Tung, K.S.; Gilman, R.H.; Rahaman, M.M.; Majid, M.A.; Islam, S.; Williams, R.C., Jr. Hemolytic-uremic syndrome after shigellosis. Relation to endotoxemia and circulating immune complexes. N. Engl. J. Med. 1978, 298, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.; Lingwood, C. Verotoxin receptor glycolipid in human renal tissue. Nephron 1989, 51, 207–210. [Google Scholar] [CrossRef]
- Koster, F.T.; Boonpucknavig, V.; Sujaho, S.; Gilman, R.H.; Rahaman, M.M. Renal histopathology in the hemolytic-uremic syndrome following shigellosis. Clin. Nephrol. 1984, 21, 126–133. [Google Scholar]
- Bauwens, A.; Betz, J.; Meisen, I.; Kemper, B.; Karch, H.; Muthing, J. Facing glycosphingolipid-Shiga toxin interaction: dire straits for endothelial cells of the human vasculature. Cell Mol. Life Sci. 2013, 70, 425–457. [Google Scholar] [CrossRef]
- Wiley, R.G.; Donohue-Rolfe, A.; Keusch, G.T. Axonally transported Shigella cytotoxin is neuronotoxic. J. Neuropathol. Exp. Neurol. 1985, 44, 496–506. [Google Scholar] [CrossRef]
- Liu, J.; Akahoshi, T.; Sasahana, T.; Kitasato, H.; Namai, R.; Sasaki, T.; Inoue, M.; Kondo, H. Inhibition of neutrophil apoptosis by verotoxin 2 derived from Escherichia coli O157:H7. Infect. Immun. 1999, 67, 6203–6205. [Google Scholar] [CrossRef] [Green Version]
- King, A.J.; Sundaram, S.; Cendoroglo, M.; Acheson, D.W.; Keusch, G.T. Shiga toxin induces superoxide production in polymorphonuclear cells with subsequent impairment of phagocytosis and responsiveness to phorbol esters. J. Infect. Dis. 1999, 179, 503–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, A.; Madrid-Marina, V.; Estrov, Z.; Freedman, M.H.; Lingwood, C.A.; Dosch, H.M. Expression of glycolipid receptors to Shiga-like toxin on human B lymphocytes: a mechanism for the failure of long-lived antibody response to dysenteric disease. Int. Immunol. 1990, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Pellino, C.; MacMaster, K.; Coyle, D.; Weiss, A.A. Shiga Toxin Mediated Neurologic Changes in Murine Model of Disease. Front. Cell. Infect. Microbiol. 2016, 6, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karmali, M.A.; Petric, M.; Lim, C.; Fleming, P.C.; Arbus, G.S.; Lior, H. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J. Infect. Dis. 1985, 151, 775–782. [Google Scholar] [CrossRef]
- Wurzner, R.; Riedl, M.; Rosales, A.; Orth-Holler, D. Treatment of enterohemorrhagic Escherichia coli-induced hemolytic uremic syndrome (eHUS). Semin. Thromb. Hemost. 2014, 40, 508–516. [Google Scholar] [CrossRef]
- Thomas, D.E.; Elliott, E.J. Interventions for preventing diarrhea-associated hemolytic uremic syndrome: systematic review. BMC Public Health 2013, 13, 799. [Google Scholar] [CrossRef] [Green Version]
- Fricke, R.; Bastert, O.; Gotter, V.; Brons, N.; Kamp, J.; Selbitz, H.J. Implementation of a vaccine against Shigatoxin 2e in a piglet producing farm with problems of Oedema disease: case study. Porcine Health Manag. 2015, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Vande Walle, K.; Vanrompay, D.; Cox, E. Bovine innate and adaptive immune responses against Escherichia coli O157:H7 and vaccination strategies to reduce faecal shedding in ruminants. Vet. Immunol. Immunopathol. 2013, 152, 109–120. [Google Scholar] [CrossRef]
- Johannes, L. Shiga Toxin-A Model for Glycolipid-Dependent and Lectin-Driven Endocytosis. Toxins (Basel) 2017, 9, 340. [Google Scholar] [CrossRef]
- Geyer, P.E.; Maak, M.; Nitsche, U.; Perl, M.; Novotny, A.; Slotta-Huspenina, J.; Dransart, E.; Holtorf, A.; Johannes, L.; Janssen, K.P. Gastric Adenocarcinomas Express the Glycosphingolipid Gb3/CD77: Targeting of Gastric Cancer Cells with Shiga Toxin B-Subunit. Mol. Cancer Ther. 2016, 15, 1008–1017. [Google Scholar] [CrossRef] [Green Version]
- Maak, M.; Nitsche, U.; Keller, L.; Wolf, P.; Sarr, M.; Thiebaud, M.; Rosenberg, R.; Langer, R.; Kleeff, J.; Friess, H.; et al. Tumor-specific targeting of pancreatic cancer with Shiga toxin B-subunit. Mol. Cancer Ther. 2011, 10, 1918–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavaliauskiene, S.; Dyve Lingelem, A.B.; Skotland, T.; Sandvig, K. Protection against Shiga Toxins. Toxins (Basel) 2017, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Pohlentz, G.; Steil, D.; Rubin, D.; Mellmann, A.; Karch, H.; Muthing, J. Pectin-derived neoglycolipids: Tools for differentiation of Shiga toxin subtypes and inhibitors of Shiga toxin-mediated cellular injury. Carbohydr. Polym. 2019, 212, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.; Barth, S.A.; Frahm, J.; Meyer, U.; Danicke, S.; Geue, L.; Menge, C. Decreased STEC shedding by cattle following passive and active vaccination based on recombinant Escherichia coli Shiga toxoids. Vet. Res. 2018, 49, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, K.J.; Rogan, D.; Finlay, B.B.; Potter, A.A.; Asper, D.J. Vaccination with type III secreted proteins leads to decreased shedding in calves after experimental infection with Escherichia coli O157. Can. J. Vet. Res. 2011, 75, 98–105. [Google Scholar]
- Wileman, B.W.; Thomson, D.U.; Olson, K.C.; Jaeger, J.R.; Pacheco, L.A.; Bolte, J.; Burkhardt, D.T.; Emery, D.A.; Straub, D. Escherichia coli O157:H7 shedding in vaccinated beef calves born to cows vaccinated prepartum with Escherichia coli O157:H7 SRP vaccine. J. Food Prot. 2011, 74, 1599–1604. [Google Scholar] [CrossRef]
- Konowalchuk, J.; Speirs, J.I.; Stavric, S. Vero response to a cytotoxin of Escherichia coli. Infect. Immun. 1977, 18, 775–779. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, A.D.; LaVeck, G.D. Purification and characterization of a Shigella dysenteriae 1-like toxin produced by Escherichia coli. Infect. Immun. 1983, 40, 675–683. [Google Scholar] [CrossRef] [Green Version]
- Scheutz, F.; Teel, L.D.; Beutin, L.; Pierard, D.; Buvens, G.; Karch, H.; Mellmann, A.; Caprioli, A.; Tozzoli, R.; Morabito, S.; et al. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J. Clin. Microbiol. 2012, 50, 2951–2963. [Google Scholar] [CrossRef] [Green Version]
- Strockbine, N.A.; Jackson, M.P.; Sung, L.M.; Holmes, R.K.; O’Brien, A.D. Cloning and sequencing of the genes for Shiga toxin from Shigella dysenteriae type 1. J. Bacteriol. 1988, 170, 1116–1122. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Bielaszewska, M.; Kuczius, T.; Karch, H. Identification, characterization, and distribution of a Shiga toxin 1 gene variant (stx(1c)) in Escherichia coli strains isolated from humans. J. Clin. Microbiol. 2002, 40, 1441–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burk, C.; Dietrich, R.; Acar, G.; Moravek, M.; Bulte, M.; Martlbauer, E. Identification and characterization of a new variant of Shiga toxin 1 in Escherichia coli ONT:H19 of bovine origin. J. Clin. Microbiol. 2003, 41, 2106–2112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, C.K.; McKee, M.L.; O’Brien, A.D. Two copies of Shiga-like toxin II-related genes common in enterohemorrhagic Escherichia coli strains are responsible for the antigenic heterogeneity of the O157:H- strain E32511. Infect. Immun. 1991, 59, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Melton-Celsa, A.R.; Kokai-Kun, J.F.; O’Brien, A.D. Activation of Shiga toxin type 2d (Stx2d) by elastase involves cleavage of the C-terminal two amino acids of the A2 peptide in the context of the appropriate B pentamer. Mol. Microbiol. 2002, 43, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinstein, D.L.; Jackson, M.P.; Samuel, J.E.; Holmes, R.K.; O’Brien, A.D. Cloning and sequencing of a Shiga-like toxin type II variant from Escherichia coli strain responsible for edema disease of swine. J. Bacteriol. 1988, 170, 4223–4230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, H.; Scheef, J.; Morabito, S.; Caprioli, A.; Wieler, L.H.; Karch, H. A new Shiga toxin 2 variant (Stx2f) from Escherichia coli isolated from pigeons. Appl. Environ. Microbiol. 2000, 66, 1205–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, P.H.; Peiris, J.S.; Ng, W.W.; Robins-Browne, R.M.; Bettelheim, K.A.; Yam, W.C. A newly discovered verotoxin variant, VT2g, produced by bovine verocytotoxigenic Escherichia coli. Appl. Environ. Microbiol. 2003, 69, 7549–7553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gannon, V.P.; Gyles, C.L. Characteristics of the Shiga-like toxin produced by Escherichia coli associated with porcine edema disease. Vet. Microbiol. 1990, 24, 89–100. [Google Scholar] [CrossRef]
- DeGrandis, S.; Law, H.; Brunton, J.; Gyles, C.; Lingwood, C.A. Globotetraosylceramide is recognized by the pig edema disease toxin. J. Biol. Chem. 1989, 264, 12520–12525. [Google Scholar]
- Gannon, V.P.; Gyles, C.L.; Wilcock, B.P. Effects of Escherichia coli Shiga-like toxins (verotoxins) in pigs. Can J. Vet. Res. 1989, 53, 306–312. [Google Scholar]
- Schmidt, H. Shiga-toxin-converting bacteriophages. Res. Microbiol. 2001, 152, 687–695. [Google Scholar] [CrossRef]
- Herold, S.; Karch, H.; Schmidt, H. Shiga toxin-encoding bacteriophages--genomes in motion. Int. J. Med. Microbiol. 2004, 294, 115–121. [Google Scholar] [CrossRef]
- DeGrandis, S.; Ginsberg, J.; Toone, M.; Climie, S.; Friesen, J.; Brunton, J. Nucleotide sequence and promoter mapping of the Escherichia coli Shiga-like toxin operon of bacteriophage H-19B. J. Bacteriol. 1987, 169, 4313–4319. [Google Scholar] [CrossRef] [Green Version]
- Jackson, M.P.; Newland, J.W.; Holmes, R.K.; O’Brien, A.D. Nucleotide sequence analysis of the structural genes for Shiga-like toxin I encoded by bacteriophage 933J from Escherichia coli. Microb. Pathog. 1987, 2, 147–153. [Google Scholar] [CrossRef]
- Ramotar, K.; Boyd, B.; Tyrrell, G.; Gariepy, J.; Lingwood, C.; Brunton, J. Characterization of Shiga-like toxin I B subunit purified from overproducing clones of the SLT-I B cistron. Biochem. J. 1990, 272, 805–811. [Google Scholar] [CrossRef] [Green Version]
- Seidah, N.G.; Donohue-Rolfe, A.; Lazure, C.; Auclair, F.; Keusch, G.T.; Chretien, M. Complete amino acid sequence of Shigella toxin B-chain. A novel polypeptide containing 69 amino acids and one disulfide bridge. J. Biol. Chem. 1986, 261, 13928–13931. [Google Scholar]
- Stein, P.E.; Boodhoo, A.; Tyrrell, G.J.; Brunton, J.L.; Read, R.J. Crystal structure of the cell-binding B oligomer of verotoxin-1 from E. coli. Nature 1992, 355, 748–750. [Google Scholar] [CrossRef] [PubMed]
- Bast, D.J.; Banerjee, L.; Clark, C.; Read, R.J.; Brunton, J.L. The identification of three biologically relevant globotriaosyl ceramide receptor binding sites on the Verotoxin 1 B subunit. Mol. Microbiol. 1999, 32, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.T.; Gariepy, J. Local conformational change in the B-subunit of Shiga-like toxin 1 at endosomal pH. Biochemistry 1993, 32, 918–922. [Google Scholar] [CrossRef]
- Fraser, M.E.; Fujinaga, M.; Cherney, M.M.; Melton-Celsa, A.R.; Twiddy, E.M.; O’Brien, A.D.; James, M.N. Structure of shiga toxin type 2 (Stx2) from Escherichia coli O157:H7. J. Biol. Chem. 2004, 279, 27511–27517. [Google Scholar] [CrossRef] [Green Version]
- Jackson, M.P.; Wadolkowski, E.A.; Weinstein, D.L.; Holmes, R.K.; O’Brien, A.D. Functional analysis of the Shiga toxin and Shiga-like toxin type II variant binding subunits by using site-directed mutagenesis. J. Bacteriol. 1990, 172, 653–658. [Google Scholar] [CrossRef] [Green Version]
- Lingwood, C.A.; Mylvaganam, M.; Arab, S.; Khine, A.A.; Magnusson, G.; Grinstein, S.; Nyholm, P.G. Shiga toxin (verotoxin) binding to its receptor glycolipid. In Escherichia coli O157:H7 and Other Shiga Toxin-Producing E. coli Strains; Kaper, J.B., O’Brien, A.D., Eds.; ASM Press: Washington, DC, USA, 1998; pp. 129–139. [Google Scholar]
- Tyrrell, G.J.; Ramotar, K.; Toye, B.; Boyd, B.; Lingwood, C.A.; Brunton, J.L. Alteration of the carbohydrate binding specificity of verotoxins from Gal alpha 1-4Gal to GalNAc beta 1-3Gal alpha 1-4Gal and vice versa by site-directed mutagenesis of the binding subunit. Proc. Natl. Acad. Sci. USA 1992, 89, 524–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyholm, P.G.; Magnusson, G.; Zheng, Z.; Norel, R.; Binnington-Boyd, B.; Lingwood, C.A. Two distinct binding sites for globotriaosyl ceramide on verotoxins: identification by molecular modelling and confirmation using deoxy analogues and a new glycolipid receptor for all verotoxins. Chem. Biol. 1996, 3, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Clark, C.; Bast, D.; Sharp, A.M.; St Hilaire, P.M.; Agha, R.; Stein, P.E.; Toone, E.J.; Read, R.J.; Brunton, J.L. Phenylalanine 30 plays an important role in receptor binding of verotoxin-1. Mol. Microbiol. 1996, 19, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Perera, L.P.; Samuel, J.E.; Holmes, R.K.; O’Brien, A.D. Identification of three amino acid residues in the B subunit of Shiga toxin and Shiga-like toxin type II that are essential for holotoxin activity. J. Bacteriol. 1991, 173, 1151–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lingwood, C.A.; Yiu, S.K. Glycolipid modification of alpha 2 interferon binding. Sequence similarity between the alpha 2 interferon receptor and verotoxin (Shiga-like toxin) B-subunit. Biochem. J. 1992, 283 Pt 1, 25–26. [Google Scholar] [CrossRef]
- Fraser, M.E.; Chernaia, M.M.; Kozlov, Y.V.; James, M.N. Crystal structure of the holotoxin from Shigella dysenteriae at 2.5 A resolution. Nat. Struct. Biol. 1994, 1, 59–64. [Google Scholar] [CrossRef]
- Perera, L.P.; Samuel, J.E.; Holmes, R.K.; O’Brien, A.D. Mapping the minimal contiguous gene segment that encodes functionally active Shiga-like toxin II. Infect. Immun. 1991, 59, 829–835. [Google Scholar] [CrossRef] [Green Version]
- Austin, P.R.; Jablonski, P.E.; Bohach, G.A.; Dunker, A.K.; Hovde, C.J. Evidence that the A2 fragment of Shiga-like toxin type I is required for holotoxin integrity. Infect. Immun. 1994, 62, 1768–1775. [Google Scholar] [CrossRef] [Green Version]
- Haddad, J.E.; Jackson, M.P. Identification of the Shiga toxin A-subunit residues required for holotoxin assembly. J. Bacteriol. 1993, 175, 7652–7657. [Google Scholar] [CrossRef] [Green Version]
- Legros, N.; Pohlentz, G.; Steil, D.; Muthing, J. Shiga toxin-glycosphingolipid interaction: Status quo of research with focus on primary human brain and kidney endothelial cells. Int. J. Med. Microbiol. 2018, 308, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Gyles, C.L.; De Grandis, S.A.; MacKenzie, C.; Brunton, J.L. Cloning and nucleotide sequence analysis of the genes determining verocytotoxin production in a porcine edema disease isolate of Escherichia coli. Microb. Pathog. 1988, 5, 419–426. [Google Scholar] [CrossRef]
- Takeda, Y.; Kurazono, H.; Yamasaki, S. Vero toxins (Shiga-like toxins) produced by enterohemorrhagic Escherichia coli (verocytotoxin-producing E. coli). Microbiol. Immunol. 1993, 37, 591–599. [Google Scholar] [CrossRef]
- Jackson, M.P. Structure-function analyses of Shiga toxin and the Shiga-like toxins. Microb. Pathog. 1990, 8, 235–242. [Google Scholar] [CrossRef]
- Basu, D.; Li, X.P.; Kahn, J.N.; May, K.L.; Kahn, P.C.; Tumer, N.E. The A1 Subunit of Shiga Toxin 2 Has Higher Affinity for Ribosomes and Higher Catalytic Activity than the A1 Subunit of Shiga Toxin 1. Infect. Immun. 2016, 84, 149–161. [Google Scholar] [CrossRef] [Green Version]
- Fuller, C.A.; Pellino, C.A.; Flagler, M.J.; Strasser, J.E.; Weiss, A.A. Shiga toxin subtypes display dramatic differences in potency. Infect. Immun. 2011, 79, 1329–1337. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Ryu, S.H.; Lee, S.H.; Lee, Y.H.; Lee, S.R.; Huh, J.W.; Kim, S.U.; Kim, E.; Kim, S.; Jon, S.; et al. Instability of toxin A subunit of AB(5) toxins in the bacterial periplasm caused by deficiency of their cognate B subunits. Biochim. Biophys. Acta 2011, 1808, 2359–2365. [Google Scholar] [CrossRef] [Green Version]
- Pellino, C.A.; Karve, S.S.; Pradhan, S.; Weiss, A.A. AB5 Preassembly Is Not Required for Shiga Toxin Activity. J. Bacteriol. 2016, 198, 1621–1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Nours, J.; Paton, A.W.; Byres, E.; Troy, S.; Herdman, B.P.; Johnson, M.D.; Paton, J.C.; Rossjohn, J.; Beddoe, T. Structural basis of subtilase cytotoxin SubAB assembly. J. Biol. Chem. 2013, 288, 27505–27516. [Google Scholar] [CrossRef] [Green Version]
- Pellizzari, A.; Pang, H.; Lingwood, C.A. Binding of verocytotoxin 1 to its receptor is influenced by differences in receptor fatty acid content. Biochemistry 1992, 31, 1363–1370. [Google Scholar] [CrossRef]
- Lindberg, A.A.; Brown, J.E.; Stromberg, N.; Westling-Ryd, M.; Schultz, J.E.; Karlsson, K.A. Identification of the carbohydrate receptor for Shiga toxin produced by Shigella dysenteriae type 1. J. Biol. Chem. 1987, 262, 1779–1785. [Google Scholar] [PubMed]
- Obrig, T.G.; Louise, C.B.; Lingwood, C.A.; Boyd, B.; Barley-Maloney, L.; Daniel, T.O. Endothelial heterogeneity in Shiga toxin receptors and responses. J. Biol. Chem. 1993, 268, 15484–15488. [Google Scholar]
- Jacewicz, M.S.; Mobassaleh, M.; Gross, S.K.; Balasubramanian, K.A.; Daniel, P.F.; Raghavan, S.; McCluer, R.H.; Keusch, G.T. Pathogenesis of Shigella diarrhea: XVII. A mammalian cell membrane glycolipid, Gb3, is required but not sufficient to confer sensitivity to Shiga toxin. J. Infect. Dis. 1994, 169, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Mobassaleh, M.; Mishra, K.; Keusch, G.T. A quantitative immunostaining method for the measurement of UDP-galactose:lactosylceramide galactosyltransferase for the synthesis of globotriaosylceramide in rabbit small intestine and HeLa cells. Anal. Biochem. 1993, 214, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Taga, S.; Tetaud, C.; Mangeney, M.; Tursz, T.; Wiels, J. Sequential changes in glycolipid expression during human B cell differentiation: enzymatic bases. Biochim. Biophys. Acta 1995, 1254, 56–65. [Google Scholar] [CrossRef]
- Lingwood, C.A. Verotoxin-binding in human renal sections. Nephron 1994, 66, 21–28. [Google Scholar] [CrossRef]
- Olsnes, S.; Reisbig, R.; Eiklid, K. Subunit structure of Shigella cytotoxin. J. Biol. Chem. 1981, 256, 8732–8738. [Google Scholar]
- Sandvig, K.; Prydz, K.; Ryd, M.; van Deurs, B. Endocytosis and intracellular transport of the glycolipid-binding ligand Shiga toxin in polarized MDCK cells. J. Cell Biol. 1991, 113, 553–562. [Google Scholar] [CrossRef] [Green Version]
- Obrig, T.G.; Del Vecchio, P.J.; Brown, J.E.; Moran, T.P.; Rowland, B.M.; Judge, T.K.; Rothman, S.W. Direct cytotoxic action of Shiga toxin on human vascular endothelial cells. Infect. Immun. 1988, 56, 2373–2378. [Google Scholar] [CrossRef] [Green Version]
- Pudymaitis, A.; Lingwood, C.A. Susceptibility to verotoxin as a function of the cell cycle. J. Cell Physiol. 1992, 150, 632–639. [Google Scholar] [CrossRef]
- Majoul, I.; Schmidt, T.; Pomasanova, M.; Boutkevich, E.; Kozlov, Y.; Soling, H.D. Differential expression of receptors for Shiga and Cholera toxin is regulated by the cell cycle. J. Cell Sci. 2002, 115, 817–826. [Google Scholar]
- Bhattacharjee, R.N.; Park, K.S.; Uematsu, S.; Okada, K.; Hoshino, K.; Takeda, K.; Takeuchi, O.; Akira, S.; Iida, T.; Honda, T. Escherichia coli verotoxin 1 mediates apoptosis in human HCT116 colon cancer cells by inducing overexpression of the GADD family of genes and S phase arrest. FEBS Lett. 2005, 579, 6604–6610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.E.; Echeverria, P.; Lindberg, A.A. Digalactosyl-containing glycolipids as cell surface receptors for shiga toxin of Shigella dysenteriae 1 and related cytotoxins of Escherichia coli. Rev. Infect. Dis. 1991, 13 Suppl 4, S298–303. [Google Scholar] [CrossRef]
- Ramegowda, B.; Tesh, V.L. Differentiation-associated toxin receptor modulation, cytokine production, and sensitivity to Shiga-like toxins in human monocytes and monocytic cell lines. Infect. Immun. 1996, 64, 1173–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pudymaitis, A.; Armstrong, G.; Lingwood, C.A. Verotoxin-resistant cell clones are deficient in the glycolipid globotriosylceramide: differential basis of phenotype. Arch. Biochem. Biophys. 1991, 286, 448–452. [Google Scholar] [CrossRef]
- Louise, C.B.; Obrig, T.G. Shiga toxin-associated hemolytic uremic syndrome: combined cytotoxic effects of shiga toxin and lipopolysaccharide (endotoxin) on human vascular endothelial cells in vitro. Infect. Immun. 1992, 60, 1536–1543. [Google Scholar] [CrossRef] [Green Version]
- van de Kar, N.C.; Monnens, L.A.; Karmali, M.A.; van Hinsbergh, V.W. Tumor necrosis factor and interleukin-1 induce expression of the verocytotoxin receptor globotriaosylceramide on human endothelial cells: implications for the pathogenesis of the hemolytic uremic syndrome. Blood 1992, 80, 2755–2764. [Google Scholar] [CrossRef] [Green Version]
- van de Kar, N.C.; Kooistra, T.; Vermeer, M.; Lesslauer, W.; Monnens, L.A.; van Hinsbergh, V.W. Tumor necrosis factor alpha induces endothelial galactosyl transferase activity and verocytotoxin receptors. Role of specific tumor necrosis factor receptors and protein kinase C. Blood 1995, 85, 734–743. [Google Scholar] [CrossRef]
- Stricklett, P.K.; Hughes, A.K.; Ergonul, Z.; Kohan, D.E. Molecular basis for up-regulation by inflammatory cytokines of Shiga toxin 1 cytotoxicity and globotriaosylceramide expression. J. Infect. Dis. 2002, 186, 976–982. [Google Scholar] [CrossRef]
- Kaye, S.A.; Louise, C.B.; Boyd, B.; Lingwood, C.A.; Obrig, T.G. Shiga toxin-associated hemolytic uremic syndrome: interleukin-1 beta enhancement of Shiga toxin cytotoxicity toward human vascular endothelial cells in vitro. Infect. Immun. 1993, 61, 3886–3891. [Google Scholar] [CrossRef] [Green Version]
- van Setten, P.A.; van Hinsbergh, V.W.; van der Velden, T.J.; van de Kar, N.C.; Vermeer, M.; Mahan, J.D.; Assmann, K.J.; van den Heuvel, L.P.; Monnens, L.A. Effects of TNF alpha on verocytotoxin cytotoxicity in purified human glomerular microvascular endothelial cells. Kidney Int. 1997, 51, 1245–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenhauer, P.B.; Chaturvedi, P.; Fine, R.E.; Ritchie, A.J.; Pober, J.S.; Cleary, T.G.; Newburg, D.S. Tumor necrosis factor alpha increases human cerebral endothelial cell Gb3 and sensitivity to Shiga toxin. Infect. Immun. 2001, 69, 1889–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramegowda, B.; Samuel, J.E.; Tesh, V.L. Interaction of Shiga toxins with human brain microvascular endothelial cells: cytokines as sensitizing agents. J. Infect. Dis. 1999, 180, 1205–1213. [Google Scholar] [CrossRef] [Green Version]
- Keusch, G.T.; Acheson, D.W.; Aaldering, L.; Erban, J.; Jacewicz, M.S. Comparison of the effects of Shiga-like toxin 1 on cytokine- and butyrate-treated human umbilical and saphenous vein endothelial cells. J. Infect. Dis. 1996, 173, 1164–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Head, S.C.; Karmali, M.A.; Lingwood, C.A. Preparation of VT1 and VT2 hybrid toxins from their purified dissociated subunits. Evidence for B subunit modulation of a subunit function. J. Biol. Chem. 1991, 266, 3617–3621. [Google Scholar] [PubMed]
- Samuel, J.E.; Perera, L.P.; Ward, S.; O’Brien, A.D.; Ginsburg, V.; Krivan, H.C. Comparison of the glycolipid receptor specificities of Shiga-like toxin type II and Shiga-like toxin type II variants. Infect. Immun. 1990, 58, 611–618. [Google Scholar] [CrossRef] [Green Version]
- Imai, Y.; Fukui, T.; Kurohane, K.; Miyamoto, D.; Suzuki, Y.; Ishikawa, T.; Ono, Y.; Miyake, M. Restricted expression of shiga toxin binding sites on mucosal epithelium of mouse distal colon. Infect. Immun. 2003, 71, 985–990. [Google Scholar] [CrossRef] [Green Version]
- Keusch, G.T.; Jacewicz, M.; Mobassaleh, M.; Donohue-Rolfe, A. Shiga toxin: intestinal cell receptors and pathophysiology of enterotoxic effects. Rev. Infect. Dis. 1991, 13, S304–S310. [Google Scholar] [CrossRef]
- Mobassaleh, M.; Donohue-Rolfe, A.; Jacewicz, M.; Grand, R.J.; Keusch, G.T. Pathogenesis of shigella diarrhea: evidence for a developmentally regulated glycolipid receptor for shigella toxin involved in the fluid secretory response of rabbit small intestine. J. Infect. Dis. 1988, 157, 1023–1031. [Google Scholar] [CrossRef]
- Pruimboom-Brees, I.M.; Morgan, T.W.; Ackermann, M.R.; Nystrom, E.D.; Samuel, J.E.; Cornick, N.A.; Moon, H.W. Cattle lack vascular receptors for Escherichia coli O157:H7 Shiga toxins. Proc. Natl. Acad. Sci. USA 2000, 97, 10325–10329. [Google Scholar] [CrossRef] [Green Version]
- Hoey, D.E.; Currie, C.; Else, R.W.; Nutikka, A.; Lingwood, C.A.; Gally, D.L.; Smith, D.G. Expression of receptors for verotoxin 1 from Escherichia coli O157 on bovine intestinal epithelium. J. Med. Microbiol. 2002, 51, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Stamm, I.; Mohr, M.; Bridger, P.S.; Schropfer, E.; Konig, M.; Stoffregen, W.C.; Dean-Nystrom, E.A.; Baljer, G.; Menge, C. Epithelial and mesenchymal cells in the bovine colonic mucosa Differ. in their responsiveness to Escherichia coli Shiga toxin 1. Infect. Immun. 2008, 76, 5381–5391. [Google Scholar] [CrossRef] [Green Version]
- Schuller, S.; Heuschkel, R.; Torrente, F.; Kaper, J.B.; Phillips, A.D. Shiga toxin binding in normal and inflamed human intestinal mucosa. Microbes Infect. 2007, 9, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Louise, C.B.; Obrig, T.G. Shiga toxin-associated hemolytic-uremic syndrome: Combined cytotoxic effects of Shiga toxin, interleukin-1 beta, and tumor necrosis factor alpha on human vascular endothelial cells in vitro. Infect. Immun. 1991, 59, 4173–4179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molostvov, G.; Morris, A.; Rose, P.; Basu, S. Interaction of cytokines and growth factor in the regulation of verotoxin-induced apoptosis in cultured human endothelial cells. Br. J. Haematol. 2001, 113, 891–897. [Google Scholar] [CrossRef]
- Ohmi, K.; Kiyokawa, N.; Takeda, T.; Fujimoto, J. Human microvascular endothelial cells are strongly sensitive to Shiga toxins. Biochem. Biophys. Res. Commun. 1998, 251, 137–141. [Google Scholar] [CrossRef]
- Yoshida, T.; Fukada, M.; Koide, N.; Ikeda, H.; Sugiyama, T.; Kato, Y.; Ishikawa, N.; Yokochi, T. Primary cultures of human endothelial cells are susceptible to low doses of Shiga toxins and undergo apoptosis. J. Infect. Dis. 1999, 180, 2048–2052. [Google Scholar] [CrossRef] [Green Version]
- Bitzan, M.M.; Wang, Y.; Lin, J.; Marsden, P.A. Verotoxin and ricin have novel effects on preproendothelin-1 expression but fail to modify nitric oxide synthase (ecNOS) expression and NO production in vascular endothelium. J. Clin. Investig. 1998, 101, 372–382. [Google Scholar] [CrossRef] [Green Version]
- Erwert, R.D.; Winn, R.K.; Harlan, J.M.; Bannerman, D.D. Shiga-like toxin inhibition of FLICE-like inhibitory protein expression sensitizes endothelial cells to bacterial lipopolysaccharide-induced apoptosis. J. Biol. Chem. 2002, 277, 40567–40574. [Google Scholar] [CrossRef] [Green Version]
- Obrig, T.G.; Seaner, R.M.; Bentz, M.; Lingwood, C.A.; Boyd, B.; Smith, A.; Narrow, W. Induction by sphingomyelinase of shiga toxin receptor and shiga toxin 2 sensitivity in human microvascular endothelial cells. Infect. Immun. 2003, 71, 845–849. [Google Scholar] [CrossRef] [Green Version]
- Pijpers, A.H.; van Setten, P.A.; van den Heuvel, L.P.; Assmann, K.J.; Dijkman, H.B.; Pennings, A.H.; Monnens, L.A.; van Hinsbergh, V.W. Verocytotoxin-induced apoptosis of human microvascular endothelial cells. J. Am. Soc. Nephrol. 2001, 12, 767–778. [Google Scholar] [PubMed]
- Jacewicz, M.S.; Acheson, D.W.; Binion, D.G.; West, G.A.; Lincicome, L.L.; Fiocchi, C.; Keusch, G.T. Responses of human intestinal microvascular endothelial cells to Shiga toxins 1 and 2 and pathogenesis of hemorrhagic colitis. Infect. Immun. 1999, 67, 1439–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, S.E.; Rotman, T.A.; Jay, V.; Smith, C.R.; Becker, L.E.; Petric, M.; Olivieri, N.F.; Karmali, M.A. Experimental verocytotoxemia in rabbits. Infect. Immun. 1992, 60, 4154–4167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaisri, U.; Nagata, M.; Kurazono, H.; Horie, H.; Tongtawe, P.; Hayashi, H.; Watanabe, T.; Tapchaisri, P.; Chongsa-nguan, M.; Chaicumpa, W. Localization of Shiga toxins of enterohaemorrhagic Escherichia coli in kidneys of paediatric and geriatric patients with fatal haemolytic uraemic syndrome. Microb. Pathog. 2001, 31, 59–67. [Google Scholar] [CrossRef]
- Louise, C.B.; Obrig, T.G. Human renal microvascular endothelial cells as a potential target in the development of the hemolytic uremic syndrome as related to fibrinolysis factor expression, in vitro. Microvasc. Res. 1994, 47, 377–387. [Google Scholar] [CrossRef]
- Winter, K.R.; Stoffregen, W.C.; Dean-Nystrom, E.A. Shiga toxin binding to isolated porcine tissues and peripheral blood leukocytes. Infect. Immun. 2004, 72, 6680–6684. [Google Scholar] [CrossRef] [Green Version]
- Legros, N.; Dusny, S.; Humpf, H.U.; Pohlentz, G.; Karch, H.; Muthing, J. Shiga toxin glycosphingolipid receptors and their lipid membrane ensemble in primary human blood-brain barrier endothelial cells. Glycobiology 2017, 27, 99–109. [Google Scholar] [CrossRef]
- Rutjes, N.W.; Binnington, B.A.; Smith, C.R.; Maloney, M.D.; Lingwood, C.A. Differential tissue targeting and pathogenesis of verotoxins 1 and 2 in the mouse animal model. Kidney Int. 2002, 62, 832–845. [Google Scholar] [CrossRef] [Green Version]
- Park, J.Y.; Jeong, Y.J.; Park, S.K.; Yoon, S.J.; Choi, S.; Jeong, D.G.; Chung, S.W.; Lee, B.J.; Kim, J.H.; Tesh, V.L.; et al. Shiga Toxins Induce Apoptosis and ER Stress in Human Retinal Pigment Epithelial Cells. Toxins (Basel) 2017, 9, 319. [Google Scholar] [CrossRef] [Green Version]
- Chark, D.; Nutikka, A.; Trusevych, N.; Kuzmina, J.; Lingwood, C. Differential carbohydrate epitope recognition of globotriaosyl ceramide by verotoxins and a monoclonal antibody. Eur. J. Biochem. 2004, 271, 405–417. [Google Scholar] [CrossRef]
- Hughes, A.K.; Stricklett, P.K.; Kohan, D.E. Cytotoxic effect of Shiga toxin-1 on human proximal tubule cells. Kidney Int. 1998, 54, 426–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiyokawa, N.; Taguchi, T.; Mori, T.; Uchida, H.; Sato, N.; Takeda, T.; Fujimoto, J. Induction of apoptosis in normal human renal tubular epithelial cells by Escherichia coli Shiga toxins 1 and 2. J. Infect. Dis. 1998, 178, 178–184. [Google Scholar] [CrossRef] [Green Version]
- Imai, Y.; Fukui, T.; Ikegaya, A.; Ishikawa, T.; Ono, Y.; Kurohane, K. Lack of Shiga-like toxin binding sites in germinal centres of mouse lymphoid tissues. Immunology 2002, 105, 509–514. [Google Scholar] [CrossRef]
- Zoja, C.; Corna, D.; Farina, C.; Sacchi, G.; Lingwood, C.; Doyle, M.P.; Padhye, V.V.; Abbate, M.; Remuzzi, G. Verotoxin glycolipid receptors determine the localization of microangiopathic process in rabbits given verotoxin-1. J. Lab. Clin. Med. 1992, 120, 229–238. [Google Scholar] [PubMed]
- Hughes, A.K.; Stricklett, P.K.; Schmid, D.; Kohan, D.E. Cytotoxic effect of Shiga toxin-1 on human glomerular epithelial cells. Kidney Int. 2000, 57, 2350–2359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Setten, P.A.; Monnens, L.A.; Verstraten, R.G.; van den Heuvel, L.P.; van Hinsbergh, V.W. Effects of verocytotoxin-1 on nonadherent human monocytes: binding characteristics, protein synthesis, and induction of cytokine release. Blood 1996, 88, 174–183. [Google Scholar] [CrossRef] [Green Version]
- Kniep, B.; Monner, D.A.; Schwulera, U.; Muhlradt, P.F. Glycosphingolipids of the globo-series are associated with the monocytic lineage of human myeloid cells. Eur. J. Biochem. 1985, 149, 187–191. [Google Scholar] [CrossRef]
- Tesh, V.L.; Ramegowda, B.; Samuel, J.E. Purified Shiga-like toxins induce expression of proinflammatory cytokines from murine peritoneal macrophages. Infect. Immun. 1994, 62, 5085–5094. [Google Scholar] [CrossRef] [Green Version]
- Menge, C.; Loos, D.; Bridger, P.S.; Barth, S.; Werling, D.; Baljer, G. Bovine macrophages sense Escherichia coli Shiga toxin 1. Innate Immun. 2015, 21, 655–664. [Google Scholar] [CrossRef] [Green Version]
- Van Setten, P.A.; van Hinsbergh, V.W.; Van den Heuvel, L.P.; van der Velden, T.J.; van de Kar, N.C.; Krebbers, R.J.; Karmali, M.A.; Monnens, L.A. Verocytotoxin inhibits mitogenesis and protein synthesis in purified human glomerular mesangial cells without affecting cell viability: evidence for two distinct mechanisms. J. Am. Soc. Nephrol. 1997, 8, 1877–1888. [Google Scholar]
- Robinson, L.A.; Hurley, R.M.; Lingwood, C.; Matsell, D.G. Escherichia coli verotoxin binding to human paediatric glomerular mesangial cells. Pediatr. Nephrol. 1995, 9, 700–704. [Google Scholar] [CrossRef]
- Mengeling, W.L.; Vorwald, A.C.; Cornick, N.A.; Lager, K.M.; Moon, H.W. In vitro detection of Shiga toxin using porcine alveolar macrophages. J. Vet. Diagn. Investig. 2001, 13, 421–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menge, C.; Stamm, I.; Wuhrer, M.; Geyer, R.; Wieler, L.H.; Baljer, G. Globotriaosylceramide (Gb(3)/CD77) is synthesized and surface expressed by bovine lymphocytes upon activation in vitro. Vet. Immunol. Immunopathol. 2001, 83, 19–36. [Google Scholar] [CrossRef]
- Menge, C.; Blessenohl, M.; Eisenberg, T.; Stamm, I.; Baljer, G. Bovine ileal intraepithelial lymphocytes represent target cells for Shiga toxin 1 from Escherichia coli. Infect. Immun. 2004, 72, 1896–1905. [Google Scholar] [CrossRef] [Green Version]
- Mangeney, M.; Richard, Y.; Coulaud, D.; Tursz, T.; Wiels, J. CD77: an antigen of germinal center B cells entering apoptosis. Eur. J. Immunol. 1991, 21, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Mangeney, M.; Rousselet, G.; Taga, S.; Tursz, T.; Wiels, J. The fate of human CD77+ germinal center B lymphocytes after rescue from apoptosis. Mol. Immunol. 1995, 32, 333–339. [Google Scholar] [CrossRef]
- Gruner, K.R.; van Eijk, R.V.; Muhlradt, P.F. Structure elucidation of marker glycolipids of alloantigen-activated murine T lymphocytes. Biochemistry 1981, 20, 4518–4522. [Google Scholar] [CrossRef]
- Kniep, B.; Hunig, T.R.; Fitch, F.W.; Heuer, J.; Kolsch, E.; Muhlradt, P.F. Neutral glycosphingolipids of murine myeloma cells and helper, cytolytic, and suppressor T lymphocytes. Biochemistry 1983, 22, 251–255. [Google Scholar] [CrossRef]
- Menge, C.; Eisenberg, T.; Stamm, I.; Baljer, G. Comparison of binding and effects of Escherichia coli Shiga toxin 1 on bovine and ovine granulocytes. Vet. Immunol. Immunopathol. 2006, 113, 392–403. [Google Scholar] [CrossRef]
- Eisenberg, T. Untersuchungen zur Wirkung von Shigatoxin 1 von Escherichia coli auf Zellen der unspezifischen Immunabwehr bei Rind, Schaf und Ziege, Dissertation ed.; Fachbereich Veterinärmedizin, Justus-Liebig-Universität Giessen: Hesse, Germany, 2003. [Google Scholar]
- Newburg, D.S.; Chaturvedi, P.; Lopez, E.L.; Devoto, S.; Fayad, A.; Cleary, T.G. Susceptibility to hemolytic-uremic syndrome relates to erythrocyte glycosphingolipid patterns. J. Infect. Dis. 1993, 168, 476–479. [Google Scholar] [CrossRef]
- Betz, J.; Dorn, I.; Kouzel, I.U.; Bauwens, A.; Meisen, I.; Kemper, B.; Bielaszewska, M.; Mormann, M.; Weymann, L.; Sibrowski, W.; et al. Shiga toxin of enterohaemorrhagic Escherichia coli directly injures developing human erythrocytes. Cell Microbiol. 2016, 18, 1339–1348. [Google Scholar] [CrossRef] [Green Version]
- Cooling, L.L.; Walker, K.E.; Gille, T.; Koerner, T.A. Shiga toxin binds human platelets via globotriaosylceramide (Pk antigen) and a novel platelet glycosphingolipid. Infect. Immun. 1998, 66, 4355–4366. [Google Scholar] [CrossRef]
- Karpman, D.; Papadopoulou, D.; Nilsson, K.; Sjogren, A.C.; Mikaelsson, C.; Lethagen, S. Platelet activation by Shiga toxin and circulatory factors as a pathogenetic mechanism in the hemolytic uremic syndrome. Blood 2001, 97, 3100–3108. [Google Scholar] [CrossRef] [Green Version]
- Steil, D.; Bonse, R.; Meisen, I.; Pohlentz, G.; Vallejo, G.; Karch, H.; Muthing, J. A Topographical Atlas of Shiga Toxin 2e Receptor Distribution in the Tissues of Weaned Piglets. Toxins (Basel) 2016, 8, 357. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; de Waard, A.A.; Wuhrer, M.; Spaapen, R.M. The Role of Glycosphingolipids in Immune Cell Functions. Front. Immunol. 2019, 10, 90. [Google Scholar] [CrossRef]
- Falguieres, T.; Mallard, F.; Baron, C.; Hanau, D.; Lingwood, C.; Goud, B.; Salamero, J.; Johannes, L. Targeting of Shiga toxin B-subunit to retrograde transport route in association with detergent-resistant membranes. Mol. Biol. Cell 2001, 12, 2453–2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katagiri, Y.U.; Mori, T.; Nakajima, H.; Katagiri, C.; Taguchi, T.; Takeda, T.; Kiyokawa, N.; Fujimoto, J. Activation of Src family kinase yes induced by Shiga toxin binding to globotriaosyl ceramide (Gb3/CD77) in low density, detergent-insoluble microdomains. J. Biol. Chem. 1999, 274, 35278–35282. [Google Scholar] [CrossRef] [Green Version]
- Kovbasnjuk, O.; Edidin, M.; Donowitz, M. Role of lipid rafts in Shiga toxin 1 interaction with the apical surface of Caco-2 cells. J. Cell Sci. 2001, 114, 4025–4031. [Google Scholar] [PubMed]
- Eiklid, K.; Olsnes, S. Interaction of Shigella shigae cytotoxin with receptors on sensitive and insensitive cells. J. Recept Res. 1980, 1, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, G.; Mobassaleh, M.; Donohue-Rolfe, A.; Montgomery, R.K.; Grand, R.J.; Keusch, G.T. Pathogenesis of Shigella diarrhea: rabbit intestinal cell microvillus membrane binding site for Shigella toxin. Infect. Immun. 1986, 53, 372–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- St Hilaire, P.M.; Boyd, M.K.; Toone, E.J. Interaction of the Shiga-like toxin type 1 B-subunit with its carbohydrate receptor. Biochemistry 1994, 33, 14452–14463. [Google Scholar] [CrossRef] [PubMed]
- Jacewicz, M.; Feldman, H.A.; Donohue-Rolfe, A.; Balasubramanian, K.A.; Keusch, G.T. Pathogenesis of Shigella diarrhea. XIV. Analysis of Shiga toxin receptors on cloned HeLa cells. J. Infect. Dis. 1989, 159, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Kiarash, A.; Boyd, B.; Lingwood, C.A. Glycosphingolipid receptor function is modified by fatty acid content. Verotoxin 1 and verotoxin 2c preferentially recognize different globotriaosyl ceramide fatty acid homologues. J. Biol. Chem. 1994, 269, 11138–11146. [Google Scholar]
- Karve, S.S.; Weiss, A.A. Glycolipid binding preferences of Shiga toxin variants. PLoS ONE 2014, 9, e101173. [Google Scholar] [CrossRef] [Green Version]
- Sandvig, K.; Olsnes, S.; Brown, J.E.; Petersen, O.W.; van Deurs, B. Endocytosis from coated pits of Shiga toxin: a glycolipid-binding protein from Shigella dysenteriae 1. J. Cell Biol. 1989, 108, 1331–1343. [Google Scholar] [CrossRef] [Green Version]
- Lingwood, C.A.; Law, H.; Richardson, S.; Petric, M.; Brunton, J.L.; De Grandis, S.; Karmali, M. Glycolipid binding of purified and recombinant Escherichia coli produced verotoxin in vitro. J. Biol. Chem. 1987, 262, 8834–8839. [Google Scholar]
- Waddell, T.; Head, S.; Petric, M.; Cohen, A.; Lingwood, C. Globotriosyl ceramide is specifically recognized by the Escherichia coli verocytotoxin 2. Biochem. Biophys. Res. Commun. 1988, 152, 674–679. [Google Scholar] [CrossRef]
- Cohen, A.; Hannigan, G.E.; Williams, B.R.; Lingwood, C.A. Roles of globotriosyl- and galabiosylceramide in verotoxin binding and high affinity interferon receptor. J. Biol. Chem. 1987, 262, 17088–17091. [Google Scholar]
- Newburg, D.S.; Ashkenazi, S.; Cleary, T.G. Human milk contains the Shiga toxin and Shiga-like toxin receptor glycolipid Gb3. J. Infect. Dis. 1992, 166, 832–836. [Google Scholar] [CrossRef]
- Kouzel, I.U.; Pohlentz, G.; Schmitz, J.S.; Steil, D.; Humpf, H.U.; Karch, H.; Muthing, J. Shiga Toxin Glycosphingolipid Receptors in Human Caco-2 and HCT-8 Colon Epithelial Cell Lines. Toxins (Basel) 2017, 9, 338. [Google Scholar] [CrossRef]
- Ito, H.; Yutsudo, T.; Hirayama, T.; Takeda, Y. Isolation and some properties of A and B subunits of Vero toxin 2 and in vitro formation of hybrid toxins between subunits of Vero toxin 1 and Vero toxin 2 from Escherichia coli O157:H7. Microb. Pathog. 1988, 5, 189–195. [Google Scholar] [CrossRef]
- Weinstein, D.L.; Jackson, M.P.; Perera, L.P.; Holmes, R.K.; O’Brien, A.D. In vivo formation of hybrid toxins comprising Shiga toxin and the Shiga-like toxins and role of the B subunit in localization and cytotoxic activity. Infect. Immun. 1989, 57, 3743–3750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacewicz, M.; Clausen, H.; Nudelman, E.; Donohue-Rolfe, A.; Keusch, G.T. Pathogenesis of shigella diarrhea. XI. Isolation of a shigella toxin-binding glycolipid from rabbit jejunum and HeLa cells and its identification as globotriaosylceramide. J. Exp. Med. 1986, 163, 1391–1404. [Google Scholar] [CrossRef] [PubMed]
- Maloney, M.D.; Lingwood, C.A. CD19 has a potential CD77 (globotriaosyl ceramide)-binding site with sequence similarity to verotoxin B-subunits: implications of molecular mimicry for B cell adhesion and enterohemorrhagic Escherichia coli pathogenesis. J. Exp. Med. 1994, 180, 191–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitova, E.N.; Kitov, P.I.; Bundle, D.R.; Klassen, J.S. The observation of multivalent complexes of Shiga-like toxin with globotriaoside and the determination of their stoichiometry by nanoelectrospray Fourier-transform ion cyclotron resonance mass spectrometry. Glycobiology 2001, 11, 605–611. [Google Scholar] [CrossRef] [Green Version]
- Soltyk, A.M.; MacKenzie, C.R.; Wolski, V.M.; Hirama, T.; Kitov, P.I.; Bundle, D.R.; Brunton, J.L. A mutational analysis of the globotriaosylceramide-binding sites of verotoxin VT1. J. Biol. Chem. 2002, 277, 5351–5359. [Google Scholar] [CrossRef] [Green Version]
- Ling, H.; Boodhoo, A.; Hazes, B.; Cummings, M.D.; Armstrong, G.D.; Brunton, J.L.; Read, R.J. Structure of the shiga-like toxin I B-pentamer complexed with an analogue of its receptor Gb3. Biochemistry 1998, 37, 1777–1788. [Google Scholar] [CrossRef]
- Wolski, V.M.; Soltyk, A.M.; Brunton, J.L. Mouse toxicity and cytokine release by verotoxin 1 B subunit mutants. Infect. Immun. 2001, 69, 579–583. [Google Scholar] [CrossRef] [Green Version]
- Tedder, T.F. CD19: A promising B cell target for rheumatoid arthritis. Nat. Rev. Rheumatol 2009, 5, 572–577. [Google Scholar] [CrossRef]
- Khine, A.A.; Firtel, M.; Lingwood, C.A. CD77-dependent retrograde transport of CD19 to the nuclear membrane: functional relationship between CD77 and CD19 during germinal center B-cell apoptosis. J. Cell Physiol. 1998, 176, 281–292. [Google Scholar] [CrossRef]
- Iwata, Y.; Yoshizaki, A.; Komura, K.; Shimizu, K.; Ogawa, F.; Hara, T.; Muroi, E.; Bae, S.; Takenaka, M.; Yukami, T.; et al. CD19, a response regulator of B lymphocytes, regulates wound healing through hyaluronan-induced TLR4 signaling. Am. J. Pathol. 2009, 175, 649–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghislain, J.; Lingwood, C.A.; Fish, E.N. Evidence for glycosphingolipid modification of the type 1 IFN receptor. J. Immunol. 1994, 153, 3655–3663. [Google Scholar] [PubMed]
- Maloney, M.D.; Binnington-Boyd, B.; Lingwood, C.A. Globotriaosyl ceramide modulates interferon-alpha-induced growth inhibition and CD19 expression in Burkitt’s lymphoma cells. Glycoconj. J. 1999, 16, 821–828. [Google Scholar] [CrossRef]
- Khine, A.A.; Lingwood, C.A. Functional significance of globotriaosyl ceramide in interferon-alpha(2)/type 1 interferon receptor-mediated antiviral activity. J. Cell Physiol. 2000, 182, 97–108. [Google Scholar] [CrossRef]
- Bukholm, G.; Degre, M. Shiga toxin inhibits the anti-invasive effect of interferons. J. Infect. Dis. 1988, 157, 849–850. [Google Scholar] [CrossRef]
- George, T.; Boyd, B.; Price, M.; Lingwood, C.; Maloney, M. MHC class II proteins contain a potential binding site for the verotoxin receptor glycolipid CD77. Cell Mol. Biol. (Noisy-le-grand) 2001, 47, 1179–1185. [Google Scholar]
- Reymond, D.; Johnson, R.P.; Karmali, M.A.; Petric, M.; Winkler, M.; Johnson, S.; Rahn, K.; Renwick, S.; Wilson, J.; Clarke, R.C.; et al. Neutralizing antibodies to Escherichia coli Vero cytotoxin 1 and antibodies to O157 lipopolysaccharide in healthy farm family members and urban residents. J. Clin. Microbiol. 1996, 34, 2053–2057. [Google Scholar] [CrossRef] [Green Version]
- Torgersen, M.L.; Engedal, N.; Pedersen, A.M.; Husebye, H.; Espevik, T.; Sandvig, K. Toll-like receptor 4 facilitates binding of Shiga toxin to colon carcinoma and primary umbilical vein endothelial cells. FEMS Immunol. Med. Microbiol. 2011, 61, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Torgersen, M.L.; Lauvrak, S.U.; Sandvig, K. The A-subunit of surface-bound Shiga toxin stimulates clathrin-dependent uptake of the toxin. FEBS J. 2005, 272, 4103–4113. [Google Scholar] [CrossRef]
- Pezeshkian, W.; Gao, H.; Arumugam, S.; Becken, U.; Bassereau, P.; Florent, J.C.; Ipsen, J.H.; Johannes, L.; Shillcock, J.C. Mechanism of Shiga Toxin Clustering on Membranes. ACS Nano 2017, 11, 314–324. [Google Scholar] [CrossRef] [Green Version]
- Sandvig, K.; van Deurs, B. Endocytosis and intracellular sorting of ricin and Shiga toxin. FEBS Lett. 1994, 346, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Marcato, P.; Vander Helm, K.; Mulvey, G.L.; Armstrong, G.D. Serum amyloid P component binding to Shiga toxin 2 requires both a subunit and B pentamer. Infect. Immun. 2003, 71, 6075–6078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pezeshkian, W.; Hansen, A.G.; Johannes, L.; Khandelia, H.; Shillcock, J.C.; Kumar, P.B.; Ipsen, J.H. Membrane invagination induced by Shiga toxin B-subunit: from molecular structure to tube formation. Soft Matter 2016, 12, 5164–5171. [Google Scholar] [CrossRef] [Green Version]
- Lauvrak, S.U.; Torgersen, M.L.; Sandvig, K. Efficient endosome-to-Golgi transport of Shiga toxin is dependent on dynamin and clathrin. J. Cell Sci. 2004, 117, 2321–2331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, M.J.; Lampe, M.; Merrifield, C.J. A feedback loop between dynamin and actin recruitment during clathrin-mediated endocytosis. PLoS Biol. 2012, 10, e1001302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, Y.; Haucke, V. Membrane shaping by the Bin/amphiphysin/Rvs (BAR) domain protein superfamily. Cell Mol. Life Sci. 2011, 68, 3983–3993. [Google Scholar] [CrossRef]
- Renard, H.F.; Simunovic, M.; Lemiere, J.; Boucrot, E.; Garcia-Castillo, M.D.; Arumugam, S.; Chambon, V.; Lamaze, C.; Wunder, C.; Kenworthy, A.K.; et al. Endophilin-A2 functions in membrane scission in clathrin-independent endocytosis. Nature 2015, 517, 493–496. [Google Scholar] [CrossRef]
- Hehnly, H.; Sheff, D.; Stamnes, M. Shiga toxin facilitates its retrograde transport by modifying microtubule dynamics. Mol. Biol. Cell 2006, 17, 4379–4389. [Google Scholar] [CrossRef] [Green Version]
- Arab, S.; Lingwood, C.A. Intracellular targeting of the endoplasmic reticulum/nuclear envelope by retrograde transport may determine cell hypersensitivity to verotoxin via globotriaosyl ceramide fatty acid isoform traffic. J. Cell Physiol. 1998, 177, 646–660. [Google Scholar] [CrossRef]
- Sandvig, K.; Ryd, M.; Garred, O.; Schweda, E.; Holm, P.K.; van Deurs, B. Retrograde transport from the Golgi complex to the ER of both Shiga toxin and the nontoxic Shiga B-fragment is regulated by butyric acid and cAMP. J. Cell Biol. 1994, 126, 53–64. [Google Scholar] [CrossRef]
- Johannes, L.; Goud, B. Facing inward from compartment shores: how many pathways were we looking for? Traffic 2000, 1, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Nichols, B.J.; Kenworthy, A.K.; Polishchuk, R.S.; Lodge, R.; Roberts, T.H.; Hirschberg, K.; Phair, R.D.; Lippincott-Schwartz, J. Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J. Cell Biol. 2001, 153, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Mallard, F.; Antony, C.; Tenza, D.; Salamero, J.; Goud, B.; Johannes, L. Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of shiga toxin B-fragment transport. J. Cell Biol. 1998, 143, 973–990. [Google Scholar] [CrossRef] [PubMed]
- Mallard, F.; Tang, B.L.; Galli, T.; Tenza, D.; Saint-Pol, A.; Yue, X.; Antony, C.; Hong, W.; Goud, B.; Johannes, L. Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J. Cell Biol. 2002, 156, 653–664. [Google Scholar] [CrossRef] [Green Version]
- Renard, H.F.; Garcia-Castillo, M.D.; Chambon, V.; Lamaze, C.; Johannes, L. Shiga toxin stimulates clathrin-independent endocytosis of the VAMP2, VAMP3 and VAMP8 SNARE proteins. J. Cell Sci. 2015, 128, 2891–2902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, G.; Lu, L.; Wang, T.L.; Tang, B.L.; Goud, B.; Johannes, L.; Hong, W. Participation of the syntaxin 5/Ykt6/GS28/GS15 SNARE complex in transport from the early/recycling endosome to the trans-Golgi network. Mol. Biol. Cell 2004, 15, 4011–4022. [Google Scholar] [CrossRef] [Green Version]
- Saint-Pol, A.; Yelamos, B.; Amessou, M.; Mills, I.G.; Dugast, M.; Tenza, D.; Schu, P.; Antony, C.; McMahon, H.T.; Lamaze, C.; et al. Clathrin adaptor epsinR is required for retrograde sorting on early endosomal membranes. Dev. Cell 2004, 6, 525–538. [Google Scholar] [CrossRef] [Green Version]
- Kouzel, I.U.; Kehl, A.; Berger, P.; Liashkovich, I.; Steil, D.; Makalowski, W.; Suzuki, Y.; Pohlentz, G.; Karch, H.; Mellmann, A.; et al. RAB5A and TRAPPC6B are novel targets for Shiga toxin 2a inactivation in kidney epithelial cells. Sci. Rep. 2020, 10, 4945. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, J.L.; Anderson, R.G.; Brown, M.S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature 1979, 279, 679–685. [Google Scholar] [CrossRef]
- Girod, A.; Storrie, B.; Simpson, J.C.; Johannes, L.; Goud, B.; Roberts, L.M.; Lord, J.M.; Nilsson, T.; Pepperkok, R. Evidence for a COP-I-independent transport route from the Golgi complex to the endoplasmic reticulum. Nat. Cell Biol. 1999, 1, 423–430. [Google Scholar] [CrossRef]
- White, J.; Johannes, L.; Mallard, F.; Girod, A.; Grill, S.; Reinsch, S.; Keller, P.; Tzschaschel, B.; Echard, A.; Goud, B.; et al. Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J. Cell Biol. 1999, 147, 743–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandvig, K.; Garred, O.; Prydz, K.; Kozlov, J.V.; Hansen, S.H.; van Deurs, B. Retrograde transport of endocytosed Shiga toxin to the endoplasmic reticulum. Nature 1992, 358, 510–512. [Google Scholar] [CrossRef] [PubMed]
- Jacewicz, M.; Keusch, G.T. Pathogenesis of Shigella diarrhea. VIII. Evidence for a translocation step in the cytotoxic action of Shiga toxin. J. Infect. Dis. 1983, 148, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Melby, E.L.; Jacobsen, J.; Olsnes, S.; Sandvig, K. Entry of protein toxins in polarized epithelial cells. Cancer Res. 1993, 53, 1755–1760. [Google Scholar]
- Marsh, M.; McMahon, H.T. The structural era of endocytosis. Science 1999, 285, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Sandvig, K.; Grimmer, S.; Lauvrak, S.U.; Torgersen, M.L.; Skretting, G.; van Deurs, B.; Iversen, T.G. Pathways followed by ricin and Shiga toxin into cells. Histochem. Cell Biol. 2002, 117, 131–141. [Google Scholar] [CrossRef]
- Arfilli, V.; Carnicelli, D.; Rocchi, L.; Ricci, F.; Pagliaro, P.; Tazzari, P.L.; Brigotti, M. Shiga toxin 1 and ricin A chain bind to human polymorphonuclear leucocytes through a common receptor. Biochem. J. 2010, 432, 173–180. [Google Scholar] [CrossRef]
- Brigotti, M.; Carnicelli, D.; Arfilli, V.; Tamassia, N.; Borsetti, F.; Fabbri, E.; Tazzari, P.L.; Ricci, F.; Pagliaro, P.; Spisni, E.; et al. Identification of TLR4 as the receptor that recognizes Shiga toxins in human neutrophils. J. Immunol. 2013, 191, 4748–4758. [Google Scholar] [CrossRef] [Green Version]
- Brigotti, M.; Carnicelli, D.; Arfilli, V.; Porcellini, E.; Galassi, E.; Valerii, M.C.; Spisni, E. Human monocytes stimulated by Shiga toxin 1a via globotriaosylceramide release proinflammatory molecules associated with hemolytic uremic syndrome. Int. J. Med. Microbiol. 2018, 308, 940–946. [Google Scholar] [CrossRef]
- O’Brien, A.D.; Tesh, V.L.; Donohue-Rolfe, A.; Jackson, M.P.; Olsnes, S.; Sandvig, K.; Lindberg, A.A.; Keusch, G.T. Shiga toxin: Biochemistry, genetics, mode of action, and role in pathogenesis. Curr. Top. Microbiol. Immunol. 1992, 180, 65–94. [Google Scholar]
- O’Brien, A.D.; Holmes, R.K. Shiga and Shiga-like toxins. Microbiol. Rev. 1987, 51, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Samuel, J.E.; Gordon, V.M. Evidence that proteolytic separation of Shiga-like toxin type IIv A subunit into A1 and A2 subunits is not required for toxin activity. J. Biol. Chem. 1994, 269, 4853–4859. [Google Scholar] [PubMed]
- Garred, O.; Dubinina, E.; Holm, P.K.; Olsnes, S.; van Deurs, B.; Kozlov, J.V.; Sandvig, K. Role of processing and intracellular transport for optimal toxicity of Shiga toxin and toxin mutants. Exp. Cell Res. 1995, 218, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Sandvig, K.; Garred, O.; van Deurs, B. Intracellular transport and processing of protein toxins produced by enteric bacteria. Adv. Exp. Med. Biol. 1997, 412, 225–232. [Google Scholar] [PubMed]
- Reisbig, R.; Olsnes, S.; Eiklid, K. The cytotoxic activity of Shigella toxin. Evidence for catalytic inactivation of the 60 S ribosomal subunit. J. Biol. Chem. 1981, 256, 8739–8744. [Google Scholar] [PubMed]
- Li, X.P.; Tumer, N.E. Differences in Ribosome Binding and Sarcin/Ricin Loop Depurination by Shiga and Ricin Holotoxins. Toxins (Basel) 2017, 9, 133. [Google Scholar] [CrossRef]
- Sandvig, K.; Brown, J.E. Ionic requirements for entry of Shiga toxin from Shigella dysenteriae 1 into cells. Infect. Immun. 1987, 55, 298–303. [Google Scholar] [CrossRef] [Green Version]
- Garred, O.; Dubinina, E.; Polesskaya, A.; Olsnes, S.; Kozlov, J.; Sandvig, K. Role of the disulfide bond in Shiga toxin A-chain for toxin entry into cells. J. Biol. Chem. 1997, 272, 11414–11419. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Haslam, D.B. Shiga toxin is transported from the endoplasmic reticulum following interaction with the luminal chaperone HEDJ/ERdj3. Infect. Immun. 2005, 73, 2524–2532. [Google Scholar] [CrossRef] [Green Version]
- Falguieres, T.; Johannes, L. Shiga toxin B-subunit binds to the chaperone BiP and the nucleolar protein B23. Biol. Cell 2005, 98, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Hazes, B.; Read, R.J. Accumulating evidence suggests that several AB-toxins subvert the endoplasmic reticulum-associated protein degradation pathway to enter target cells. Biochemistry 1997, 36, 11051–11054. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yan, Q.; Lennarz, W.J. Complex, two-way traffic of molecules across the membrane of the endoplasmic reticulum. J. Biol. Chem. 1998, 273, 10083–10086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haicheur, N.; Bismuth, E.; Bosset, S.; Adotevi, O.; Warnier, G.; Lacabanne, V.; Regnault, A.; Desaymard, C.; Amigorena, S.; Ricciardi-Castagnoli, P.; et al. The B subunit of Shiga toxin fused to a tumor antigen elicits CTL and targets dendritic cells to allow MHC class I-restricted presentation of peptides derived from exogenous antigens. J. Immunol. 2000, 165, 3301–3308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haicheur, N.; Benchetrit, F.; Amessou, M.; Leclerc, C.; Falguieres, T.; Fayolle, C.; Bismuth, E.; Fridman, W.H.; Johannes, L.; Tartour, E. The B subunit of Shiga toxin coupled to full-size antigenic protein elicits humoral and cell-mediated immune responses associated with a Th1-dominant polarization. Int. Immunol. 2003, 15, 1161–1171. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.S.; Tartour, E.; van der Bruggen, P.; Vantomme, V.; Joyeux, I.; Goud, B.; Fridman, W.H.; Johannes, L. Major histocompatibility complex class I presentation of exogenous soluble tumor antigen fused to the B-fragment of Shiga toxin. Eur. J. Immunol. 1998, 28, 2726–2737. [Google Scholar] [CrossRef]
- Endo, Y.; Tsurugi, K.; Yutsudo, T.; Takeda, Y.; Ogasawara, T.; Igarashi, K. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur. J. Biochem. 1988, 171, 45–50. [Google Scholar] [CrossRef]
- Moazed, D.; Robertson, J.M.; Noller, H.F. Interaction of elongation factors EF-G and EF-Tu with a conserved loop in 23S RNA. Nature 1988, 334, 362–364. [Google Scholar] [CrossRef]
- Ogasawara, T.; Ito, K.; Igarashi, K.; Yutsudo, T.; Nakabayashi, N.; Takeda, Y. Inhibition of protein synthesis by a Vero toxin (VT2 or Shiga-like toxin II) produced by Escherichia coli O157:H7 at the level of elongation factor 1-dependent aminoacyl-tRNA binding to ribosomes. Microb. Pathog. 1988, 4, 127–135. [Google Scholar] [CrossRef]
- Yamasaki, S.; Furutani, M.; Ito, K.; Igarashi, K.; Nishibuchi, M.; Takeda, Y. Importance of arginine at position 170 of the A subunit of Vero toxin 1 produced by enterohemorrhagic Escherichia coli for toxin activity. Microb. Pathog. 1991, 11, 1–9. [Google Scholar] [CrossRef]
- Deresiewicz, R.L.; Calderwood, S.B.; Robertus, J.D.; Collier, R.J. Mutations affecting the activity of the Shiga-like toxin I A-chain. Biochemistry 1992, 31, 3272–3280. [Google Scholar] [CrossRef]
- Iordanov, M.S.; Pribnow, D.; Magun, J.L.; Dinh, T.H.; Pearson, J.A.; Chen, S.L.; Magun, B.E. Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the alpha-sarcin/ricin loop in the 28S rRNA. Mol. Cell Biol. 1997, 17, 3373–3381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obrig, T.G.; Moran, T.P.; Brown, J.E. The mode of action of Shiga toxin on peptide elongation of eukaryotic protein synthesis. Biochem. J. 1987, 244, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.E.; Rothman, S.W.; Doctor, B.P. Inhibition of protein synthesis in intact HeLa cells by Shigella dysenteriae 1 toxin. Infect. Immun. 1980, 29, 98–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez, A.; Vazquez, D. Plant and fungal protein and glycoprotein toxins inhibiting eukaryote protein synthesis. Annu Rev. Microbiol. 1985, 39, 649–672. [Google Scholar] [CrossRef] [PubMed]
- Facchini, L.M.; Lingwood, C.A. A verotoxin 1 B subunit-lambda CRO chimeric protein specifically binds both DNA and globotriaosylceramide (Gb(3)) to effect nuclear targeting of exogenous DNA in Gb(3) positive cells. Exp. Cell Res. 2001, 269, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, I.; Nakata, M.; Kawabata, S.; Hamada, S. Regulated expression of the Shiga toxin B gene induces apoptosis in mammalian fibroblastic cells. Mol. Microbiol. 1999, 33, 1190–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brigotti, M.; Accorsi, P.; Carnicelli, D.; Rizzi, S.; Gonzalez Vara, A.; Montanaro, L.; Sperti, S. Shiga toxin 1: damage to DNA in vitro. Toxicon 2001, 39, 341–348. [Google Scholar] [CrossRef]
- Brigotti, M.; Alfieri, R.; Sestili, P.; Bonelli, M.; Petronini, P.G.; Guidarelli, A.; Barbieri, L.; Stirpe, F.; Sperti, S. Damage to nuclear DNA induced by Shiga toxin 1 and ricin in human endothelial cells. FASEB J. 2002, 16, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Sandvig, K.; van Deurs, B. Toxin-induced cell lysis: protection by 3-methyladenine and cycloheximide. Exp. Cell Res. 1992, 200, 253–262. [Google Scholar] [CrossRef]
- Fujii, J.; Matsui, T.; Heatherly, D.P.; Schlegel, K.H.; Lobo, P.I.; Yutsudo, T.; Ciraolo, G.M.; Morris, R.E.; Obrig, T. Rapid apoptosis induced by Shiga toxin in HeLa cells. Infect. Immun. 2003, 71, 2724–2735. [Google Scholar] [CrossRef] [Green Version]
- Arends, M.J.; Wyllie, A.H. Apoptosis: mechanisms and roles in pathology. Int. Rev. Exp. Pathol. 1991, 32, 223–254. [Google Scholar] [PubMed]
- Chen, Y.; Zychlinsky, A. Apoptosis induced by bacterial pathogens. Microb. Pathog. 1994, 17, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Erwert, R.D.; Eiting, K.T.; Tupper, J.C.; Winn, R.K.; Harlan, J.M.; Bannerman, D.D. Shiga toxin induces decreased expression of the anti-apoptotic protein Mcl-1 concomitant with the onset of endothelial apoptosis. Microb. Pathog. 2003, 35, 87–93. [Google Scholar] [CrossRef]
- Inward, C.D.; Williams, J.; Chant, I.; Crocker, J.; Milford, D.V.; Rose, P.E.; Taylor, C.M. Verocytotoxin-1 induces apoptosis in vero cells. J. Infect. 1995, 30, 213–218. [Google Scholar] [CrossRef]
- Garibal, J.; Hollville, E.; Renouf, B.; Tetaud, C.; Wiels, J. Caspase-8-mediated cleavage of Bid and protein phosphatase 2A-mediated activation of Bax are necessary for Verotoxin-1-induced apoptosis in Burkitt’s lymphoma cells. Cell Signal 2010, 22, 467–475. [Google Scholar] [CrossRef]
- Tetaud, C.; Falguieres, T.; Carlier, K.; Lecluse, Y.; Garibal, J.; Coulaud, D.; Busson, P.; Steffensen, R.; Clausen, H.; Johannes, L.; et al. Two distinct Gb3/CD77 signaling pathways leading to apoptosis are triggered by anti-Gb3/CD77 mAb and verotoxin-1. J. Biol. Chem. 2003, 278, 45200–45208. [Google Scholar] [CrossRef] [Green Version]
- Smith, W.E.; Kane, A.V.; Campbell, S.T.; Acheson, D.W.; Cochran, B.H.; Thorpe, C.M. Shiga toxin 1 triggers a ribotoxic stress response leading to p38 and JNK activation and induction of apoptosis in intestinal epithelial cells. Infect. Immun. 2003, 71, 1497–1504. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, M.; Gunji, Y.; Yamasaki, S.; Takeda, Y. Shiga toxin activates p38 MAP kinase through cellular Ca(2+) increase in Vero cells. FEBS Lett. 2000, 485, 94–98. [Google Scholar] [CrossRef]
- Nelin, L.D.; White, H.A.; Jin, Y.; Trittmann, J.K.; Chen, B.; Liu, Y. The Src family tyrosine kinases src and yes have differential effects on inflammation-induced apoptosis in human pulmonary microvascular endothelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L880–L888. [Google Scholar] [CrossRef] [Green Version]
- Ching, J.C.; Jones, N.L.; Ceponis, P.J.; Karmali, M.A.; Sherman, P.M. Escherichia coli shiga-like toxins induce apoptosis and cleavage of poly(ADP-ribose) polymerase via in vitro activation of caspases. Infect. Immun. 2002, 70, 4669–4677. [Google Scholar] [CrossRef] [Green Version]
- Kiyokawa, N.; Mori, T.; Taguchi, T.; Saito, M.; Mimori, K.; Suzuki, T.; Sekino, T.; Sato, N.; Nakajima, H.; Katagiri, Y.U.; et al. Activation of the caspase cascade during Stx1-induced apoptosis in Burkitt’s lymphoma cells. J. Cell Biochem. 2001, 81, 128–142. [Google Scholar] [CrossRef]
- Sahara, S.; Aoto, M.; Eguchi, Y.; Imamoto, N.; Yoneda, Y.; Tsujimoto, Y. Acinus is a caspase-3-activated protein required for apoptotic chromatin condensation. Nature 1999, 401, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Kojio, S.; Zhang, H.; Ohmura, M.; Gondaira, F.; Kobayashi, N.; Yamamoto, T. Caspase-3 activation and apoptosis induction coupled with the retrograde transport of shiga toxin: inhibition by brefeldin A. FEMS Immunol. Med. Microbiol. 2000, 29, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Alnemri, E.S.; Lazebnik, Y.A.; Fernandes-Alnemri, T.; Litwack, G.; Moir, R.D.; Goldman, R.D.; Poirier, G.G.; Kaufmann, S.H.; Earnshaw, W.C. Cleavage of lamin A by Mch2 alpha but not CPP32: multiple interleukin 1 beta-converting enzyme-related proteases with distinct substrate recognition properties are active in apoptosis. Proc. Natl. Acad. Sci. USA 1996, 93, 8395–8400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.Y.; Cherla, R.P.; Caliskan, I.; Tesh, V.L. Shiga Toxin 1 Induces Apoptosis in the Human Myelogenous Leukemia Cell Line THP-1 by a Caspase-8-Dependent, Tumor Necrosis Factor Receptor-Independent Mechanism. Infect. Immun. 2005, 73, 5115–5126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sood, A.; Mathew, R.; Trachtman, H. Cytoprotective effect of curcumin in human proximal tubule epithelial cells exposed to shiga toxin. Biochem. Biophys. Res. Commun. 2001, 283, 36–41. [Google Scholar] [CrossRef]
- Edelmann, B.; Bertsch, U.; Tchikov, V.; Winoto-Morbach, S.; Perrotta, C.; Jakob, M.; Adam-Klages, S.; Kabelitz, D.; Schutze, S. Caspase-8 and caspase-7 sequentially mediate proteolytic activation of acid sphingomyelinase in TNF-R1 receptosomes. EMBO J. 2011, 30, 379–394. [Google Scholar] [CrossRef]
- Suzuki, A.; Doi, H.; Matsuzawa, F.; Aikawa, S.; Takiguchi, K.; Kawano, H.; Hayashida, M.; Ohno, S. Bcl-2 antiapoptotic protein mediates verotoxin II-induced cell death: possible association between bcl-2 and tissue failure by E. coli O157:H7. Genes Dev. 2000, 14, 1734–1740. [Google Scholar]
- Gordon, J.; Challa, A.; Levens, J.M.; Gregory, C.D.; Williams, J.M.; Armitage, R.J.; Cook, J.P.; Roberts, L.M.; Lord, J.M. CD40 ligand, Bcl-2, and Bcl-xL spare group I Burkitt lymphoma cells from CD77-directed killing via Verotoxin-1 B chain but fail to protect against the holotoxin. Cell Death Differ. 2000, 7, 785–794. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.S.; Cherla, R.P.; Leyva-Illades, D.; Tesh, V.L. Bcl-2 regulates the onset of shiga toxin 1-induced apoptosis in THP-1 cells. Infect. Immun. 2009, 77, 5233–5244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.S.; Cherla, R.P.; Lentz, E.K.; Leyva-Illades, D.; Tesh, V.L. Signaling through C/EBP homologous protein and death receptor 5 and calpain activation differentially regulate THP-1 cell maturation-dependent apoptosis induced by Shiga toxin type 1. Infect. Immun. 2010, 78, 3378–3391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsunaga, T.; Nakajima, T.; Sonoda, M.; Kawai, S.; Kobayashi, J.; Inoue, I.; Satomi, A.; Katayama, S.; Hara, A.; Hokari, S.; et al. Reactive oxygen species as a risk factor in verotoxin-1-exposed rats. Biochem. Biophys. Res. Commun. 1999, 260, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Platnich, J.M.; Chung, H.; Lau, A.; Sandall, C.F.; Bondzi-Simpson, A.; Chen, H.M.; Komada, T.; Trotman-Grant, A.C.; Brandelli, J.R.; Chun, J.; et al. Shiga Toxin/Lipopolysaccharide Activates Caspase-4 and Gasdermin D to Trigger Mitochondrial Reactive Oxygen Species Upstream of the NLRP3 Inflammasome. Cell Rep. 2018, 25, 1525–1536.e1527. [Google Scholar] [CrossRef] [Green Version]
- Herson, P.S.; Lee, K.; Pinnock, R.D.; Hughes, J.; Ashford, M.L. Hydrogen peroxide induces intracellular calcium overload by activation of a non-selective cation channel in an insulin-secreting cell line. J. Biol. Chem. 1999, 274, 833–841. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.M.; Lea, N.; Lord, J.M.; Roberts, L.M.; Milford, D.V.; Taylor, C.M. Comparison of ribosome-inactivating proteins in the induction of apoptosis. Toxicol. Lett. 1997, 91, 121–127. [Google Scholar] [CrossRef]
- Katagiri, Y.U.; Kiyokawa, N.; Fujimoto, J. The effect of shiga toxin binding to globotriaosylceramidein rafts of human kidney cells and Burkitt´slymphoma cells. Trends Glycosci. Glycotech. 2001, 13, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Mangeney, M.; Lingwood, C.A.; Taga, S.; Caillou, B.; Tursz, T.; Wiels, J. Apoptosis induced in Burkitt’s lymphoma cells via Gb3/CD77, a glycolipid antigen. Cancer Res. 1993, 53, 5314–5319. [Google Scholar]
- Taga, S.; Carlier, K.; Mishal, Z.; Capoulade, C.; Mangeney, M.; Lecluse, Y.; Coulaud, D.; Tetaud, C.; Pritchard, L.L.; Tursz, T.; et al. Intracellular signaling events in CD77-mediated apoptosis of Burkitt’s lymphoma cells. Blood 1997, 90, 2757–2767. [Google Scholar] [CrossRef]
- Mori, T.; Kiyokawa, N.; Katagiri, Y.U.; Taguchi, T.; Suzuki, T.; Sekino, T.; Sato, N.; Ohmi, K.; Nakajima, H.; Takeda, T.; et al. Globotriaosyl ceramide (CD77/Gb3) in the glycolipid-enriched membrane domain participates in B-cell receptor-mediated apoptosis by regulating lyn kinase activity in human B cells. Exp. Hematol. 2000, 28, 1260–1268. [Google Scholar] [CrossRef]
- Liu, F.; Huang, J.; Sadler, J.E. Shiga toxin (Stx)1B and Stx2B induce von Willebrand factor secretion from human umbilical vein endothelial cells through different signaling pathways. Blood 2011, 118, 3392–3398. [Google Scholar] [CrossRef] [Green Version]
- Hedlund, M.; Duan, R.D.; Nilsson, A.; Svanborg, C. Sphingomyelin, glycosphingolipids and ceramide signalling in cells exposed to P-fimbriated Escherichia coli. Mol. Microbiol. 1998, 29, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, P.A.; Takamatsu, H.H.; Lam, E.W.; Parkhouse, R.M. Ligation of the WC1 receptor induces gamma delta T cell growth arrest through fumonisin B1-sensitive increases in cellular ceramide. J. Immunol. 2000, 165, 3564–3570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, H.; Ellstrom, P.; Ekstrom, K.; Gustafsson, L.; Gustafsson, M.; Svanborg, C. Ceramide as a TLR4 agonist; a putative signalling intermediate between sphingolipid receptors for microbial ligands and TLR4. Cell Microbiol. 2007, 9, 1239–1251. [Google Scholar] [CrossRef]
- Garcia-Ruiz, C.; Colell, A.; Paris, R.; Fernandez-Checa, J.C. Direct interaction of GD3 ganglioside with mitochondria generates reactive oxygen species followed by mitochondrial permeability transition, cytochrome c release, and caspase activation. FASEB J. 2000, 14, 847–858. [Google Scholar] [CrossRef] [Green Version]
- Andrieu-Abadie, N.; Gouaze, V.; Salvayre, R.; Levade, T. Ceramide in apoptosis signaling: relationship with oxidative stress. Free Radic. Biol. Med. 2001, 31, 717–728. [Google Scholar] [CrossRef]
- Jandhyala, D.M.; Ahluwalia, A.; Obrig, T.; Thorpe, C.M. ZAK: a MAP3Kinase that transduces Shiga toxin- and ricin-induced proinflammatory cytokine expression. Cell Microbiol. 2008, 10, 1468–1477. [Google Scholar] [CrossRef]
- Thorpe, C.M.; Hurley, B.P.; Lincicome, L.L.; Jacewicz, M.S.; Keusch, G.T.; Acheson, D.W. Shiga toxins stimulate secretion of interleukin-8 from intestinal epithelial cells. Infect. Immun. 1999, 67, 5985–5993. [Google Scholar] [CrossRef] [Green Version]
- Cameron, P.; Bingham, D.; Paul, A.; Pavelka, M.; Cameron, S.; Rotondo, D.; Plevin, R. Essential role for verotoxin in sustained stress-activated protein kinase and nuclear factor kappa B signaling, stimulated by Escherichia coli O157:H7 in Vero cells. Infect. Immun. 2002, 70, 5370–5380. [Google Scholar] [CrossRef] [Green Version]
- Kojima, S.; Yanagihara, I.; Kono, G.; Sugahara, T.; Nasu, H.; Kijima, M.; Hattori, A.; Kodama, T.; Nagayama, K.I.; Honda, T. mkp-1 encoding mitogen-activated protein kinase phosphatase 1, a verotoxin 1 responsive gene, detected by differential display reverse transcription-PCR in Caco-2 cells. Infect. Immun. 2000, 68, 2791–2796. [Google Scholar] [CrossRef] [Green Version]
- Foster, G.H.; Armstrong, C.S.; Sakiri, R.; Tesh, V.L. Shiga toxin-induced tumor necrosis factor alpha expression: requirement for toxin enzymatic activity and monocyte protein kinase C and protein tyrosine kinases. Infect. Immun. 2000, 68, 5183–5189. [Google Scholar] [CrossRef] [Green Version]
- Cameron, P.; Smith, S.J.; Giembycz, M.A.; Rotondo, D.; Plevin, R. Verotoxin activates mitogen-activated protein kinase in human peripheral blood monocytes: role in apoptosis and proinflammatory cytokine release. Br. J. Pharmacol. 2003, 140, 1320–1330. [Google Scholar] [CrossRef] [Green Version]
- Leyva-Illades, D.; Cherla, R.P.; Lee, M.S.; Tesh, V.L. Regulation of cytokine and chemokine expression by the ribotoxic stress response elicited by Shiga toxin type 1 in human macrophage-like THP-1 cells. Infect. Immun. 2012, 80, 2109–2120. [Google Scholar] [CrossRef] [Green Version]
- Cherla, R.P.; Lee, S.Y.; Mulder, R.A.; Lee, M.S.; Tesh, V.L. Shiga toxin 1-induced proinflammatory cytokine production is regulated by the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin signaling pathway. Infect. Immun. 2009, 77, 3919–3931. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Lee, M.S.; Cherla, R.P.; Tesh, V.L. Shiga toxin 1 induces apoptosis through the endoplasmic reticulum stress response in human monocytic cells. Cell Microbiol. 2008, 10, 770–780. [Google Scholar] [CrossRef]
- Lentz, E.K.; Leyva-Illades, D.; Lee, M.S.; Cherla, R.P.; Tesh, V.L. Differential response of the human renal proximal tubular epithelial cell line HK-2 to Shiga toxin types 1 and 2. Infect. Immun. 2011, 79, 3527–3540. [Google Scholar] [CrossRef] [Green Version]
- Tang, B.; Li, Q.; Zhao, X.H.; Wang, H.G.; Li, N.; Fang, Y.; Wang, K.; Jia, Y.P.; Zhu, P.; Gu, J.; et al. Shiga toxins induce autophagic cell death in intestinal epithelial cells via the endoplasmic reticulum stress pathway. Autophagy 2015, 11, 344–354. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.S.; Cherla, R.P.; Jenson, M.H.; Leyva-Illades, D.; Martinez-Moczygemba, M.; Tesh, V.L. Shiga toxins induce autophagy leading to differential signalling pathways in toxin-sensitive and toxin-resistant human cells. Cell Microbiol. 2011, 13, 1479–1496. [Google Scholar] [CrossRef] [Green Version]
- Harrison, L.M.; van Haaften, W.C.; Tesh, V.L. Regulation of proinflammatory cytokine expression by Shiga toxin 1 and/or lipopolysaccharides in the human monocytic cell line THP-1. Infect. Immun. 2004, 72, 2618–2627. [Google Scholar] [CrossRef] [Green Version]
- Sakiri, R.; Ramegowda, B.; Tesh, V.L. Shiga toxin type 1 activates tumor necrosis factor-alpha gene transcription and nuclear translocation of the transcriptional activators nuclear factor-kappaB and activator protein-1. Blood 1998, 92, 558–566. [Google Scholar] [CrossRef]
- Yamasaki, C.; Natori, Y.; Zeng, X.T.; Ohmura, M.; Yamasaki, S.; Takeda, Y. Induction of cytokines in a human colon epithelial cell line by Shiga toxin 1 (Stx1) and Stx2 but not by non-toxic mutant Stx1 which lacks N-glycosidase activity. FEBS Lett. 1999, 442, 231–234. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, C.; Nishikawa, K.; Zeng, X.T.; Katayama, Y.; Natori, Y.; Komatsu, N.; Oda, T. Induction of cytokines by toxins that have an identical RNA N-glycosidase activity: Shiga toxin, ricin, and modeccin. Biochim. Biophys. Acta 2004, 1671, 44–50. [Google Scholar] [CrossRef]
- Brandelli, J.R.; Griener, T.P.; Laing, A.; Mulvey, G.; Armstrong, G.D. The Effects of Shiga Toxin 1, 2 and Their Subunits on Cytokine and Chemokine Expression by Human Macrophage-Like THP-1 Cells. Toxins (Basel) 2015, 7, 4054–4066. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, A.; Johns, E.J.; Imaizumi, A.; Yanagawa, Y.; Kohsaka, T. Activation of beta(2)-adrenoceptor prevents shiga toxin 2-induced TNF-alpha gene transcription. J. Am. Soc. Nephrol. 2001, 12, 2288–2299. [Google Scholar]
- Dahan, S.; Busuttil, V.; Imbert, V.; Peyron, J.F.; Rampal, P.; Czerucka, D. Enterohemorrhagic Escherichia coli infection induces interleukin-8 production via activation of mitogen-activated protein kinases and the transcription factors NF-kappaB and AP-1 in T84 cells. Infect. Immun. 2002, 70, 2304–2310. [Google Scholar] [CrossRef] [Green Version]
- Berin, M.C.; Darfeuille-Michaud, A.; Egan, L.J.; Miyamoto, Y.; Kagnoff, M.F. Role of EHEC O157:H7 virulence factors in the activation of intestinal epithelial cell NF-kappaB and MAP kinase pathways and the upregulated expression of interleukin 8. Cell Microbiol. 2002, 4, 635–648. [Google Scholar] [CrossRef]
- Thorpe, C.M.; Smith, W.E.; Hurley, B.P.; Acheson, D.W. Shiga toxins induce, superinduce, and stabilize a variety of C-X-C chemokine mRNAs in intestinal epithelial cells, resulting in increased chemokine expression. Infect. Immun. 2001, 69, 6140–6147. [Google Scholar] [CrossRef] [Green Version]
- Harrison, L.M.; van den Hoogen, C.; van Haaften, W.C.; Tesh, V.L. Chemokine expression in the monocytic cell line THP-1 in response to purified shiga toxin 1 and/or lipopolysaccharides. Infect. Immun. 2005, 73, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.S.; Kwon, H.; Lee, E.Y.; Kim, D.J.; Park, J.H.; Tesh, V.L.; Oh, T.K.; Kim, M.H. Shiga Toxins Activate the NLRP3 Inflammasome Pathway To Promote Both Production of the Proinflammatory Cytokine Interleukin-1beta and Apoptotic Cell Death. Infect. Immun. 2016, 84, 172–186. [Google Scholar] [CrossRef] [Green Version]
- Hughes, A.K.; Stricklett, P.K.; Kohan, D.E. Shiga toxin-1 regulation of cytokine production by human glomerular epithelial cells. Nephron 2001, 88, 14–23. [Google Scholar] [CrossRef]
- Hughes, A.K.; Stricklett, P.K.; Kohan, D.E. Shiga toxin-1 regulation of cytokine production by human proximal tubule cells. Kidney Int. 1998, 54, 1093–1106. [Google Scholar] [CrossRef] [Green Version]
- Eisenhauer, P.B.; Jacewicz, M.S.; Conn, K.J.; Koul, O.; Wells, J.M.; Fine, R.E.; Newburg, D.S. Escherichia coli Shiga toxin 1 and TNF-alpha induce cytokine release by human cerebral microvascular endothelial cells. Microb. Pathog. 2004, 36, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Matussek, A.; Lauber, J.; Bergau, A.; Hansen, W.; Rohde, M.; Dittmar, K.E.; Gunzer, M.; Mengel, M.; Gatzlaff, P.; Hartmann, M.; et al. Molecular and functional analysis of Shiga toxin-induced response patterns in human vascular endothelial cells. Blood 2003, 102, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Ou, Z.L.; Gondaira, F.; Ohmura, M.; Kojio, S.; Yamamoto, T. Protective effect of anisodamine against Shiga toxin-1: Inhibition of cytokine production and increase in the survival of mice. J. Lab. Clin. Med. 2001, 137, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Ohmura, M.; Gondaira, F.; Yamamoto, T. Inhibition of Shiga toxin-induced tumor necrosis factor-alpha production and gene expression in human monocytic cells by CV6209. Life Sci. 2001, 68, 1931–1937. [Google Scholar] [CrossRef]
- Barrett, T.J.; Potter, M.E.; Strockbine, N.A. Evidence for participation of the macrophage in Shiga-like toxin II-induced lethality in mice. Microb. Pathog. 1990, 9, 95–103. [Google Scholar] [CrossRef]
- Simon, M.; Cleary, T.G.; Hernandez, J.D.; Abboud, H.E. Shiga toxin 1 elicits diverse biologic responses in mesangial cells. Kidney Int. 1998, 54, 1117–1127. [Google Scholar] [CrossRef] [Green Version]
- Moussay, E.; Stamm, I.; Taubert, A.; Baljer, G.; Menge, C. Escherichia coli Shiga toxin 1 enhances il-4 transcripts in bovine ileal intraepithelial lymphocytes. Vet. Immunol. Immunopathol. 2006, 113, 367–382. [Google Scholar] [CrossRef]
- Balsinde, J.; Balboa, M.A.; Dennis, E.A. Inflammatory activation of arachidonic acid signaling in murine P388D1 macrophages via sphingomyelin synthesis. J. Biol. Chem. 1997, 272, 20373–20377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, J.W.; Finkelstein, R.A.; Cantu, J.; Gessell, D.L.; Chopra, A.K. Cholera toxin B subunit activates arachidonic acid metabolism. Infect. Immun. 1999, 67, 794–799. [Google Scholar] [CrossRef] [Green Version]
- Schmid, D.I.; Kohan, D.E. Effect of shigatoxin-1 on arachidonic acid release by human glomerular epithelial cells. Kidney Int. 2001, 60, 1026–1036. [Google Scholar] [CrossRef] [Green Version]
- Orth, D.; Khan, A.B.; Naim, A.; Grif, K.; Brockmeyer, J.; Karch, H.; Joannidis, M.; Clark, S.J.; Day, A.J.; Fidanzi, S.; et al. Shiga toxin activates complement and binds factor H: evidence for an active role of complement in hemolytic uremic syndrome. J. Immunol. 2009, 182, 6394–6400. [Google Scholar] [CrossRef] [Green Version]
- Poolpol, K.; Orth-Holler, D.; Speth, C.; Zipfel, P.F.; Skerka, C.; de Cordoba, S.R.; Brockmeyer, J.; Bielaszewska, M.; Wurzner, R. Interaction of Shiga toxin 2 with complement regulators of the factor H protein family. Mol. Immunol. 2014, 58, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Brigotti, M.; Orth-Holler, D.; Carnicelli, D.; Porcellini, E.; Galassi, E.; Tazzari, P.L.; Ricci, F.; Manoli, F.; Manet, I.; Talasz, H.; et al. The structure of the Shiga toxin 2a A-subunit dictates the interactions of the toxin with blood components. Cell Microbiol. 2019, 21, e13000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrlenbach, S.; Rosales, A.; Posch, W.; Wilflingseder, D.; Hermann, M.; Brockmeyer, J.; Karch, H.; Satchell, S.C.; Wurzner, R.; Orth-Holler, D. Shiga toxin 2 reduces complement inhibitor CD59 expression on human renal tubular epithelial and glomerular endothelial cells. Infect. Immun. 2013, 81, 2678–2685. [Google Scholar] [CrossRef] [Green Version]
- Kimura, T.; Tani, S.; Matsumoto Yi, Y.; Takeda, T. Serum amyloid P component is the Shiga toxin 2-neutralizing factor in human blood. J. Biol. Chem. 2001, 276, 41576–41579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, G.D.; Mulvey, G.L.; Marcato, P.; Griener, T.P.; Kahan, M.C.; Tennent, G.A.; Sabin, C.A.; Chart, H.; Pepys, M.B. Human serum amyloid P component protects against Escherichia coli O157:H7 Shiga toxin 2 in vivo: therapeutic implications for hemolytic-uremic syndrome. J. Infect. Dis. 2006, 193, 1120–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brigotti, M.; Arfilli, V.; Carnicelli, D.; Ricci, F.; Tazzari, P.L.; Ardissino, G.; Scavia, G.; Morabito, S.; He, X. Soluble Toll-Like Receptor 4 Impairs the Interaction of Shiga Toxin 2a with Human Serum Amyloid P Component. Toxins (Basel) 2018, 10, 379. [Google Scholar] [CrossRef] [Green Version]
- Kieckens, E.; Rybarczyk, J.; Barth, S.A.; Menge, C.; Cox, E.; Vanrompay, D. Effect of lactoferrin on release and bioactivity of Shiga toxins from different Escherichia coli O157:H7 strains. Vet. Microbiol. 2017, 202, 29–37. [Google Scholar] [CrossRef]
- Malyukova, I.; Murray, K.F.; Zhu, C.; Boedeker, E.; Kane, A.; Patterson, K.; Peterson, J.R.; Donowitz, M.; Kovbasnjuk, O. Macropinocytosis in Shiga toxin 1 uptake by human intestinal epithelial cells and transcellular transcytosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G78–G92. [Google Scholar] [CrossRef] [Green Version]
- Laiko, M.; Murtazina, R.; Malyukova, I.; Zhu, C.; Boedeker, E.C.; Gutsal, O.; O’Malley, R.; Cole, R.N.; Tarr, P.I.; Murray, K.F.; et al. Shiga toxin 1 interaction with enterocytes causes apical protein mistargeting through the depletion of intracellular galectin-3. Exp. Cell Res. 2010, 316, 657–666. [Google Scholar] [CrossRef] [Green Version]
- Maluykova, I.; Gutsal, O.; Laiko, M.; Kane, A.; Donowitz, M.; Kovbasnjuk, O. Latrunculin B facilitates Shiga toxin 1 transcellular transcytosis across T84 intestinal epithelial cells. Biochim. Biophys. Acta 2008, 1782, 370–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuller, S. Shiga toxin interacion with human intestinal epithelium. Toxins (Basel) 2011, 3, 626–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
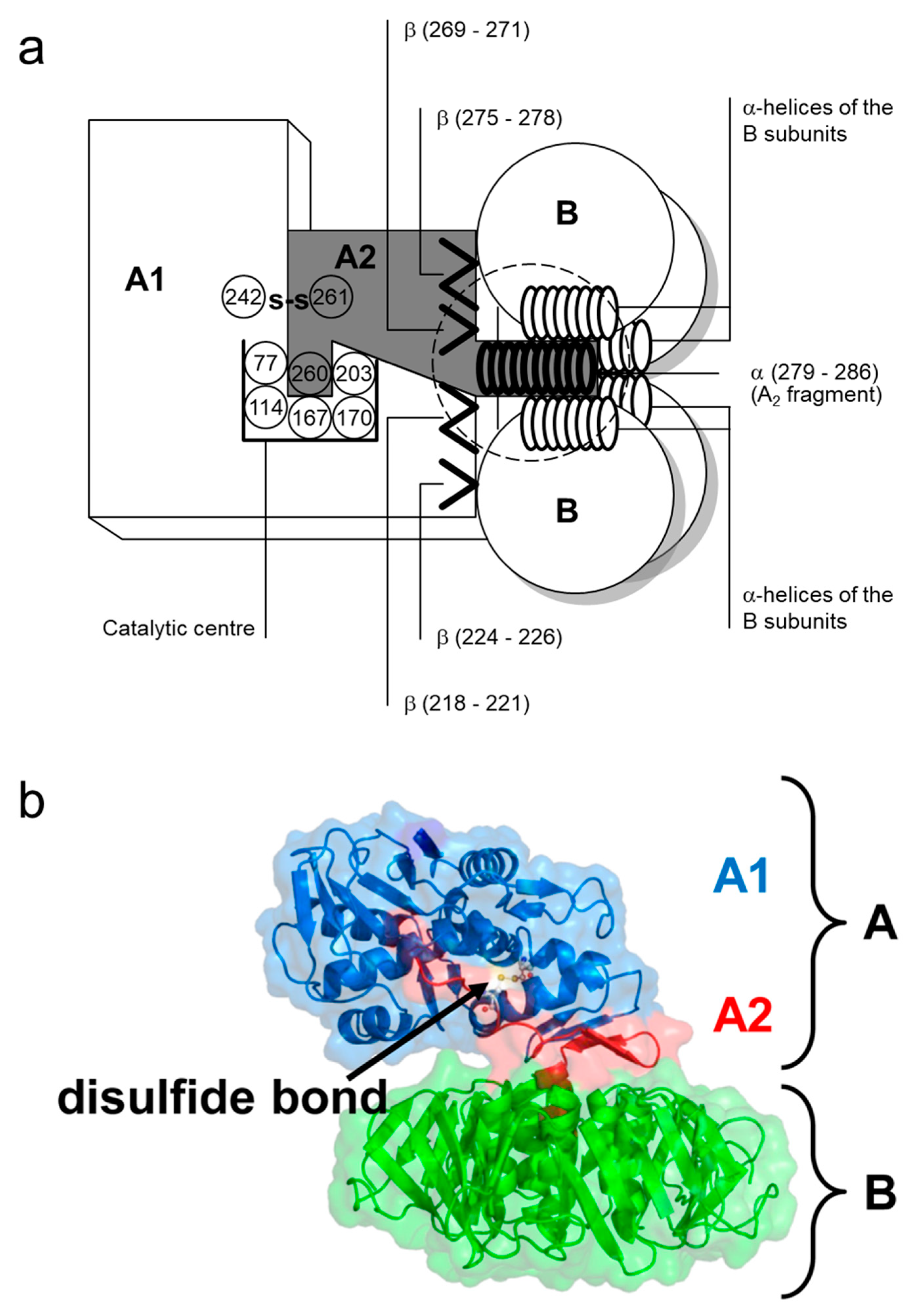

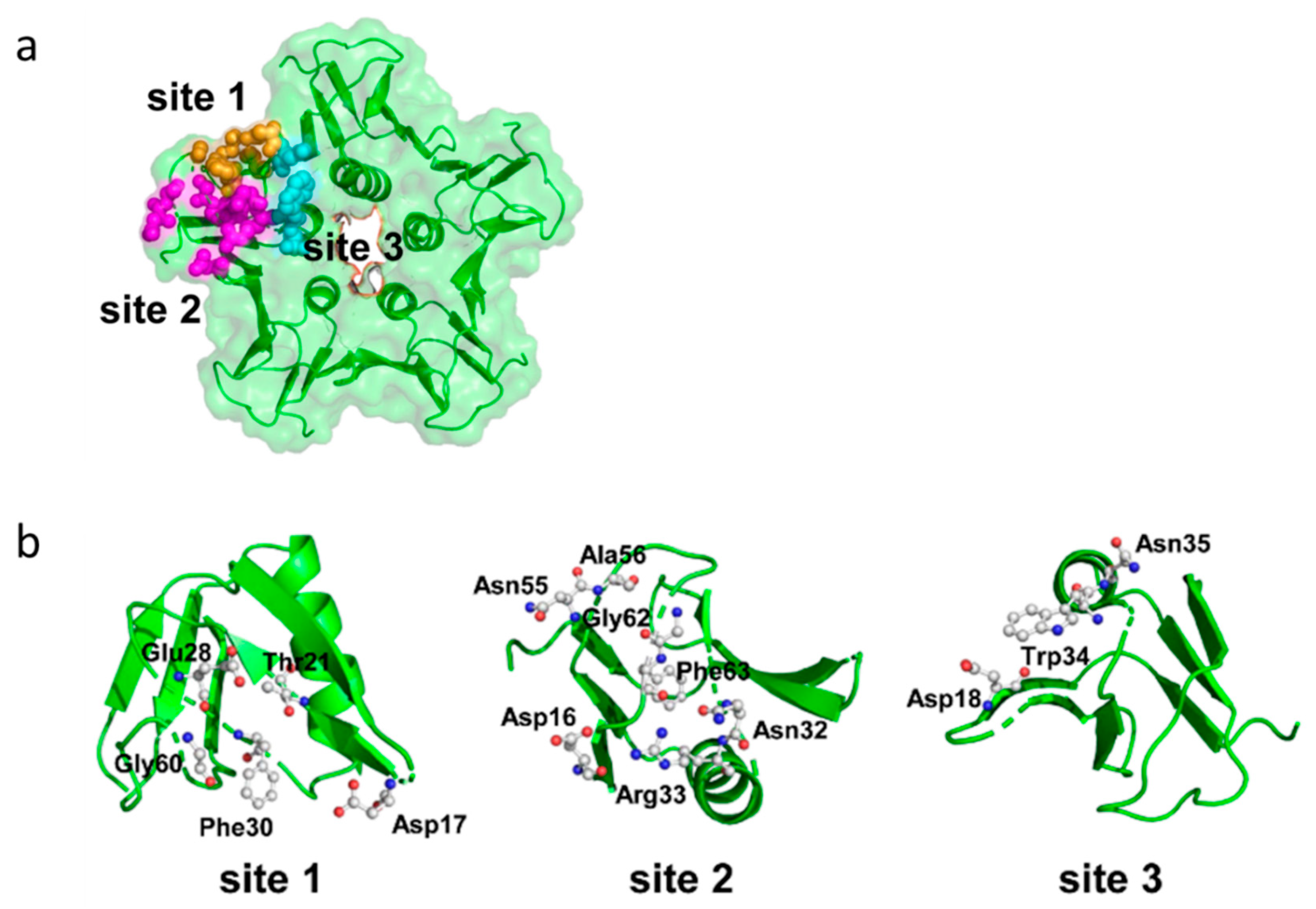

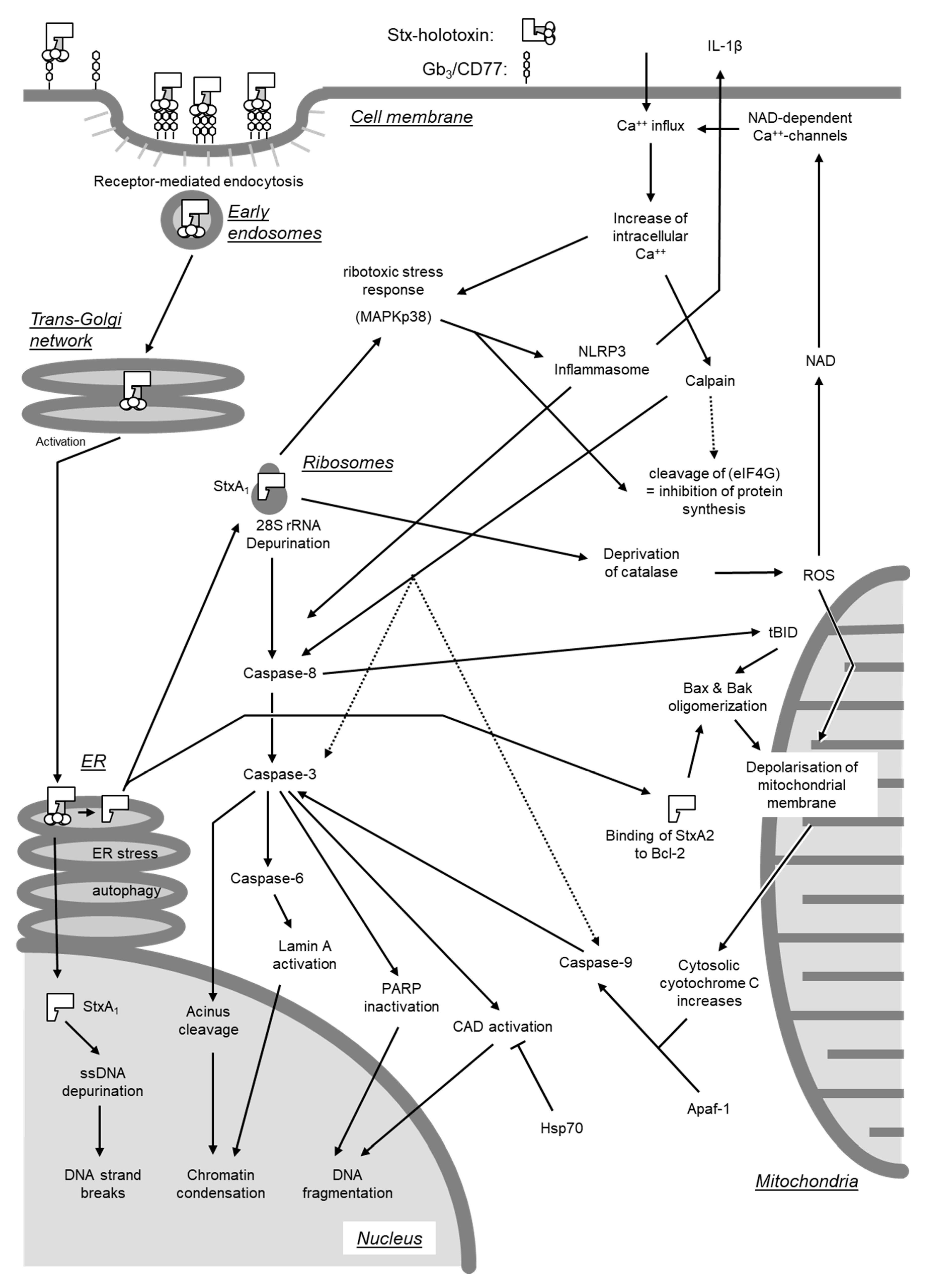
| Amino Acid in Toxin: | Allocated Function: | Reference: | ||
|---|---|---|---|---|
| Stx1 | Stx2 1 | Stx2e | ||
| (-20)-(-1) | (-19)-(-1) | (-19)-(-1) | signal sequence | [63,73,74] |
| Ala1 | hydrogen bond to Ser53; part of receptor binding site II | [80] | ||
| 3–8 | β1-sheet | [77] | ||
| Cys4 | Cys3 | disulfide bond to Cys57 (essential for receptor binding); part of receptor binding site II | [76,80,81] | |
| 9–14 | β2-sheet, interaction with β6-sheet of the adjacent monomer (receptor binding site I 2) | [77] | ||
| Lys13 | part of receptor binding site I 2 | [82] | ||
| 10–20 | hydrophilic domain: receptor binding, binding of neutralizing antibodies, highly conserved in all Stxs (antigenic domain?) | [76] | ||
| Asp16 | receptor binding | [83] | ||
| Asp17 | part of receptor binding site I 2 | [82,83] | ||
| Asn17 | receptor specificity for Gb4 | [83] | ||
| Asp18 | Asp17 | receptor specificity for Gb3/CD77 | [81] | |
| 20–24 | β3-sheet | [77] | ||
| Thr21 | part of receptor binding site I 2 | [82,84] | ||
| 27–31 | β4-sheet | [77] | ||
| Glu28 | part of receptor binding site I 2 | [82,84] | ||
| Phe30 | located between receptor binding site I and II; essential for both binding sites | [85] | ||
| Asn32 | part of receptor binding site II | [84] | ||
| Arg33 | Arg32 | part of receptor binding sites II and III | [80,84,86] | |
| Trp34 | Trp33 | part of receptor binding site III | [78,80,87] | |
| Asn35 | Asn34 | part of receptor binding site III? | [80] | |
| 36–46 | α-helix, forming the central pore of the pentamer together with the α-helices of the other four B subunits; in contact with the anti-parallel α-helix of the A2 fragment; conformational changes occur here at low pH (impacting on the translocation of the A2 fragment) | [77,79,88] | ||
| Ala43 | Ala42 | receptor binding | [86] | |
| 49–53 | β5-sheet | [77] | ||
| Lys53 | Lys52 | receptor binding (involved in determination of receptor specificity?) | [81] | |
| Ser53 | hydrogen bond to Ala1; part of receptor binding site II | [80] | ||
| Cys57 | Cys56 | Disulfide bond to Cys4 (essential for receptor binding); part of receptor binding site II | [76,80,81] | |
| Gly60 | Gly59 | part of receptor binding site I 2 | [82,86] | |
| 65–68 | β-sheet, interaction with the β2-sheet of the adjacent monomer (receptor binding site I 2) | [77] | ||
| Glu65 | receptor specificity for Gb3; part of receptor binding site I 2 | [82,84] | ||
| Gln64 | localization of the toxin in the E. coli cell | [81] | ||
| receptor specificity for Gb4 | [83] | |||
| Lys66 | receptor specificity for Gb4 | [81,83] | ||
| Phe68 | Phe67 | Phe67 | receptor binding | [89] |
| Arg69 | Asn68 | Asn68 | receptor binding | [89] |
| C-terminal 4 (-5) | receptor binding | [83] | ||
| Cell Type | Man | Mice | Rabbit | Pig | Cattle | Sheep | Goat |
|---|---|---|---|---|---|---|---|
| Intestinal epithelial cells | (+) only for Stx2 [21] + only cell lines [21] + intestinal organoids [20] | + only distal colon [128] | + [129,130] | ? [131] | + [132,133] | ||
| Paneth cells | + [134] | ||||||
| Endothelial cells | |||||||
| in large blood vessels | + [118,135,136,137,138] | + [139]; − [137] | |||||
| in the microvasculature | + [137,140,141,142] | ||||||
| intestinal | + [143] | + [144] | − [131] | ||||
| in renal glomeruli | + [122,145,146] | + [147] | − [131] | ||||
| in the CNS | + [120,123,124,148] | − [43] | + [144] | + [147] | |||
| in the lung | + [149] | ||||||
| Nerve cells | ? [22] | ||||||
| retinal pigment epithelial | + [150] | ||||||
| Renal tubular epithelial cells | + [107,145,151,152,153] | ? [154] | − [155] | + [147] | + [131,132] | ||
| Renal glomerular epithelial cells | + [156] | − [155] | |||||
| Fibroblasts | |||||||
| intestinal myofibroblasts | + [21] | + [133] | |||||
| Monocytes/macrophages | (+) [157] + [158] | + [159] | + [147] | + [160] | |||
| Mesangium cells | (+) [161] + [161,162] | − [155] | − [132] | ||||
| Tissue macrophages | + [147,163] | + [133] | |||||
| Lymphocytes | − [147] | + [164,165] | |||||
| B cells | + [42,166,167] | + [168] | + [164,165] | ||||
| αβT cells | − [42,166] | − [154,169] | + [164,165] | ||||
| γδT cells | + [164,165] | ||||||
| Granulocytes | (+) [32] | + [147] | − [170] | + [170] | + [171] | ||
| Erythrocytes | + [31,172] | − [22] | |||||
| Hematopoetic stem cells | + [173] | ||||||
| Platelets | + [174,175] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menge, C. Molecular Biology of Escherichia coli Shiga Toxins’ Effects on Mammalian Cells. Toxins 2020, 12, 345. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12050345
Menge C. Molecular Biology of Escherichia coli Shiga Toxins’ Effects on Mammalian Cells. Toxins. 2020; 12(5):345. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12050345
Chicago/Turabian StyleMenge, Christian. 2020. "Molecular Biology of Escherichia coli Shiga Toxins’ Effects on Mammalian Cells" Toxins 12, no. 5: 345. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12050345




