Mycotoxins and the Enteric Nervous System
Abstract
:1. Introduction
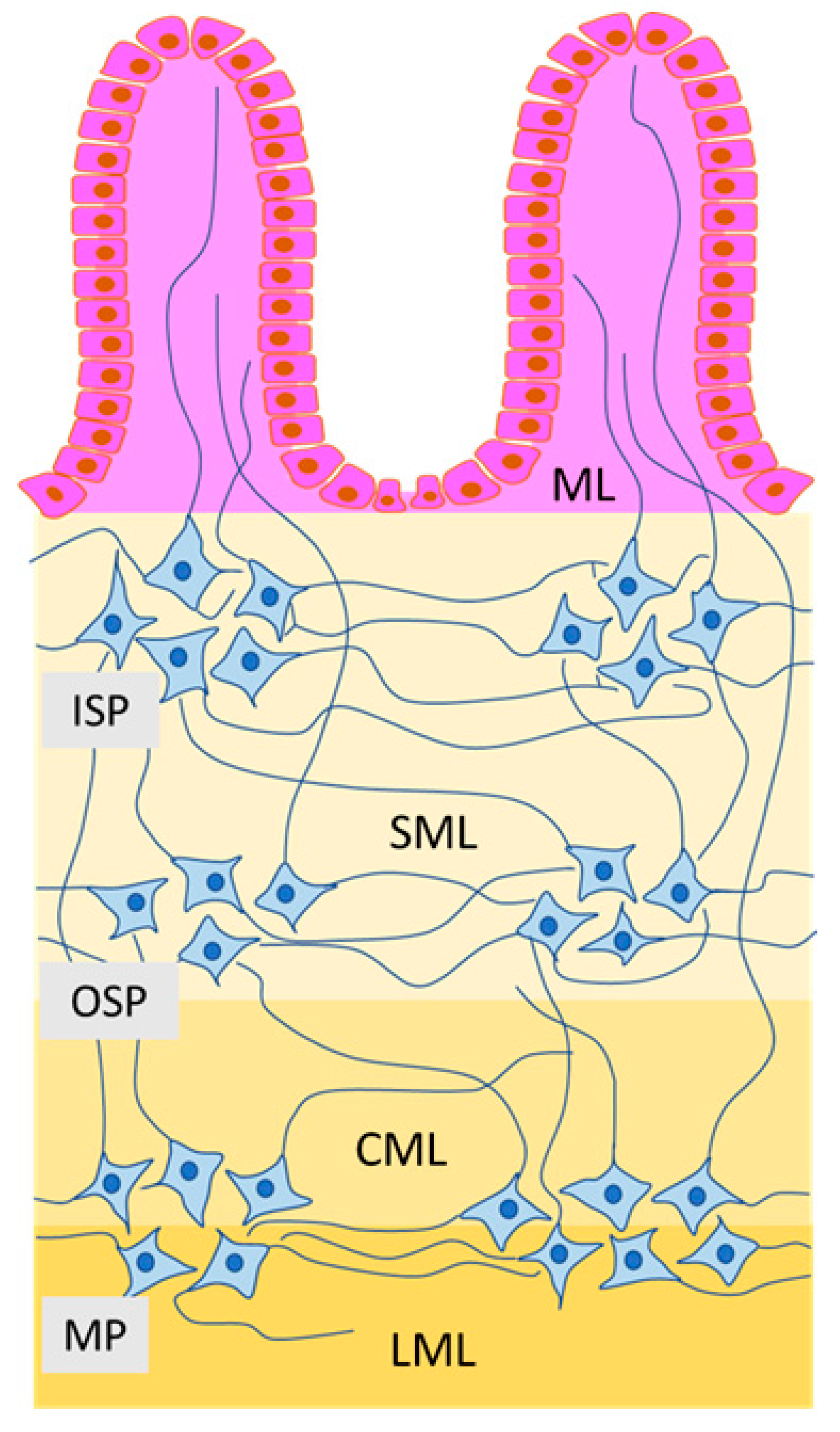
2. Anatomy of the Enteric Nervous System
3. Mycotoxins Affecting the Enteric Neurons
3.1. Deoxynivalenol
3.2. T2 Toxin
3.3. Zearalenon
3.4. Patulin
3.5. Fumonisins
4. Mycotoxin Consumption and Human Gastrointestinal Diseases
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cimbalo, A.; Alonso-Garrido, M.; Font, G.; Manyes, L. Toxicity of mycotoxins in vivo on vertebrate organisms: A review. Food Chem. Toxicol. 2020, 137, 111161. [Google Scholar] [CrossRef] [PubMed]
- De Ruyck, K.; De Boevre, M.; Huybrechts, I.; De Saeger, S. Dietary mycotoxins, co-exposure, and carcinogenesis in humans: Short review. Mutat. Res. 2015, 766, 32–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, N.; Marquès, M.; Nadal, M.; Domingo, J.L. Occurrence of environmental pollutants in foodstuffs: A review of organic vs. conventional food. Food Chem. Toxicol. 2019, 125, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S.; Obremski, K.; Makowska, K.; Rytel, L.; Mwaanga, E.S. Levels of Zearalenone and its metabolites in sun-dried kapenta fish and water of Lake Kariba in Zambi—A preliminary study. Sci. Total Environ. 2018, 637–638, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Milićević, D.R.; Skrinjar, M.; Baltić, T. Real and perceived risks for mycotoxin contamination in foods and feeds: Challenges for food safety control. Toxins 2010, 2, 572–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alshannaq, A.; Yu, J.H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rykaczewska, A.; Gajęcka, M.; Onyszek, E.; Cieplińska, K.; Dąbrowski, M.; Lisieska-Żołnierczyk, S.; Bulińska, M.; Babuchowski, A.; Gajęcki, M.T.; Zielonka, Ł. Imbalance in the blood concentrations of selected steroids in prepubertal gilts depending on the time of exposure to low doses of zearalenone. Toxins 2019, 11, 561. [Google Scholar] [CrossRef] [Green Version]
- Gajęcka, M.; Zielonka, Ł.; Gajęcki, M. Activity of zearalenone in the porcine intestinal tract. Molecules 2017, 22, 18. [Google Scholar] [CrossRef] [Green Version]
- Khoshal, A.K.; Novak, B.; Martin, P.G.P.; Jenkins, T.; Neves, M.; Schatzmayr, G.; Oswald, I.P.; Pinton, P. Co-Occurrence of DON and emerging mycotoxins in worldwide finished pig feed and their combined toxicity in intestinal cells. Toxins 2019, 11, 727. [Google Scholar] [CrossRef] [Green Version]
- Przybylska-Gornowicz, B.; Tarasiuk, M.; Lewczuk, B.; Prusik, M.; Ziółkowska, N.; Zielonka, Ł.; Gajęcki, M.; Gajęcka, M. The effects of low doses of two Fusarium toxins, zearalenone and deoxynivalenol, on the pig jejunum. A light and electron microscopic study. Toxins 2015, 7, 4684–4705. [Google Scholar] [CrossRef] [Green Version]
- Przybylska-Gornowicz, B.; Lewczuk, B.; Prusik, M.; Hanuszewska, M.; Petrusewicz-Kosińska, M.; Gajęcka, M.; Zielonka, Ł.; Gajęcki, M. The effects of deoxynivalenol and zearalenone on the pig large intestine. A light and electron microscopic study. Toxins 2018, 10, 148. [Google Scholar] [CrossRef] [Green Version]
- Sousa, F.C.; Schamber, C.R.; Amorin, S.S.; Natali, M.R. Effect of fumonisin-containing diet on the myenteric plexus of the jejunum in rats. Auton. Neurosci. 2014, 185, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Rudyk, H.; Tomaszewska, E.; Arciszewski, M.B.; Muszyński, S.; Tomczyk-Warunek, A.; Dobrowolski, P.; Donaldson, J.; Brezvyn, O.; Kotsyumbas, I. Histomorphometrical changes in intestine structure and innervation following experimental fumonisins intoxication in male Wistar rats. Pol. J. Vet. Sci. 2020, 23, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Rissato, D.F.; de Santi Rampazzo, A.P.; Borges, S.C.; Sousa, F.C.; Busso, C.; Buttow, N.C.; Natali, M.R.M. Chronic ingestion of deoxynivalenol-contaminated diet dose-dependently decreases the area of myenteric neurons and gliocytes of rats. Neurogastroenterol. Motil. 2020, 32, e13770. [Google Scholar] [CrossRef] [PubMed]
- Makowska, K.; Obremski, K.; Gonkowski, S. The impact of T-2 toxin on vasoactive intestinal polypeptide-like immunoreactive (VIP-LI) nerve structures in the wall of the porcine stomach and duodenum. Toxins 2018, 10, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makowska, K.; Obremski, K.; Zielonka, L.; Gonkowski, S. The influence of low doses of zearalenone and T-2 toxin on calcitonin gene related peptide-like immunoreactive (CGRP-LI) neurons in the ENS of the porcine descending colon. Toxins 2017, 9, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alassane-Kpembi, I.; Pinton, P.; Oswald, I.P. Effects of mycotoxins on the intestine. Toxins 2019, 11, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liew, W.P.; Mohd-Redzwan, S. Mycotoxin: Its impact on gut health and microbiota. Front. Cell Infect. Microbiol. 2018, 8, 60. [Google Scholar] [CrossRef] [Green Version]
- Gonkowski, S.; Obremski, K.; Calka, J. The influence of low doses of zearalenone on distribution of selected active substances in nerve fibers within the circular muscle layer of porcine ileum. J. Mol. Neurosci. 2015, 56, 878–886. [Google Scholar] [CrossRef] [Green Version]
- Bouhet, S.; Oswald, I. The intestine as a possible target for fumonisin toxicity. Mol. Nutr. Food Res. 2007, 51, 925–931. [Google Scholar] [CrossRef]
- Obremski, K.; Gonkowski, S.; Wojtacha, P. Zearalenone-induced changes in the lymphoid tissue and mucosal nerve fibers in the porcine ileum. Pol. J. Vet. Sci. 2015, 18, 357–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinton, P.; Oswald, I.P. Effect of deoxynivalenol and other Type B trichothecenes on the intestine: A review. Toxins 2014, 6, 1615–1643. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar] [CrossRef] [PubMed]
- Gershon, M.D. The enteric nervous system: A second brain. Hosp. Pract. 1999, 34, 31–52. [Google Scholar] [CrossRef]
- Furness, J.B. Extrinsic and intrinsic sources of calcitonin gene-related peptide immunoreactivity in the lamb ileum: A morphometric and neurochemical investigation. Cell Tissue Res. 2006, 323, 183–196. [Google Scholar]
- Schneider, S.; Wright, C.M.; Heuckeroth, R.O. Unexpected roles for the second brain: Enteric nervous system as master regulator of bowel function. Annu. Rev. Physiol. 2019, 81, 235–259. [Google Scholar] [CrossRef]
- Morikawa, S.; Komuro, T. Distribution of myenteric NO neurons along the guinea-pig esophagus. J. Auton. Nerv. Syst. 1998, 74, 91–99. [Google Scholar] [CrossRef]
- Reiche, D.; Michel, K.; Pfannkuche, H.; Schemann, M. Projections and neurochemistry of interneurones in the myenteric plexus of the guinea-pig gastric corpus. Neurosci. Lett. 2000, 295, 109–112. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Yang, S.; Li, X.S.; Zhou, D.S. Expression and possible role of IGF-IR in the mouse gastric myenteric plexus and smooth muscles. Acta Histochem. 2014, 116, 788–794. [Google Scholar] [CrossRef]
- Zimmermann, J.; Neuhuber, W.L.; Raab, M. Homer1 (VesL-1) in the rat esophagus: Focus on myenteric plexus and neuromuscular junction. Histochem. Cell Biol. 2017, 148, 189–206. [Google Scholar] [CrossRef]
- Furness, J.B. The Enteric Nervous System; Blackwell Publishing: Oxford, UK, 2006; pp. 1–274. [Google Scholar]
- Kamikawa, Y.; Shimo, Y. Pharmacological characterization of the opioid receptor in the submucous plexus of the guinea-pig oesophagus. Br. J. Pharmacol. 1983, 78, 693–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunisawa, Y.; Komuro, T. Interstitial cells of Cajal associated with the submucosal plexus of the Guinea-pig stomach. Neurosci. Lett. 2008, 434, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Heinicke, E.A.; Kiernan, J.A. An immunohistochemical study of the myenteric plexus of the colon in the rat and mouse. J. Anat. 1990, 170, 51–62. [Google Scholar] [PubMed]
- Sayegh, A.I.; Ritter, R.C. Morphology and distribution of nitric oxide synthase-, neurokinin-1 receptor-, calretinin-, calbindin-, and neurofilament-M-immunoreactive neurons in the myenteric and submucosal plexuses of the rat small intestine. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2003, 271, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Monro, R.L.; Bornstein, J.C.; Bertrand, P.P. Synaptic transmission from the submucosal plexus to the myenteric plexus in Guinea-pig ileum. Neurogastroenterol. Motil. 2008, 20, 1165–1173. [Google Scholar] [CrossRef]
- Li, J.P.; Zhang, T.; Gao, C.J.; Kou, Z.Z.; Jiao, X.W.; Zhang, L.X.; Wu, Z.Y.; He, Z.Y.; Li, Y.Q. Neurochemical features of endomorphin-2-containing neurons in the submucosal plexus of the rat colon. World J. Gastroenterol. 2015, 21, 9936–9944. [Google Scholar] [CrossRef]
- Rekawek, W.; Sobiech, P.; Gonkowski, S.; Żarczyńska, K.; Snarska, A.; Waśniewski, T.; Wojtkiewicz, J. Distribution and chemical coding patterns of cocaine- and amphetamine-regulated transcript-like immunoreactive (CART-LI) neurons in the enteric nervous system of the porcine stomach cardia. Pol. J. Vet. Sci. 2015, 18, 515–522. [Google Scholar] [CrossRef]
- Bulc, M.; Palus, K.; Całka, J.; Zielonka, Ł. Changes in immunoreactivity of sensory substances within the enteric nervous system of the porcine stomach during experimentally induced diabetes. J. Diabetes Res. 2018, 2018, 4735659. [Google Scholar] [CrossRef]
- Makowska, K.; Rytel, L.; Lech, P.; Osowski, A.; Kruminis-Kaszkiel, E.; Gonkowski, S. Cocaine- and amphetamine-regulated transcript (CART) peptide in the enteric nervous system of the porcine esophagus. Comptes Rendus Biol. 2018, 341, 325–333. [Google Scholar] [CrossRef]
- Kaleczyc, J.; Klimczuk, M.; Franke-Radowiecka, A.; Sienkiewicz, W.; Majewski, M.; Łakomy, M. The distribution and chemical coding of intramural neurons supplying the porcine stomach—The study on normal pigs and on animals suffering from swine dysentery. Anat. Histol. Embryol. 2007, 36, 186–193. [Google Scholar] [CrossRef]
- Teixeira, A.F.; Wedel, T.; Krammer, H.J.; Kühnel, W. Structural differences of the enteric nervous system in the cattle forestomach revealed by whole mount immunohistochemistry. Ann. Anat. 1998, 180, 393–400. [Google Scholar] [CrossRef]
- Arciszewski, M.B.; Barabasz, S.; Skobowiat, C.; Maksymowicz, W.; Majewski, M. Immunodetection of cocaine- and amphetamine-regulated transcript in the rumen, reticulum, omasum and abomasum of the sheep. Anat. Histol. Embryol. 2009, 38, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, J.P.; Barbiers, M.; Scheuermann, D.W.; Stach, W.; Adriaensen, D.; Mayer, B.; De Groodt-Lasseel, M.H. Distribution pattern, neurochemical features and projections of nitrergic neurons in the pig small intestine. Ann. Anat. 1994, 176, 515–525. [Google Scholar] [CrossRef]
- Makowska, K. Chemically induced inflammation and nerve damage affect the distribution of vasoactive intestinal polypeptide-like immunoreactive (VIP-LI) nervous structures in the descending colon of the domestic pig. Neurogastroenterol. Motil. 2018, 30, e13439. [Google Scholar] [CrossRef]
- Makowska, K.; Gonkowski, S. Age and sex-dependent differences in the neurochemical characterization of calcitonin gene-related peptide-like immunoreactive (CGRP-LI) nervous structures in the porcine descending colon. Int. J. Mol. Sci. 2019, 20, 1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapp, S.; Schrödl, F.; Neuhuber, W.; Brehmer, A. Chemical coding of submucosal type V neurons in porcine ileum. Cells Tissues Organs 2006, 184, 31–41. [Google Scholar] [CrossRef]
- Gonkowski, S.; Całka, J. Changes in the somatostatin (SOM)-like immunoreactivity within nervous structures of the porcine descending colon under various pathological factors. Exp. Mol. Pathol. 2010, 88, 416–423. [Google Scholar] [CrossRef]
- Gonkowski, S. Substance P as a neuronal factor in the enteric nervous system of the porcine descending colon in physiological conditions and during selected pathogenic processes. Biofactors 2013, 39, 542–551. [Google Scholar] [CrossRef]
- Scheuermann, D.W.; Stach, W. Fluorescence microscopic study of the architecture and structure of an adrenergic network in the plexus myentericus (Auerbach), plexus submucosus externus (Schabadasch) and plexus submucosus internus (Meissner) of the porcine small intestine. Acta Anat. 1984, 119, 49–59. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Takahashi, H.; Ohama, E.; Ikuta, F. Tyrosine hydroxylase-immunoreactive intrinsic neurons in the Auerbach’s and Meissner’s plexuses of humans. Neurosci. Lett. 1989, 96, 259–263. [Google Scholar] [CrossRef]
- Hwang, S.E.; Hieda, K.; Kim, J.H.; Murakami, G.; Abe, S.; Matsubara, A.; Cho, B.H. Region-specific differences in the human myenteric plexus: An immunohistochemical study using donated elderly cadavers. Int. J. Colorectal Dis. 2014, 29, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Mandić, P.; Filipović, T.; Gasić, M.; Djukić-Macut, N.; Filipović, M.; Bogosavljević, I. Quantitative morphometric analysis of the myenteric nervous plexus ganglion structures along the human digestive tract. Vojnosanit. Pregl. 2016, 73, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Ibba-Manneschi, L.; Martini, M.; Zecchi-Orlandini, S.; Faussone-Pellegrini, M.S. Structural organization of enteric nervous system in human colon. Histol. Histopathol. 1995, 10, 17–25. [Google Scholar] [PubMed]
- Wedel, T.; Roblick, U.; Gleiss, J.; Schiedeck, T.; Bruch, H.P.; Kühnel, W.; Krammer, H.J. Organization of the enteric nervous system in the human colon demonstrated by wholemount immunohistochemistry with special reference to the submucous plexus. Ann. Anat. 1999, 181, 327–337. [Google Scholar] [CrossRef]
- Brehmer, A.; Rupprecht, H.; Neuhuber, W. Two submucosal nerve plexus in human intestines. Histochem. Cell Biol. 2010, 133, 149–161. [Google Scholar] [CrossRef]
- Jabari, S.; de Oliveira, E.C.; Brehmer, A.; da Silveira, A.B. Chagasic megacolon: Enteric neurons and related structures. Histochem. Cell Biol. 2014, 142, 235–244. [Google Scholar] [CrossRef] [Green Version]
- Zetzmann, K.; Strehl, J.; Geppert, C.; Kuerten, S.; Jabari, S.; Brehmer, A. Calbindin D28k-immunoreactivity in human enteric neurons. Int. J. Mol. Sci. 2018, 19, 194. [Google Scholar] [CrossRef] [Green Version]
- Oponowicz, A.; Kozłowska, A.; Gonkowski, S.; Godlewski, J.; Majewski, M. Changes in the distribution of cocaine- and amphetamine-regulated transcript-containing neural structures in the human colon affected by the neoplastic process. Int. J. Mol. Sci. 2018, 19, 414. [Google Scholar] [CrossRef] [Green Version]
- Graham, K.D.; López, S.H.; Sengupta, R.; Shenoy, A.; Schneider, S.; Wright, C.M.; Feldman, M.; Furth, E.; Valdivieso, F.; Lemke, A.; et al. Robust, 3-Dimensional visualization of human colon enteric nervous system without tissue sectioning. Gastroenterology 2020, 158, 2221–2235.e5. [Google Scholar] [CrossRef]
- Crowe, R.; Burnstock, G. The subserosal ganglia of the human taenia. Neurosci. Lett. 1990, 119, 203–206. [Google Scholar] [CrossRef]
- Timmermans, J.P.; Scheuermann, D.W.; Stach, W.; Adriaensen, D.; De Groodt Lesseal, M.H.A. Functional morphology of the enteric nervous system with special reference to large mammals. Eur. J. Morphol. 1992, 30, 113–122. [Google Scholar]
- Timmermans, J.P.; Adriaensen, D.; Cornelissen, W.; Scheuermann, D.W. Structural organization and neuropeptide distribution in the mammalian enteric nervous system, with special attention to those components involved in mucosal reflexes. Comp. Biochem. Physiol. 1997, 118, 331–340. [Google Scholar] [CrossRef]
- Arciszewski, M.B.; Barabasz, S.; Całka, J. Expression of substance P, vasoactive intestinal peptide and galanin in cultured myenteric neurons from the ovine abomasum. Vet. Med. 2009, 3, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Gonkowski, S.; Burliński, P.; Skobowiat, C.; Majewski, M.; Całka, J. Inflammation- and axotomy-induced changes in galanin-like immunoreactive (GAL-LI) nerve structures in the porcine descending colon. Acta Vet. Hung. 2010, 58, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Botella, A.; Delvaux, M.; Frexinos, J.; Bueno, L. Comparative effects of galanin on isolated smooth muscle cells from ileum in five mammalian species. Life Sci. 1992, 50, 1253–1261. [Google Scholar] [CrossRef]
- Fox-Threlkeld, J.E.T.; McDonald, T.J.; Cipris, S.; Woskowska, Z.; Daniel, E.E. Galanin inhibition of vasoactive intestinal polypeptide release and circular muscle motility in the isolated perfused canine ileum. Gastroenterology 1991, 101, 1471–1476. [Google Scholar] [CrossRef]
- Lördal, M.; Johansson, C.; Hellström, P.M. Myoelectric pattern and effects on small bowel transit induced by the tachykinins neurokinin A, neurokinin B, substance P and neuropedtide K in the rat. Neurogastroenterol. Motil. 1993, 5, 33–40. [Google Scholar] [CrossRef]
- Lördal, M.; Theodorsson, E.; Hellström, P.M. Tachykinins influence interdigestive rhythm and contractile strength of human small intestine. Dig. Dis. Sci. 1997, 42, 1940–1949. [Google Scholar] [CrossRef]
- Thor, P.J.; Sendur, R.; Konturek, S.J. Influence of substance P on myoelectric activity of the small bowel. Am. J. Physiol. 1982, 243, G493–G496. [Google Scholar] [CrossRef]
- Roza, C.; Reeh, P.W.; Substance, P. calcitonin gene related peptide and PGE2 co-released from the mouse colon: A new model to study nociceptive and inflammatory responses in viscera, in vitro. Pain 2001, 93, 213–219. [Google Scholar] [CrossRef]
- Wolf, M.; Schrödl, F.; Neuhuber, W.; Brehmer, A. Calcitonin gene-related peptide: A marker for putative primary afferent neurons in the pig small intestinal myenteric plexus? Anat. Rec. 2007, 290, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, N.; Burchert, M.; Respondek, M.; Muller, K.M.; Peskar, B.M. Role of calcitonin gene-related peptide and nitric oxide in the gastroprotective effect of capsaicin in the rat. Gastroenterology 1993, 104, 1371–1380. [Google Scholar] [CrossRef]
- Barada, K.A.; Saade, N.E.; Atweh, S.F.; Khoury, C.I.; Nassar, C.F. Calcitonin gene-related peptide regulates amino acid absorption across rat jejunum. Regul. Pept. 2000, 90, 39–45. [Google Scholar] [CrossRef]
- Leung, F.W.; Iwata, F.; Seno, K.; Leung, J.W. Acid-induced mesenteric hyperemia in rats: Role of CGRP, substance P, prostaglandin, adenosine, and histamine. Dig. Dis. Sci. 2003, 48, 523–532. [Google Scholar] [CrossRef]
- De Fontgalland, D.; Wattchow, D.A.; Costa, M.; Brookes, S.J.H. Immunohistochemical characterization of the innervation of human colonic mesenteric and submucosal blood vessels. Neurogastroenterol. Motil. 2008, 20, 1212–1226. [Google Scholar] [CrossRef]
- Kaiser, E.A.; Rea, B.J.; Kuburas, A.; Kovacevich, B.R.; Garcia-Martinez, L.F.; Recober, A.; Russo, A.F. Anti-CGRP antibodies block CGRP-induced diarrhea in mice. Neuropeptides 2017, 64, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Delvalle, N.M.; Fried, D.E.; Rivera-Lopez, G.; Gaudette, L.; Gulbransen, B.D. Cholinergic activation of enteric glia is a physiological mechanism that contributes to the regulation of gastrointestinal motility. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G473–G483. [Google Scholar] [CrossRef] [PubMed]
- Scheurer, U.; Drack, E.; Halter, F. Cyclooxygenase inhibitors affect Met-enkephalin- and acetylcholine-stimulated motility of the isolated rat colon. J. Pharmacol. Exp. Ther. 1985, 234, 742–746. [Google Scholar] [PubMed]
- Johnson, C.D.; Barlow-Anacker, A.J.; Pierre, J.F.; Touw, K.; Erickson, C.S.; Furness, J.B.; Epstein, M.L.; Gosain, A. Deletion of choline acetyltransferase in enteric neurons results in postnatal intestinal dysmotility and dysbiosis. FASEB J. 2018, 32, 4744–4752. [Google Scholar] [CrossRef] [Green Version]
- Aikawa, N.; Kishibayashi, N.; Karasawa, A.; Ohmori, K. The effect of zaldaride maleate, an antidiarrheal compound, on acetylcholine-induced intestinal electrolyte secretion. Biol. Pharm. Bull. 2000, 23, 1377–1378. [Google Scholar] [CrossRef] [Green Version]
- Ogata, H.; Podolsky, D.K. Trefoil peptide expression and secretion is regulated by neuropeptides and acetylcholine. Am. J. Physiol. 1997, 273, G348–G354. [Google Scholar] [CrossRef]
- Specian, R.D.; Neutra, M.R. Mechanism of rapid mucus secretion in goblet cells stimulated by acetylcholine. J. Cell Biol. 1980, 85, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.; Lampert, S.; Mineo, H.; Holst, J.J. Neural regulation of glucagon-like peptide-1 secretion in pigs. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E939–E947. [Google Scholar] [CrossRef] [Green Version]
- Al-Barazie, R.M.; Bashir, G.H.; Qureshi, M.M.; Mohamed, Y.A.; Al-Sbiei, A.; Tariq, S.; Lammers, W.J.; Al-Ramadi, B.K.; Fernandez-Cabezudo, M.J. Cholinergic activation enhances resistance to oral Salmonella infection by modulating innate immune defense mechanisms at the intestinal barrier. Front. Immunol. 2018, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, S.; Hiemstra, I.H.; Verseijden, C.; Hilbers, F.W.; Te Velde, A.A.; Willemsen, L.E.; Stap, J.; den Haan, J.M.; de Jonge, W.J. Cholinergic receptor activation on epithelia protects against cytokine-induced barrier dysfunction. Acta Physiol. 2015, 213, 846–859. [Google Scholar] [CrossRef]
- Matteoli, G.; Boeckxstaens, G.E. The vagal innervation of the gut and immune homeostasis. Gut 2013, 62, 1214–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Zanden, E.P.; Boeckxstaens, G.E.; de Jonge, W.J. The vagus nerve as a modulator of intestinal inflammation. Neurogastroenterol. Motil. 2009, 21, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Nijhuis, L.E.; Olivier, B.J.; de Jonge, W.J. Neurogenic regulation of dendritic cells in the intestine. Biochem. Pharmacol. 2010, 80, 2002–2008. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.S.; Fox-Threlkeld, J.E.; Furness, J.B. Innervation of intestinal arteries by axons with immunoreactivity for the vesicular acetylcholine transporter (VAChT). J. Anat. 1998, 192, 107–117. [Google Scholar] [CrossRef]
- Okumura, T.; Yamada, H.; Motomura, W.; Kohgo, Y. Cocaine- amphetamine-regulated transcript (CART) acts in the central nervous system to inhibit gastric acid secretion via brain corticotrophin-releasing factor system. Endocrinology 2000, 141, 2854–2860. [Google Scholar] [CrossRef]
- Tebbe, J.J.; Ortmann, E.; Schumacher, K.; Mönnikes, H.; Kobelt, P.; Arnold, R.; Schäffer, M.K.H. Cocaine- and amphetamine-regulated transcript stimulates colonic motility via central CRF receptor activation and peripheral cholinergic pathways in fed conscious rats. Neurogastroenterol. Motil. 2004, 16, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Brehmer, A.; Croner, R.; Dimmler, A.; Papadopoulos, T.; Schrödl, F.; Neuhuber, W. Immunohistochemical characterization of putative primary afferent (sensory) myenteric neurons in human small intestine. Auton. Neurosci. 2004, 112, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Grider, J.R. CGRP as a transmitter in the sensory pathway mediating peristaltic reflex. Am. J. Physiol. 1994, 266, G1139–G1145. [Google Scholar] [CrossRef] [PubMed]
- Kuramoto, H.; Kadowaki, M. Enhancement of CGRP sensory afferent innervation in the gut during the development of food allergy in an experimental murine model. Biochem. Biophys. Res. Commun. 2013, 430, 895–900. [Google Scholar]
- Holzer, P.; Guth, P.H. Neuropeptide control of rat gastric mucosal blood flow. Increase by calcitonin gene-related peptide and vasoactive intestinal polypeptide, but not substance P and neurokinin A. Circ. Res. 1991, 68, 100–105. [Google Scholar] [CrossRef] [Green Version]
- Bulut, K.; Felderbauer, P.; Deters, S.; Hoeck, K.; Schmidt-Choudhury, A.; Schmidt, W.E.; Hoffmann, P. Sensory neuropeptides and epithelial cell restitution: The relevance of SP- and CGRP-stimulated mast cells. Int. J. Colorectal Dis. 2008, 23, 535–541. [Google Scholar] [CrossRef]
- Tam, C.; Brain, S.D. The assessment of vasoactive properties of CGRP and adrenomedullin in the microvasculature: A study using in vivo and in vitro assays in the mouse. J. Mol. Neurosci. 2004, 22, 117–124. [Google Scholar] [CrossRef]
- Pawlik, W.W.; Obuchowicz, R.; Biernat, J.; Sendur, R.; Jaworek, J. Role of calcitonin gene related peptide in the modulation of intestinal circulatory, metabolic, and myoelectric activity during ischemia/reperfusion. J. Physiol. Pharmacol. 2000, 51, 933–942. [Google Scholar]
- Holzer, P. Role of visceral afferent neurons in mucosal inflammation and defense. Curr. Opin. Pharmacol. 2007, 7, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Reinshagen, M.; Flämig, G.; Ernst, S.; Geerling, I.; Wong, H.; Walsh, J.H.; Eysselein, V.E.; Adler, G.J. Calcitonin gene-related peptide mediates the protective effect of sensory nerves in a model of colonic injury. Pharmacol. Exp. Ther. 1998, 286, 657–661. [Google Scholar]
- Bálint, A.; Fehér, E.; Kisfalvi, I.J.; Máté, M.; Zelles, T.; Vizi, E.S.; Varga, G. Functional and immunocytochemical evidence that galanin is a physiological regulator of human jejunal motility. J. Physiol. Paris 2001, 95, 129–135. [Google Scholar] [CrossRef]
- Matkowskyj, K.A.; Nathaniel, R.; Prasad, R.; Weihrauch, D.; Rao, M.; Benya, R.V. Galanin contributes to the excess colonic fluid secretion observed in dextran sulfate sodium murine colitis. Inflamm. Bowel Dis. 2004, 10, 408–416. [Google Scholar] [CrossRef]
- Piqueras, L.; Taché, Y.; Martinez, V. Galanin inhibits gastric acid secretion through a somatostatin-independent mechanism in mice. Peptides 2004, 25, 1287–1295. [Google Scholar] [CrossRef]
- Matkowskyj, K.; Royan, S.V.; Blunier, A.; Hecht, G.; Rao, M.; Benya, R.V. Age-dependent differences in galanin-dependent colonic fluid secretion after infection with Salmonella typhimurium. Gut 2009, 58, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Daniel, E.E.; Haugh, C.; Woskowska, Z.; Cipris, S.; Jury, J.; Fox-Threlkeld, J.E.T. Role of nitric oxide—Related inhibition in intestinal function: Relation to vasoactive intestinal polypeptide. Am. J. Physiol. 1994, 266, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Grider, J.R. Interplay of VIP and nitric oxide in regulation of the descending relaxation phase of peristalsis. Am. J. Physiol. 1993, 264, G334–G340. [Google Scholar] [CrossRef]
- Groneberg, D.; Voussen, B.; Friebe, A. Integrative control of gastrointestinal motility by nitric oxide. Curr. Med. Chem. 2016, 23, 2715–2735. [Google Scholar] [CrossRef]
- Walker, M.Y.; Pratap, S.; Southerland, J.H.; Farmer-Dixon, C.M.; Lakshmyya, K.; Gangula, P.R. Role of oral and gut microbiome in nitric oxide-mediated colon motility. Nitric Oxide 2018, 73, 81–88. [Google Scholar] [CrossRef]
- Mourad, F.H.; O’Donnell, L.J.; Andre, E.A.; Bearcroft, C.P.; Owen, R.A.; Clark, M.L.; Farthing, M.J. L-Arginine, nitric oxide, and intestinal secretion: Studies in rat jejunum in vivo. Gut 1996, 39, 539–544. [Google Scholar] [CrossRef] [Green Version]
- Izzo, A.A.; Mascolo, N.; Capasso, F. Nitric oxide as a modulator of intestinal water and electrolyte transport. Dig. Dis. Sci. 1998, 43, 1605–1620. [Google Scholar] [CrossRef]
- Mourad, F.H.; Barada, K.A.; Abdel-Malak, N.; Bou Rached, N.A.; Khoury, C.I.; Saade, N.E.; Nassar, C.F. Interplay between nitric oxide and vasoactive intestinal polypeptide in inducing fluid secretion in rat jejunum. J. Physiol. 2003, 550, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.K.; Alois, J.D.; Pickering, S.P.; Yeo, C.J. Nitric oxide modulates water and electrolyte transport in the ileum. Ann. Surg. 1994, 219, 382–388. [Google Scholar] [CrossRef]
- Fan, W.Q.; Smolich, J.J.; Wild, J.; Yu, V.Y.; Walker, A.M. Nitric oxide modulates regional blood flow differences in the fetal gastrointestinal tract. Am. J. Physiol. 1996, 271, G598–G604. [Google Scholar] [CrossRef]
- Jansson, L.; Carlsson, P.O.; Bodin, B.; Andersson, A.; Källskog, O. Neuronal nitric oxide synthase and splanchnic blood flow in anaesthetized rats. Acta. Physiol. Scand. 2005, 183, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Roediger, W.E. Nitric oxide damage to colonocytes in colitis-by-association: Remote transfer of nitric oxide to the colon. Digestion 2002, 65, 191–195. [Google Scholar] [CrossRef]
- Varga, S.; Juhász, L.; Gál, P.; Bogáts, G.; Boro, M.; Palásthy, Z.; Szabó, A.; Kaszaki, J. Neuronal nitric oxide mediates the anti-inflammatory effects of intestinal ischemic preconditioning. J. Surg. Res. 2019, 244, 241–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsoulis, S.; Schmidt, W.E. Role of PACAP in the regulation of gastrointestinal motility. Ann. N. Y. Acad. Sci. 1996, 805, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Aono, M.; Moriga, M. Central effects of pituitary adenylate cyclase activating polypeptide (PACAP) on gastric motility and emptying in rats. Dig. Dis. Sci. 1999, 44, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Felley, C.P.; Qian, J.M.; Mantey, S.; Pradhan, T.; Jensen, R.T. Chief cells possess a receptor with high affinity for PACAP and VIP that stimulates pepsinogen release. Am. J. Physiol. 1992, 263, G901–G907. [Google Scholar] [CrossRef]
- Läuff, J.M.; Modlin, I.M.; Tang, L.H. Biological relevance of pituitary adenylate cyclase-activating polypeptide (PACAP) in the gastrointestinal tract. Regul. Pept. 1999, 84, 1–12. [Google Scholar] [CrossRef]
- Kuwahara, A.; Kuwahara, Y.; Mochizuki, T.; Yanaihara, N. Action of pituitary adenylate cyclase-activating polypeptide on ion transport in guinea pig distal colon. Am. J. Physiol. 1993, 264, G433–G441. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, M.; Adermann, K.; Raab, H.R.; Forssmann, W.G.; Kuhn, M. Pituitary adenylate cyclase-activating polypeptide: A potent activator of human intestinal ion transport. Ann. N. Y. Acad. Sci. 1996, 805, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.X.; Hu, P.; Wang, P.; Naruse, S.; Nokihara, K.; Wray, V.; Ozaki, T. Possible key residues that determine left gastric artery blood flow response to PACAP in dogs. World. J. Gastroenterol. 2010, 16, 4865–4870. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Matsuyama, H.; Shiina, T.; Takewaki, T.; Furness, J.B. Tachykinins and their functions in the gastrointestinal tract. Cell. Mol. Life Sci. 2008, 65, 295–311. [Google Scholar] [CrossRef]
- Shibata, C.; Sasaki, I.; Naito, H.; Ohtani, N.; Matsuno, S.; Mizumoto, A.; Iwanaga, Y.; Itoh, Z.; Tohoku, J. Effects of substance P on gastric motility differ depending on the sites and vagal innervation in conscious dogs. Exp. Med. 1994, 174, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Donnerer, J.; Barthó, L.; Holzer, P.; Lembeck, F. Intestinal peristalsis associated with release of immunoreactive substance P. Neuroscience 1984, 11, 913–918. [Google Scholar] [CrossRef]
- Greenwood, B.; Doolittle, T.; See, N.A.; Koch, T.R.; Dodds, W.J.; Davison, J.S. Effects of substance P and vasoactive intestinal polypeptide on contractile activity and epithelial transport in the ferret jejunum. Gastroenterology 1990, 98, 1509–1517. [Google Scholar] [CrossRef]
- Perdue, M.H.; Galbraith, R.; Davison, J.S. Evidence for substance P as a functional neurotransmitter inguinea pig small intestinal mucosa. Regul. Pept. 1987, 18, 63–74. [Google Scholar] [CrossRef]
- Pothoulakis, C.; Castagliuolo, I.; LaMont, J.T.; Jaffer, A.; OKeane, J.C.; Snider, R.M.; Leeman, S.E. CP-96,345, a substance P antagonist, inhibits rat intestinal responses to Clostridium difficile toxin A but not choleratoxin. Proc. Natl. Acad. Sci. USA 1994, 91, 947–951. [Google Scholar] [CrossRef] [Green Version]
- Arciszewski, M.B.; Ekblad, E. Effects of vasoactive intestinal peptide and galanin on survival of cultured porcine myenteric neurons. Regul. Pept. 2005, 125, 185–192. [Google Scholar] [CrossRef]
- Eklund, S.; Jodal, M.; Lundgren, O.; Sjöqvist, A. Effects of vasoactive intestinal polypeptide on blood flow, motility and fluid transport in the gastrointestinal tract of the cat. Acta Physiol. Scand. 1979, 105, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Krantis, A.; Mattar, K.; Glasgow, I. Rat gastroduodenal motility in vivo: Interaction of GABA and VIP in control of spontaneous relaxations. Am. J. Physiol. 1998, 275, G897–G903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasaki, M.; Akiba, Y.; Kaunitz, J.D. Recent advances in vasoactive intestinal peptide physiology and pathophysiology: Focus on the gastrointestinal system. F1000Resrarch 2019, 8, F1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talbot, J.; Hahn, P.; Kroehling, L.; Nguyen, H.; Li, D.; Littman, D.R. Feeding-dependent VIP neuron-ILC3 circuit regulates the intestinal barrier. Nature 2020, 579, 575–580. [Google Scholar] [CrossRef]
- Kovsca Janjatovic, A.; Valpotic, H.; Kezic, D.; Lacković, G.; Gregorovic, G.; Sladoljev, S.; Mršić, G.; Popovic, M.; Valpotic, I. Secretion of immunomodulating neuropeptides (VIP, SP) and nitric oxide synthase in porcine small intestine during postnatal development. Eur. J. Histochem. 2012, 56, e30. [Google Scholar] [CrossRef] [Green Version]
- Ottaway, C.A. Neuroimmunomodulation in the intestinal mucosa. Gastroenterol. Clin. N. Am. 1991, 20, 511–529. [Google Scholar]
- Burleigh, D.E.; Banks, M.R. Stimulation of intestinal secretion by vasoactive intestinal peptide and cholera toxin. Auton. Neurosci. Basic Clin. 2007, 133, 64–75. [Google Scholar] [CrossRef]
- Nassar, C.F.; Abdallah, L.E.; Barada, K.A.; Atweh, S.F.; Saadé, N.F. Effects of intravenous vasoactive intestinal peptide injection on jejunal alanine absorption and gastric acid secretion in rats. Regul. Pept. 1995, 55, 261–267. [Google Scholar] [CrossRef]
- Mourad, F.H.; Nassar, C.F. Effect of vasoactive intestinal polypeptide (VIP) antagonism on rat jejunal fluid and electrolyte secretion induced by cholera and Escherichia coli enterotoxins. Gut 2000, 47, 382–386. [Google Scholar] [CrossRef] [Green Version]
- Jessen, K.R.; Mirsky, R. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature 1980, 286, 736–737. [Google Scholar] [CrossRef]
- Pochard, C.; Coquenlorge, S.; Freyssinet, M.; Naveilhan, P.; Bourreille, A.; Neunlist, M.; Rolli-Derkinderen, M. The multiple faces of inflammatory enteric glial cells: Is Crohn’s disease a gliopathy? Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G1–G11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seguella, L.; Capuano, R.; Sarnelli, G.; Esposito, G. Play in advance against neurodegeneration: Exploring enteric glial cells in gut-brain axis during neurodegenerative diseases. Expert Rev. Clin. Pharmacol. 2019, 12, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Gulbransen, B.D.; Sharkey, K.A. Novel functional roles for enteric glia in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 625–632. [Google Scholar] [CrossRef]
- de Mattos Coelho-Aguiar, J.; Bon-Frauches, A.C.; Gomes, A.L.; Veríssimo, C.P.; Aguiar, D.P.; Matias, D.; Thomasi, B.B.; Gomes, A.S.; Brito, G.A.; Moura-Neto, V. The enteric glia: Identity and functions. Glia 2015, 63, 921–935. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R. Glial cells. Int. J. Biochem. Cell Biol. 2004, 36, 1861–1867. [Google Scholar] [CrossRef] [PubMed]
- Grubišić, V.; Gulbransen, B.D. Enteric glia: The most alimentary of all glia. J. Physiol. 2017, 595, 557–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdo, H.; Derkinderen, P.; Gomes, P.; Chevalier, J.; Aubert, P.; Masson, D.; Galmiche, J.P.; Vanden Berghe, P.; Neunlist, M.; Lardeux, B. Enteric glial cells protect neurons from oxidative stress in part via reduced glutathione. FASEB J. 2010, 24, 1082–1094. [Google Scholar] [CrossRef]
- Neunlist, M.; Rolli-Derkinderen, M.; Latorre, R.; Van Landeghem, L.; Coron, E.; Derkinderen, P.; De Giorgio, R. Enteric glial cells: Recent developments and future directions. Gastroenterology 2014, 147, 1230–1237. [Google Scholar] [CrossRef]
- Ruhl, A. Glial cells in the gut. Neurogastroenterol. Motil. 2005, 17, 777–790. [Google Scholar] [CrossRef]
- Boesmans, W.; Cirill, C.; Van den Abbeel, V.; Van den Haute, C.; Depoortere, I.; Tack, J.; Vanden Berghe, P. Neuro-transmitters involved in fast excitatory neurotransmission directly activate enteric glial cells. Neurogastroenterol. Motil. 2013, 25, e151–e160. [Google Scholar] [CrossRef] [Green Version]
- Vergnolle, N.; Cirillo, C. Neurons and glia in the enteric nervous system and epithelial barrier function. Physiology 2018, 33, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Bauman, B.D.; Meng, J.; Zhang, L.; Louiselle, A.; Zheng, E.; Banerjee, S.; Roy, S.; Segura, B.J. Enteric glial-mediated enhancement of intestinal barrier integrity is compromised by morphine. J. Surg. Res. 2017, 219, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Bush, T.G.; Savidge, T.C.; Freeman, T.C.; Cox, H.J.; Campbell, E.A.; Mucke, L.; Johnson, M.H.; Sofroniew, M.V. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell 1998, 93, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Joseph, N.M.; He, S.; Quintana, E.; Kim, Y.G.; Núñez, G.; Morrison, S.J. Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J. Clin. Investig. 2011, 121, 3398–3411. [Google Scholar] [CrossRef]
- Da Silveira, A.B.; de Oliveira, E.C.; Neto, S.G.; Luquetti, A.O.; Fujiwara, R.T.; Oliveira, T.C.; Brehmer, A. Enteroglial cells act as antigen-presenting cells in chagasic megacolon. Hum. Pathol. 2011, 42, 522–553. [Google Scholar] [CrossRef]
- Rühl, A.; Franzke, S.; Collins, S.M.; Stremmel, W. Interleukin-6 expression and regulation in rat enteric glial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G1163–G1171. [Google Scholar] [CrossRef] [Green Version]
- Murakami, M.; Ohta, T.; Ito, S. Lipopolysaccharides enhance the action of bradykinin in enteric neurons via secretion of interleukin-1beta from enteric glial cells. J. Neurosci. Res. 2009, 87, 2095–2104. [Google Scholar] [CrossRef]
- Kermarrec, L.; Durand, T.; Gonzales, J.; Pabois, J.; Hulin, P.; Neunlist, M.; Neveu, I.; Naveilhan, P. Rat enteric glial cells express novel isoforms of Interleukine-7 regulated during inflammation. Neurogastroenterol. Motil. 2019, 31, e13467. [Google Scholar] [CrossRef] [Green Version]
- von Boyen, G.B.; Steinkamp, M.; Reinshagen, M.; Schäfer, K.H.; Adler, G.; Kirsch, J. Nerve growth factor secretion in cultured enteric glia cells is modulated by proinflammatory cytokines. J. Neuroendocrinol. 2006, 18, 820–825. [Google Scholar] [CrossRef]
- Rosenbaum, C.; Schick, M.A.; Wollborn, J.; Heider, A.; Scholz, C.S.; Cecil, A.; Niesler, B.; Hirrlinger, J.; Walles, H.; Metzger, M. Activation of myenteric glia during acute inflammation in vitro and in vivo. PLoS ONE 2016, 11, e0151335. [Google Scholar] [CrossRef]
- Cirillo, C.; Sarnelli, G.; Esposito, G.; Grosso, M.; Petruzzelli, R.; Izzo, G.; D’Armiento, C.F.P.; Rocco, A.; Nardone, G.; Iuvone, T.; et al. Increased mucosal nitric oxide production in ulcerative colitis is mediated in part by the enteroglial-derived S100B protein. Neurogastroenterol. Motil. 2009, 21, 1209-e112. [Google Scholar] [CrossRef]
- Caputi, V.; Giron, M.C. Microbiome-gut-brain axis and toll-like receptors in Parkinson’s disease. Int. J. Mol. Sci. 2018, 19, 1689. [Google Scholar] [CrossRef] [Green Version]
- Vasina, V.; Barbara, G.; Talamonti, L.; Stanghellini, V.; Corinaldesi, R.; Tonini, M.; De Ponti, F.; De Georgio, R. Enteric neuroplasticy evoked by inflammation. Auton. Neurosci. 2006, 126–127, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Obata, Y.; Pachnis, V. The Effect of microbiota and the immune system on the development and organization of the enteric nervous system. Gastroenterology 2016, 151, 836–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szymanska, K.; Gonkowski, S. Neurochemical characterization of the enteric neurons within the porcine jejunum in physiological conditions and under the influence of bisphenol A (BPA). Neurogastroenterol. Motil. 2019, 31, e13580. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shao, H.; Luo, X.; Wang, R.; Li, Y.; Li, Y.; Luo, Y.; Chen, Z. Effect of ozone treatment on deoxynivalenol and wheat quality. PLoS ONE 2016, 11, e0147613. [Google Scholar] [CrossRef] [PubMed]
- Habrowska-Górczyńska, D.E.; Kowalska, K.; Urbanek, K.A.; Domińska, K.; Sakowicz, A.; Piastowska-Ciesielska, A.W. Deoxynivalenol modulates the viability, ROS production and apoptosis in prostate cancer cells. Toxins 2019, 11, 265. [Google Scholar] [CrossRef] [Green Version]
- Payros, D.; Alassane-Kpembi, I.; Pierron, A.; Loiseau, N.; Pinton, P.; Oswald, I.P. Toxicology of deoxynivalenol and its acetylated and modified forms. Arch. Toxicol. 2016, 90, 2931–2957. [Google Scholar] [CrossRef] [PubMed]
- Matejova, I.; Modra, H.; Blahova, J.; Franc, A.; Fictum, P.; Sevcikova, M.; Svobodova, Z. The effect of mycotoxin deoxynivalenol on haematological and biochemical indicators and histopathological changes in rainbow trout (Oncorhynchus Mykiss). BioMed Res. Int. 2014, 2014, 310680. [Google Scholar] [CrossRef] [Green Version]
- Faeste, C.K.; Pierre, F.; Ivanova, L.; Sayyari, A.; Massotte, D. Behavioural and metabolomic changes from chronic dietary exposure to low-level deoxynivalenol reveal impact on mouse well-being. Arch. Toxicol. 2019, 93, 2087–2102. [Google Scholar] [CrossRef]
- Girardet, C.; Bonnet, M.S.; Jdir, R.; Sadoud, M.; Thirion, S.; Tardivel, C.; Roux, J.; Lebrun, B.; Mounien, L.; Trouslard, J.; et al. Central inflammation and sickness-like behavior induced by the food contaminant deoxynivalenol: A PGE2-independent mechanism. Toxicol. Sci. 2011, 124, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Momonaka, Y.; Yokose, C.; Tadaishi, M.; Shimizu, M.; Yamane, T.; Oishi, Y.; Kobayashi-Hattori, K. Anorexic action of deoxynivalenol in hypothalamus and intestine. Toxicon 2016, 118, 54–60. [Google Scholar] [CrossRef]
- Hussein, H.S.; Brasel, J.M. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology 2001, 167, 101–134. [Google Scholar] [CrossRef]
- Obremski, K.; Zielonka, L.; Gajecka, M.; Jakimiuk, E.; Bakuła, T.; Baranowski, M.; Gajecki, M. Histological estimation of the small intestine wall after administration of feed containing deoxynivalenol, T-2 toxin and zearalenone in the pig. Pol. J. Vet. Sci. 2008, 11, 339–345. [Google Scholar]
- Goossens, J.; Pasmans, F.; Verbrugghe, E.; Vandenbroucke, V.; De Baere, S.; Meyer, E.; Haesebrouck, F.; De Backer, P.; Croubels, S. Porcine intestinal epithelial barrier disruption by the Fusarium mycotoxins deoxynivalenol and T-2 toxin promotes transepithelial passage of doxycycline and paromomycin. BMC Vet. Res. 2012, 8, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osselaere, A.; Li, S.J.; De Bock, L.; Devreese, M.; Goossens, J.; Vandenbroucke, V.; Van Bocxlaer, J.; Boussery, K.; Pasmans, F.; Martel, A.; et al. Toxic effects of dietary exposure to T-2 toxin on intestinal and hepatic biotransformation enzymes and drug transporter systems in broiler chickens. Food Chem. Toxicol. 2013, 55, 150–155. [Google Scholar] [CrossRef]
- Lin, R.; Sun, Y.; Ye, W.; Zheng, T.; Wen, J.; Deng, Y. T-2 toxin inhibits the production of mucin via activating the IRE1/XBP1 pathway. Toxicology 2019, 424, 152230. [Google Scholar] [CrossRef]
- Sheng, K.; Lu, X.; Yue, J.; Gu, W.; Gu, C.; Zhang, H.; Wu, W. Role of neurotransmitters 5-hydroxy-tryptamine and substance P in anorexia induction following oral exposure to the trichothecene T-2 toxin. Food Chem. Toxicol. 2019, 123, 1–8. [Google Scholar] [CrossRef]
- Guo, P.; Liu, A.; Huang, D.; Wu, Q.; Fatima, Z.; Tao, Y.; Cheng, G.; Wang, X.; Yuan, Z. Brain damage and neurological symptoms induced by T-2 toxin in rat brain. Toxicol. Lett. 2018, 286, 96–107. [Google Scholar] [CrossRef]
- Chaudhary, M.; Rao, P.V. Brain oxidative stress after dermal and subcutaneous exposure of T-2 toxin in mice. Food Chem. Toxicol. 2010, 48, 3436–3442. [Google Scholar] [CrossRef]
- Dai, C.; Xiao, X.; Sun, F.; Zhang, Y.; Hoyer, D.; Shen, J.; Tang, S.; Velkov, T. T-2 toxin neurotoxicity: Role of oxidative stress and mitochondrial dysfunction. Arch. Toxicol. 2019, 93, 3041–3056. [Google Scholar] [CrossRef]
- Makowska, K.; Gonkowski, S.; Zielonka, L.; Dabrowski, M.; Calka, J. T2 toxin-induced changes in cocaine- and amphetamine-regulated transcript (CART)-like immunoreactivity in the enteric nervous system within selected fragments of the porcine digestive tract. Neurotox. Res. 2017, 31, 136–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makowska, K.; Gonkowski, S. Cocaine- and amphetamine-regulated transcript (CART) peptide in mammals gastrointestinal system—A review. Ann. Anim. Sci. 2017, 1, 2–21. [Google Scholar] [CrossRef] [Green Version]
- Stanley, S.A.; Small, C.J.; Murphy, K.G.; Rayes, E.; Abbott, C.R.; Seal, L.J.; Morgan, D.G.; Sunter, D.; Dakin, C.L.; Kim, M.S.; et al. Actions of cocaine- and amphetamine-regulated transcript (CART) peptide on regulation of appetite and hypothalamo-pituitary axes in vitro and in vivo in male rats. Brain Res. 2001, 893, 186–194. [Google Scholar] [CrossRef]
- Helton, W.S.; Mulholland, M.M.; Bunnett, N.W.; Debas, H.T. Inhibition of gastric and pancreatic secretion in dogs by CGRP: Role of somatostatin. Am. J. Physiol. 1989, 256, G715–G720. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, H.-K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Risks for animal health related to the presence of zearalenone and its modified forms in feed. EFSA J. 2017, 15, 4851. [Google Scholar]
- Lephart, E.D.; Thompson, J.M.; Setchell, K.D.; Adlercreutz, H.; Weber, K.S. Phytoestrogens decrease brain calcium-binding proteins but do not alter hypothalamic androgen metabolizing enzymes in adult male rats. Brain Res. 2000, 859, 123–131. [Google Scholar] [CrossRef]
- Venkataramana, M.; Chandra Nayaka, S.; Anand, T.; Rajesh, R.; Aiyaz, M.; Divakara, S.T.; Murali, H.S.; Prakash, H.S.; Lakshmana Rao, P.V. Zearalenone induced toxicity in SHSY-5Y cells: The role of oxidative stress evidenced by N-acetyl cysteine. Food Chem. Toxicol. 2014, 65, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.H.; Deng, H.D.; Deng, Y.T.; Deng, J.L.; Zuo, Z.C.; Yu, S.M.; Hu, Y.C. Effect of the Fusarium toxins, zearalenone and deoxynivalenol, on the mouse brain. Environ. Toxicol. Pharmacol. 2016, 46, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Kiss, D.S.; Ioja, E.; Toth, I.; Barany, Z.; Jocsak, G.; Bartha, T.; Horvath, T.L.; Zsarnovszky, A. Comparative analysis of zearalenone effects on thyroid receptor alpha (TRα) and beta (TRβ) expression in rat primary cerebellar cell cultures. Int. J. Mol. Sci. 2018, 19, 1440. [Google Scholar] [CrossRef] [Green Version]
- Khezri, A.; Herranz-Jusdado, J.G.; Ropstad, E.; Fraser, T.W. Mycotoxins induce developmental toxicity and behavioural aberrations in zebrafish larvae. Environ. Pollut. 2018, 242, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Gajęcka, M.; Stopa, E.; Tarasiuk, M.; Zielonka, Ł.; Gajęcki, M. The expression of type-1 and type-2 nitric oxide synthase in selected tissues of the gastrointestinal tract during mixed mycotoxicosis. Toxins 2013, 5, 2281–2292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahjouji, T.; Bertaccini, A.; Neves, M.; Puel, S.; Oswald, I.P.; Soler, L. Acute exposure to zearalenone disturbs intestinal homeostasis by modulating the Wnt/β-Catenin signaling pathway. Toxins 2020, 12, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cieplińska, K.; Gajęcka, M.; Dąbrowski, M.; Rykaczewska, A.; Lisieska-Żołnierczyk, S.; Bulińska, M.; Zielonka, Ł.; Gajęcki, M.T. Time-dependent changes in the intestinal microbiome of gilts exposed to low zearalenone doses. Toxins 2019, 11, 296. [Google Scholar] [CrossRef] [Green Version]
- Artigot, M.P.; Loiseau, N.; Laffitte, J.; Mas-Reguieg, L.; Tadrist, S.; Oswald, I.P.; Puel, O. Molecular cloning and functional characterization of two CYP619 cytochrome P450s involved in biosynthesis of patulin in Aspergillus clavatus. Microbiology 2009, 155, 1738–1747. [Google Scholar] [CrossRef] [Green Version]
- Biango-Daniels, M.N.; Hodge, K.T. Paecilomyces rot: A New apple disease. Plant Dis. 2018, 102, 1581–1587. [Google Scholar] [CrossRef]
- Assunção, R.; Pinhão, M.; Loureiro, S.; Alvito, P.; João Silva, M. A Multi-endpoint approach to the combined toxic effects of patulin and ochratoxin a in human intestinal cells. Toxicol. Lett. 2019, 313, 120–129. [Google Scholar] [CrossRef]
- Mohan, H.M.; Collins, D.; Maher, S.; Walsh, E.G.; Winter, D.C.; O’Brien, P.J.; Brayden, D.J.; Baird, A.W. The mycotoxin patulin increases colonic epithelial permeability in vitro. Food. Chem. Toxicol. 2012, 50, 4097–4102. [Google Scholar] [CrossRef]
- Malekinejad, H.; Aghazadeh-Attari, J.; Rezabakhsh, A.; Sattari, M.; Ghasemsoltani-Momtaz, B. Neurotoxicity of mycotoxins produced in vitro by Penicillium roqueforti isolated from maize and grass silage. Hum. Exp. Toxicol. 2015, 34, 997–1005. [Google Scholar] [CrossRef]
- Vidal, A.; Ouhibi, S.; Gali, R.; Hedhili, A.; De Saeger, S.; De Boevre, M. The mycotoxin patulin: An updated short review on occurrence, toxicity and analytical challenges. Food. Chem. Toxicol. 2019, 129, 249–256. [Google Scholar] [CrossRef]
- Brand, B.; Stoye, N.M.; Guilherme, M.D.S.; Nguyen, V.T.T.; Baumgaertner, J.C.; Schüffler, A.; Thines, E.; Endres, K. Identification of patulin from Penicillium coprobium as a toxin for enteric neurons. Molecules 2019, 24, 2776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peltomaa, R.; Vaghini, S.; Patiño, B.; Benito-Peña, E.; Moreno-Bondi, M.C. Species-specific optical genosensors for the detection of mycotoxigenic Fusarium fungi in food samples. Anal. Chim. Acta 2016, 935, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Palencia, E.R.; Mitchell, T.R.; Snook, M.E.; Glenn, A.E.; Gold, S.; Hinton, D.M.; Riley, R.T.; Bacon, C.W. Analyses of black Aspergillus species of peanut and maize for ochratoxins and fumonisins. J. Food Prot. 2014, 77, 805–813. [Google Scholar] [CrossRef]
- Waes, J.G.; Starr, L.; Maddox, J.; Aleman, F.; Voss, K.A.; Wilberding, J.; Riley, R.T. Maternal fumonisin exposure and risk for neural tube defects: Mechanisms in an in vivo mouse model. Birth Defects Res. A Clin. Mol. Teratol. 2005, 73, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Purzycki, C.B.; Shain, D.H. Fungal toxins and multiple sclerosis: A compelling connection. Brain Res. Bull. 2010, 82, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G.R.; Tolleson, W.H.; Newkirk, D.K.; Roberts, D.W.; Rowland, K.L.; Saheki, T.; Kobayashi, K.; Howard, P.C.; Melchior, W.B., Jr. Identification of fumonisin B1 as an inhibitor of argininosuccinate synthetase using fumonisin affinity chromatography and in vitro kinetic studies. J. Biochem. Mol. Toxicol. 2000, 14, 320–328. [Google Scholar] [CrossRef]
- Kolf-Clauw, M.; Castellote, J.; Joly, B.; Bourges-Abella, N.; Raymond-Letron, I.; Pinton, P.; Oswald, I.P. Development of a pig jejunal explant culture for studying the gastrointestinal toxicity of the mycotoxin deoxynivalenol: Histopathological analysis. Toxicol. In Vitro 2009, 23, 1580–1584. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef]
- Pestka, J.J.; Smolinski, A.T. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Health B Crit. Rev. 2005, 8, 39–69. [Google Scholar] [CrossRef]
- Pestka, J.J.; Lin, W.S.; Miller, E.R. Emetic activity of the trichothecene 15-acetyldeoxynivalenol in swine. Food Chem. Toxicol. 1987, 25, 855–858. [Google Scholar] [CrossRef]
- Young, L.G.; McGirr, L.; Valli, V.E.; Lumsden, J.H.; Lun, A. Vomitoxin in corn fed to young pigs. J. Anim. Sci. 1983, 57, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Prelusky, D.B.; Trenholm, H.L. The efficacy of various classes of anti-emetics in preventing deoxynivalenol-induced vomiting in swine. Nat. Toxins 1983, 1, 296–302. [Google Scholar] [CrossRef]
- Lee, H.J.; Ryu, D. Worldwide occurrence of mycotoxins in cereals and cereal-derived food products: Public health perspectives of their co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef]
- Pierron, A.; Mimoun, S.; Murate, L.S.; Loiseau, N.; Lippi, Y.; Bracarense, A.P.; Liaubet, L.; Schatzmayr, G.; Berthiller, F.; Moll, W.D.; et al. Intestinal toxicity of the masked mycotoxin deoxynivalenol-3-β-D-glucoside. Arch. Toxicol. 2016, 90, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Braber, S.; Akbari, P.; Garssen, J.; Fink-Gremmels, J. Deoxynivalenol impairs weight gain and affects markers of gut health after low-dose, short-term exposure of growing pigs. Toxins 2015, 7, 2071–2095. [Google Scholar] [CrossRef]
- Bracarense, A.P.; Lucioli, J.; Grenier, B.; Pacheco, G.D.; Moll, W.D.; Schatzmayr, G.; Oswald, I.P. Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction, induces morphological and immunological changes in the intestine of piglets. Br. J. Nutr. 2012, 107, 1776–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerez, J.R.; Pinton, P.; Callu, P.; Grosjean, F.; Oswald, I.P.; Bracarense, A.P. Deoxynivalenol alone or in combination with nivalenol and zearalenone induce systemic histological changes in pigs. Exp. Toxicol. Pathol. 2015, 67, 89–98. [Google Scholar] [CrossRef]
- Kasuga, F.; Hara-Kudo, Y.; Saito, N.; Kumagai, S.; Sugita-Konishi, Y. In vitro effect of deoxynivalenol on the differentiation of human colonic cell lines Caco-2 and T84. Mycopathologia 1998, 142, 161–167. [Google Scholar] [CrossRef]
- Bensassi, F.; El Golli-Bennour, E.; Abid-Essefi, S.; Bouaziz, C.; Hajlaoui, M.R.; Bacha, H. Pathway of deoxynivalenol-induced apoptosis in human colon carcinoma cells. Toxicology 2009, 264, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Kouadio, J.H.; Mobio, T.A.; Baudrimont, I.; Moukha, S.; Dano, S.D.; Creppy, E. Comparative study of cytotoxicity and oxidative stress induced by deoxynivalenol, zearalenone or fumonisin B1 in human intestinal cell line Caco-2. Toxicology 2005, 213, 56–65. [Google Scholar] [CrossRef]
- Awad, W.A.; Böhm, J.; Razzazi-Fazeli, E.; Zentek, J. Effects of feeding deoxynivalenol contaminated wheat on growth performance, organ weights and histological parameters of the intestine of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2006, 90, 32–37. [Google Scholar] [CrossRef]
- Girish, C.K.; Smith, T.K. Effects of feeding blends of grains naturally contaminated with Fusarium mycotoxins on small intestinal morphology of turkeys. Poult. Sci. 2008, 87, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Tremel, H.; Strugala, G.; Forth, W.; Fichtl, B. Dexamethasone decreases lethality of rats in acute poisoning with T-2 toxin. Arch. Toxicol. 1985, 57, 74–75. [Google Scholar] [CrossRef]
- Chi, M.S.; Robison, T.S.; Mirocha, C.J.; Reddy, K.R. Acute toxicity of 12,13-epoxytrichothecenes in one-day-old broiler chicks. Appl. Environ. Microbiol. 1978, 35, 636–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richard, J.L. Some major mycotoxins and their mycotoxicoses—An overview. Int. J. Food Microbiol. 2007, 119, 3–10. [Google Scholar] [CrossRef]
- Pang, V.F.; Lorenzana, R.M.; Beasley, V.R.; Buck, W.B.; Haschek, W.M. Experimental T-2 toxicosis in swine. III. Morphologic changes following intravascular administration of T-2 toxin. Fundam. Appl. Toxicol. 1987, 8, 298–309. [Google Scholar] [CrossRef]
- Beasley, V.R.; Lundeen, G.R.; Poppenga, R.H.; Buck, W.B. Distribution of blood flow to the gastrointestinal tract of swine during T-2 toxin-induced shock. Fundam. Appl. Toxicol. 1987, 9, 588–594. [Google Scholar] [CrossRef]
- Ványi, A.; Glávits, R.; Gajdács, E.; Sándor, G.; Kovács, F. Changes induced in newborn piglets by the trichothecene toxin T-2. Acta. Vet. Hung. 1991, 39, 29–37. [Google Scholar] [PubMed]
- Bratich, P.M.; Buck, W.B.; Haschek, W.M. Prevention of T-2 toxin-induced morphologic effects in the rat by highly activated charcoal. Arch. Toxicol. 1990, 64, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, S.; Shimizu, T. Effects of fusarenon-X and T-2 toxin on intestinal absorption of monosaccharide in rats. Arch. Toxicol. 1988, 61, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.L.; Wannemacher, R.W., Jr. In vivo effects of T-2 mycotoxin on synthesis of proteins and DNA in rat tissues. Toxicol. Appl. Pharmacol. 1990, 105, 483–491. [Google Scholar] [CrossRef]
- Gao, X.; Sun, L.; Zhang, N.; Li, C.; Zhang, J.; Xiao, Z.; Qi, D. Gestational zearalenone exposure causes reproductive and developmental toxicity in pregnant rats and female offspring. Toxins 2017, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obremski, K.; Gajęcka, M.; Zielonka, Ł.; Jakimiuk, E.; Gajęcki, M. Morphology and ultrastructure of small intestine mucosa in gilts with zearalenone mycotoxicosis. Pol. J. Vet. Sci. 2005, 8, 301–307. [Google Scholar] [PubMed]
- Obremski, K.; Poniatowska-Broniek, G. Zearalenone induces apoptosis and inhibits proliferation in porcine ileal Peyer’s patch lymphocytes. Pol. J. Vet. Sci. 2015, 18, 153–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taranu, I.; Braicu, C.; Marin, D.E.; Pistol, G.C.; Motiu, M.; Balacescu, L.; Beridan Neagoe, I.; Burlacu, R. Exposure to zearalenone mycotoxin alters in vitro porcine intestinal epithelial cells by differential gene expression. Toxicol. Lett. 2015, 232, 310–325. [Google Scholar] [CrossRef] [PubMed]
- Girgis, G.N.; Barta, J.R.; Brash, M.; Smith, T.K. Morphologic changes in the intestine of broiler breeder pullets fed diets naturally contaminated with Fusarium mycotoxins with or without coccidial challenge. Avian Dis. 2010, 54, 67–73. [Google Scholar] [CrossRef]
- McKinley, E.R.; Carlton, W.W.; Boon, G.D. Patulin mycotoxicosis in the rat: Toxicology, pathology and clinical pathology. Food Chem. Toxicol. 1982, 20, 289–300. [Google Scholar] [CrossRef]
- Escoula, L.; More, J.; Baradat, C. The toxins by Byssochlamys nivea Westling. I. Acute toxicity of patulin in adult rats and mice. Ann. Rech. Vet. 1977, 8, 41–49. [Google Scholar]
- Speijers, G.J.; Franken, M.A.; van Leeuwen, F.X. Subacute toxicity study of patulin in the rat: Effects on the kidney and the gastro-intestinal tract. Food Chem. Toxicol. 1988, 26, 23–30. [Google Scholar] [CrossRef]
- McKinley, E.R.; Carlton, W.W. Patulin mycotoxicosis in Swiss ICR mice. Food Cosmet. Toxicol. 1980, 18, 181–187. [Google Scholar] [CrossRef]
- McKinley, E.R.; Carlton, W.W. Patulin mycotoxicosis in the Syrian hamster. Food Cosmet. Toxicol. 1980, 18, 173–179. [Google Scholar] [CrossRef]
- Dailey, R.E.; Brouwer, E.; Blaschka, A.M.; Reynaldo, E.F.; Green, S.; Monlux, W.S.; Ruggles, D.I. Intermediate-duration toxicity study of patulin in rats. J. Toxicol. Environ. Health 1977, 2, 713–725. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.; Lambert, D.; Padfield, P.J.; Burt, J.P.; O’Neill, C.A. The mycotoxin patulin, modulates tight junctions in caco-2 cells. Toxicol. In Vitro 2009, 23, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Maidana, L.; Gerez, J.R.; El Khoury, R.; Pinho, F.; Puel, O.; Oswald, I.P.; Bracarense, A.P. Effects of patulin and ascladiol on porcine intestinal mucosa: An ex vivo approach. Food Chem. Toxicol. 2016, 98, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Rotter, B.A.; Thompson, B.K.; Prelusky, D.B.; Trenholm, H.L.; Stewart, B.; Miller, J.D.; Savard, M.E. Response of growing swine to dietary exposure to pure fumonisin B1 during an eight-week period: Growth and clinical parameters. Nat. Toxins 1996, 4, 42–50. [Google Scholar] [CrossRef]
- Dilkin, P.; Zorzete, P.; Mallmann, C.A.; Gomes, J.D.; Utiyama, C.E.; Oetting, L.L.; Corrêa, B. Toxicological effects of chronic low doses of aflatoxin B(1) and fumonisin B(1)-containing Fusarium moniliforme culture material in weaned piglets. Food Chem. Toxicol. 2003, 41, 1345–1353. [Google Scholar] [CrossRef]
- Ledoux, D.R.; Brown, T.P.; Weibking, T.S.; Rottinghaus, G.E. Fumonisin toxicity in broiler chicks. J. Vet. Diagn. Investig. 1992, 4, 330–333. [Google Scholar] [CrossRef] [Green Version]
- Bhat, R.V.; Shetty, P.H.; Rao, P.A.; Rao, V.S. A foodborne disease outbreak due to the consumption of moldy sorghum and maize containing fumonisin mycotoxins. J. Toxicol. Clin. Toxicol. 1997, 35, 249–255. [Google Scholar] [CrossRef]
- Schmelz, E.M.; Dombrink-Kurtzman, M.A.; Roberts, P.C.; Kozutsumi, Y.; Kawasaki, T.; Merrill, A.H., Jr. Induction of apoptosis by fumonisin B1in HT29 cells is mediated by the accumulation of endogenous free sphingoid bases. Toxicol. Appl. Pharmacol. 1998, 148, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Theumer, M.G.; Lopez, A.G.; Masih, D.T.; Chulze, S.N.; Rubinstein, H.R. Immunobiological effects of fumonisin B1in experimental subchronic mycotoxicoses in rats. Clin. Diagn. Lab. Immunol. 2002, 9, 149–155. [Google Scholar]
- Casado, J.M.; Theumer, M.; Masih, D.T.; Chulze, S.; Rubinstein, H.R. Experimental subchronic mycotoxicoses in mice: Individual and combined effects of dietary exposure to fumonisins and aflatoxin B1. Food Chem. Toxicol. 2001, 39, 579–586. [Google Scholar] [CrossRef]
- Bouhet, S.; Hourcade, E.; Loiseau, N.; Fikry, A.; Martinez, S.; Roselli, M.; Galtier, P.; Mengheri, E.; Oswald, I.P. The mycotoxin, fumonisin B1alters the proliferation and the barrier function of porcine intestinal epithelial cells. Toxicol. Sci. 2004, 77, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Carballo, D.; Tolosa, J.; Ferrer, E.; Berrada, H. Dietary exposure assessment to mycotoxins through total diet studies. A review. Food Chem. Toxicol. 2019, 128, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Maresca, M.; Fantini, J. Some food-associated mycotoxins as potential risk factors in humans predisposed to chronic intestinal inflammatory diseases. Toxicon 2010, 56, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, A.L.; Caruso, G.; Cavaliere, C.; Foglia, P.; Samperi, R.; Laganà, A. Multiclass mycotoxin analysis in food, environmental and biological matrices with chromatography/mass spectrometry. Mass Spectrom. Rev. 2012, 31, 466–503. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ma, Y.; Xue, C.; Ma, J.; Xie, Q.; Wang, G.; Bi, Y.; Cao, Y. The combination of deoxynivalenol and zearalenone at permitted feed concentrations causes serious physiological effects in young pigs. J. Vet. Sci. 2008, 9, 39–44. [Google Scholar] [CrossRef]
- Sergent, T.; Ribonnet, L.; Kolosova, A.; Garsou, S.; Schaut, A.; De Saeger, S.; Van Peteghem, C.; Larondelle, Y.; Pussemier, L.; Schneider, Y.J. Molecular and cellular effects of food contaminants and secondary plant components and their plausible interactions at the intestinal level. Food Chem. Toxicol. 2008, 46, 813–841. [Google Scholar] [CrossRef]
- Harrison, J.C.; Carvajal, M.; Garner, R.C. Does aflatoxin exposure in the United Kingdom constitute a cancer risk? Environ. Health Perspect. 1993, 99, 99–105. [Google Scholar] [CrossRef]
- Eom, S.Y.; Yim, D.H.; Zhang, Y.; Yun, J.K.; Moon, S.I.; Yun, H.Y.; Song, Y.J.; Youn, S.J.; Hyun, T.; Park, J.S.; et al. Dietary aflatoxin B1 intake, genetic polymorphisms of CYP1A2, CYP2E1, EPHX1, GSTM1, and GSTT1, and gastric cancer risk in Korean. Cancer Causes Control 2013, 24, 1963–1972. [Google Scholar] [CrossRef]
- Przybyłowicz, K.E.; Arłukowicz, T.; Danielewicz, A.; Morze, J.; Gajęcka, M.; Zielonka, Ł.; Fotschki, B.; Sawicki, T. Association between mycotoxin exposure and dietary habits in colorectal cancer development among a Polish population: A study protocol. Int. J. Environ. Res. Public Health 2020, 17, 698. [Google Scholar] [CrossRef] [Green Version]
- Roy, R.N.; Russell, R.I. Crohn’s Disease and Aflatoxins. J. R. Soc. Health 1992, 112, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Ouhibi, S.; Vidal, A.; Martins, C.; Gali, R.; Hedhili, A.; De Saeger, S.; De Boevre, M. LC-MS/MS methodology for simultaneous determination of patulin and citrinin in urine and plasma applied to a pilot study in colorectal cancer patients. Food Chem. Toxicol. 2020, 136, 110994. [Google Scholar] [CrossRef] [PubMed]
| Active Neuronal Substance in the ENS (Alphabetical Order) | Selected Functions | References |
|---|---|---|
| Acetylcholine (Ach) | Stimulation of the intestinal motility | [78,79,80] |
| Stimulation of electrolyte, water, enzymes and hormones secretion | [81,82,83,84] | |
| Participation in protective mechanisms | [82,85,86] | |
| Ant-inflammatory and immunostymulatory effects | [87,88,89] | |
| Blood flow regulation | [90] | |
| Cocaine and Amphetamine Regulated Transcript (CART) | Inhibition of gastric acid secretion | [91] |
| Regulation of the intestinal motility | [92] | |
| Calcitonin Gene-Related Peptide (CGRP) | Participation in sensory and pain stimuli conduction | [71,72,93,94,95] |
| Regulation of the intestinal motility | [94] | |
| Blood flow regulation | [96,97,98,99] | |
| Protective roles | [73,99,100,101] | |
| The influence on intestinal absorption | [74] | |
| Galanin (GAL) | Intestinal motility regulation | [66,67,68,69,70,102] |
| Influence on secretory activity | [103,104,105] | |
| Participation in inflammatory processes | [103,105,106] | |
| Nitric Oxide (NO) | Inhibition of the intestinal motility | [106,107,108] |
| Participation in inflammatory processes | [109] | |
| Regulation of intestinal secretion, water and electrolyte transport | [110,111,112,113] | |
| Regulation of blood flow | [114,115] | |
| Participation in inflammatory processes | [116,117] | |
| Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) | Inhibition of the intestinal motility | [118,119] |
| Stimulation of gastric secretory activity | [120,121] | |
| Regulation of ion transport and Luminal fluid regulation in the large intestine | [121,122,123] | |
| Regulation of blood flow | [124] | |
| Substance P (SP) | Protective roles | [100] |
| Sensory stimuli conduction | [93,125] | |
| Regulation of the intestinal motility | [125,126,127] | |
| Regulation of water and electrolytes secretion | [125,128,129] | |
| Participation in inflammatory processes | [125,130] | |
| Vasoactive Intestinal Polypeptide (VIP) | Neuroprotective functions | [131] |
| Regulation of the intestinal motility | [132,133] | |
| Vasodialtory activity | [132,134] | |
| Participation in intestinal immunomodulation | [135,136,137] | |
| Influences on intestinal secretion | [138,139,140] |
| Mycotoxin | Gastrointestinal Signs of Toxicity | References | Influence on the Digestive Tract | References |
|---|---|---|---|---|
| Doxynivalenol (DON) | Abdominal pain, increased salivation, diarrhea, vomiting, anorexia, decrease body weight gain | [169,209,210,211,212,213,214] | IPEC-J2 cell line from porcine jejunal epithelium: cytotoxicity, decrease in transepithelial electrical resistance, disruption of epithelial integrity | [176] |
| Porcine jejunal explant samples: shortened and coalescent villi, lysis of enterocytes, edema, upregulation of proinflammatory cytokines expression | [215,216] | |||
| Pigs of White Large Polish Breed: increase in the mucosal thickness and the intestinal crypt depth, atrophy of the villi, changes in the number of goblet cells, inflammatory infiltration, intensification of apoptosis, changes in ultrastructure of intestinal cells | [10,11,175,214,217,218] | |||
| Human Colonic Cell Lines Caco-2, T84, HT-29: decrease in cell proliferation, changes in permeability, genotoxicity, intensification of apoptosis, increase in the expression of proinflammatory cytokines, influence on DNA synthesis | [215,219,220,221] | |||
| Poultry: decrease in the high of villi | [222,223] | |||
| T2 Toxin | Gastrointestinal bleeding, diarrhea, vomiting, decreased feed consumption and weight gain | [224,225,226] | IPEC-J2 cell line from porcine jejunal epithelium: cytotoxic effects, disruption of intestinal barrier integrity | [176] |
| human intestinal Caco-2 cells disturbances in intestinal barrier, enzymatic activity of enteric cells, inhibition of mucin production | [178] | |||
| Pigs of White Large Polish Breed or crossbred pigs: congestion and hemorrhage of the gastrointestinal mucosal layer, inflammatory infiltration, in high doses—necrotic changes | [175,227,228,229] | |||
| Sprague-Daw-ley rats: inflammatory and necrotic changes in, lymphocytic necrosis in intestinal Peyer’s patches, influence on nutrients absorption, influence on DNA synthesis | [230,231,232] | |||
| Zearalenone (ZEN) | Gastrointestinal symptoms are not typical for ZEN toxicity. Decrease in feed intake and body weight, changes in intestinal microbiome | [195,233] | Pigs of various breeds: increase in the mucosal thickness, increase in the number of goblet cells, increase in lymphocyte number in epithelium, intensification of apoptosis, influence on enzymatic activity of mucosal cells, changes in intestinal microbiome | [10,11,175,193,194,195,234,235] |
| Intestinal porcine epithelial cell line (IPEC-1): influence on cell activity by changes in gene expression | [236] | |||
| Poultry: changes in the high of intestinal villi | [237] | |||
| Patulin (PAT) | Anorexia, salivation, distended abdomen loss of body weight, bleeding from the digestive tract and diarrhea | [238,239,240,241,242,243] | Human intestinal Caco-2 cells: the influence on permeability and ion transport in the mucosa, epithelial desquamation and sub mucosal swelling, genotoxicity effects, modulation of tight junctions | [198,199,244] |
| Rodents: mucosal layer injury, ulceration, fibrosis in the sub mucosa, necrosis | [238,239,240,241,242] | |||
| Porcine jejunal explant samples: villi atrophy and necrosis, decrease in the number of goblet cells, increase in cell apoptosis | [245] | |||
| Fumonisins (FUM) | reduction of feed consumption and body weight, abdominal pain, diarrhea | [246,247,248,249] | Human Colonic Cell Lines Caco-2, HT-29: growth inhibition and apoptosis induction, impact on mitochondrial metabolism, necrosis | [221,250] |
| Rodents: inflammatory infiltration increase in the number of mitotic figures in the intestinal crypts, necrotic changes | [251,252] | |||
| Intestinal porcine epithelial cell line (IPEC-1): inhibition of cell proliferation, intestinal barrier dysfunction | [253] |
| Mycotoxin | Dose Examined | Animal Species or Kind of Tissues | Experimental Method Used in the Study | Character of Changes in the ENS | References |
|---|---|---|---|---|---|
| Doxynivalenol | from 0.2 mg/kg of chow to 2 mg/kg of chow | Wistar rats (Rattus novergicus) | immunohistochemistry and microscopic analysis | Reduction of the area of general population of the myenteric neurons, glial cells in the myenteric plexus and whole myenteric ganglia. | [14] |
| T2 Toxin | 12 µg/kg body weight/day | domestic pig of the White Large Polish Breed | Immunofluorescence method and microscopic analysis | Increase in the number of VIP-positive enteric neurons and intramucosal and intramuscular nerve fibers containing VIP in the stomach and duodenum. | [15] |
| 200 µg/kg of feed | domestic pig of the White Large Polish Breed | Immunofluorescence method and microscopic analysis | Increase in the number of CART-positive enteric neurons and intramucosal and intramuscular nerve fibers containing CART in the stomach, duodenum and descending colon. | [55] | |
| 12 µg/kg body weight/day | domestic pig of the White Large Polish Breed | Immunofluorescence method and microscopic analysis | Increase in the number and changes in neurochemical character of CGRP-positive enteric neurons in the descending colon. | [16] | |
| Zearalenon | 10 μg/kg body weight/day | domestic pig of the White Large Polish Breed | Immunofluorescence method and microscopic analysis | Increase in the number of nerve fibers immunoreactive to CART, SP, NOS, VIP, PACAP and decrease in the number of GAL-positive nerve fibers in the muscular layer of the ileum. | [19] |
| 0.1 mg/kg of chow/day | domestic pig of the White Large Polish Breed | Immunofluorescence method and microscopic analysis | Increase in the number of nerve fibers immunoreactive to SP and VIP with changes in their morphology | [21] | |
| 12 µg/kg body weight/day | domestic pig of the White Large Polish Breed | Immunofluorescence method and microscopic analysis | Increase in the number and changes in neurochemical character of neurons immunoreactive to CGRP in the descending colon. | [16] | |
| Patulin | EC50 = 1 ng/µL | culture of the enteric neurons from C57B6/J OlaHsd mice | Growth and viability testing, cytotoxicity test, evaluation of calcium signaling, measurement of glucose content, neurite outgrowth measurement and reactive oxygen species (ROS) test | Reduction of ATP levels and glucose concentration, disorders in calcium signaling in the enteric neurons, changes in their morphology. | [71] |
| Fumonisins | 1 and 3 mg/kg body weight | Wistar rats (Rattus novergicus) | immunohistochemistry method | Reduction of the size of neurons in the enteric ganglia. | [12] |
| 90 mg/kg body weight | Wistar rats (Rattus novergicus) | immunohistochemistry method and histomorphometrical analysis | Reduction of area and mean diameter of the submucous plexuses in duodenum. Reduction of area and mean diameter of myenteric and submucous plexuses in the jejunum, increase of sphericity of the enteric ganglia. | [13] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonkowski, S.; Gajęcka, M.; Makowska, K. Mycotoxins and the Enteric Nervous System. Toxins 2020, 12, 461. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12070461
Gonkowski S, Gajęcka M, Makowska K. Mycotoxins and the Enteric Nervous System. Toxins. 2020; 12(7):461. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12070461
Chicago/Turabian StyleGonkowski, Sławomir, Magdalena Gajęcka, and Krystyna Makowska. 2020. "Mycotoxins and the Enteric Nervous System" Toxins 12, no. 7: 461. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12070461





