Effects of Deoxynivalenol and Zearalenone on the Histology and Ultrastructure of Pig Liver
Abstract
:1. Introduction
2. Results and Discussion
2.1. Light Microscopy Study
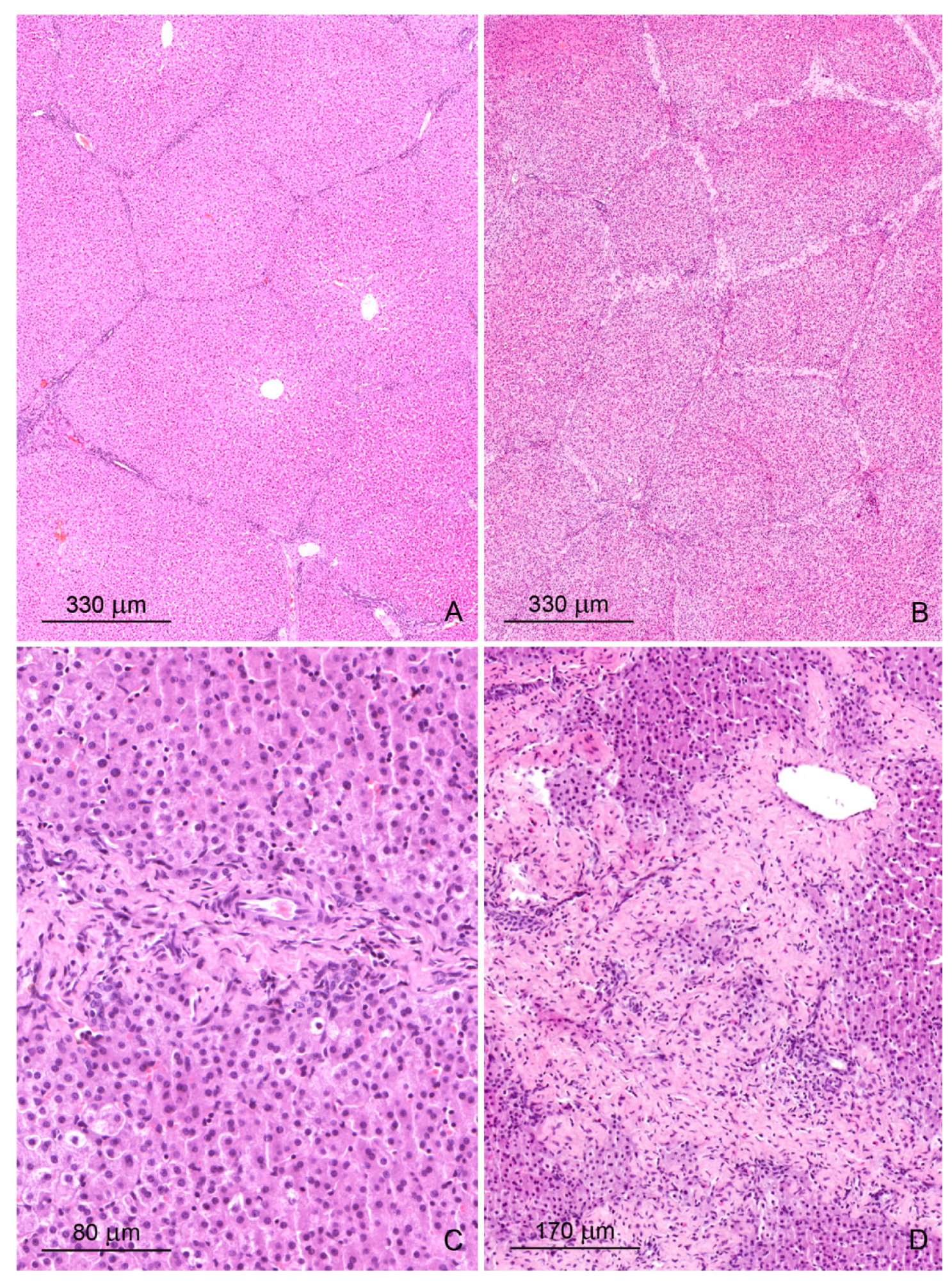
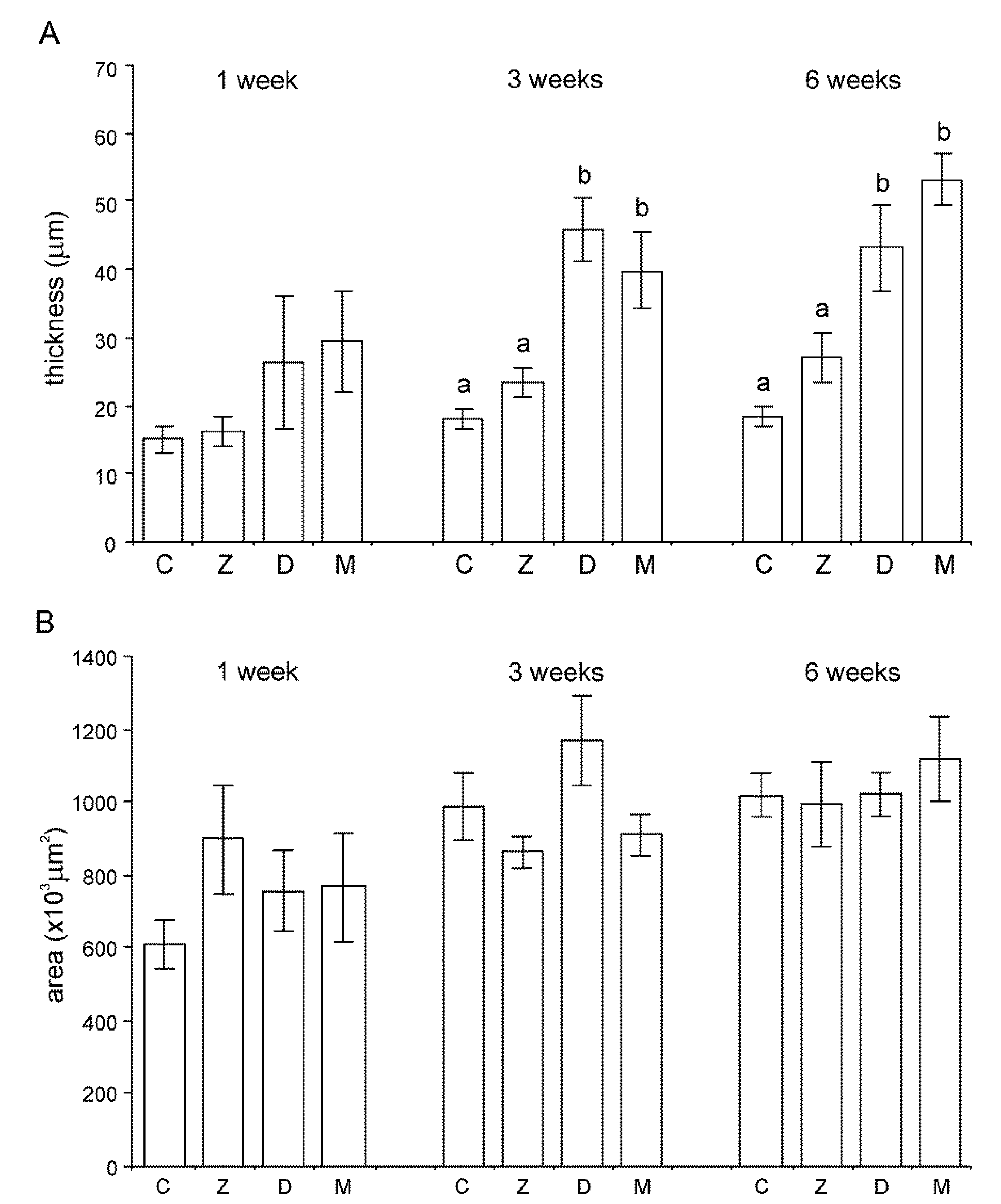
2.1.1. Architecture of the Liver
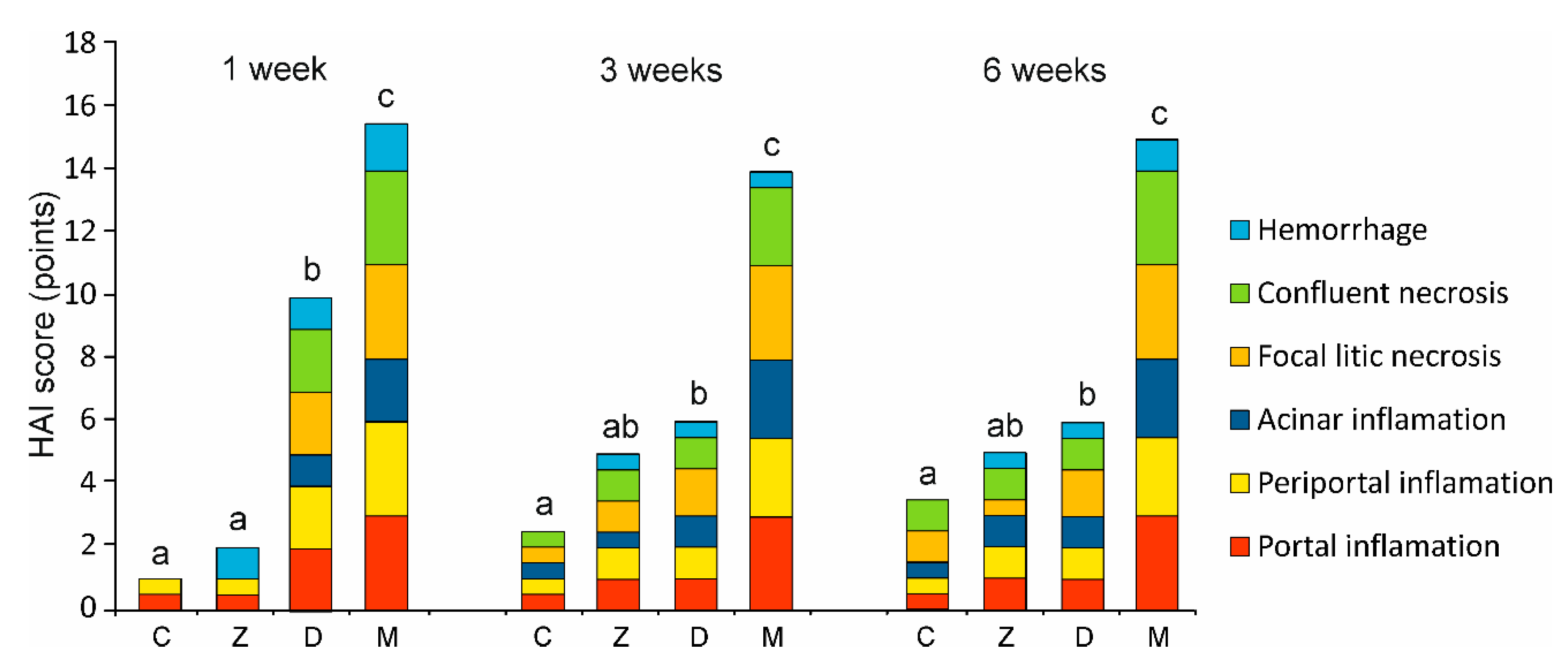
2.1.2. Microscopic Liver Scoring
2.1.3. Hepatic Sinusoids
2.1.4. Glycogen Storage
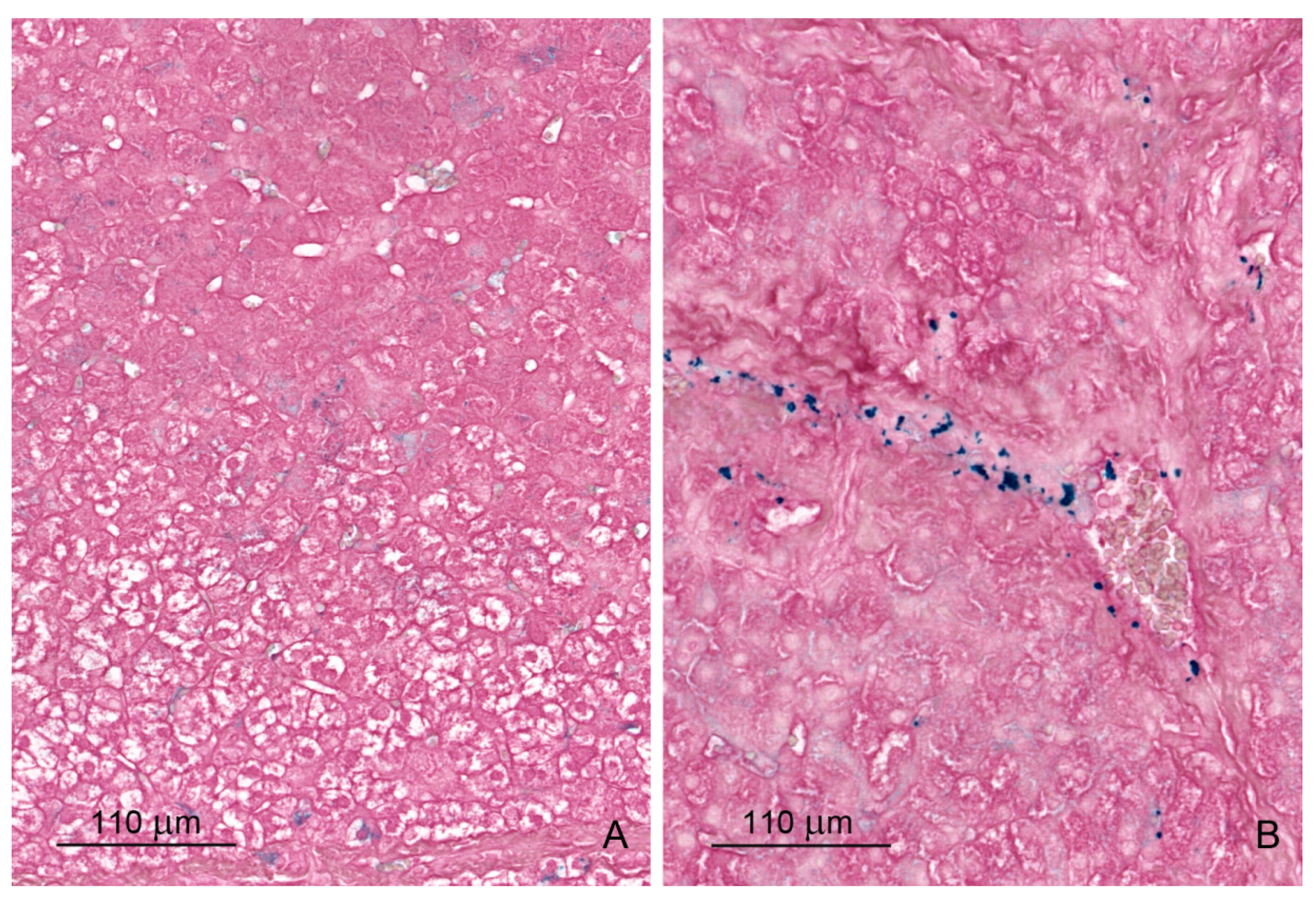
2.1.5. Iron Deposits
2.2. Ultrastructural Study
2.2.1. Hepatocytes
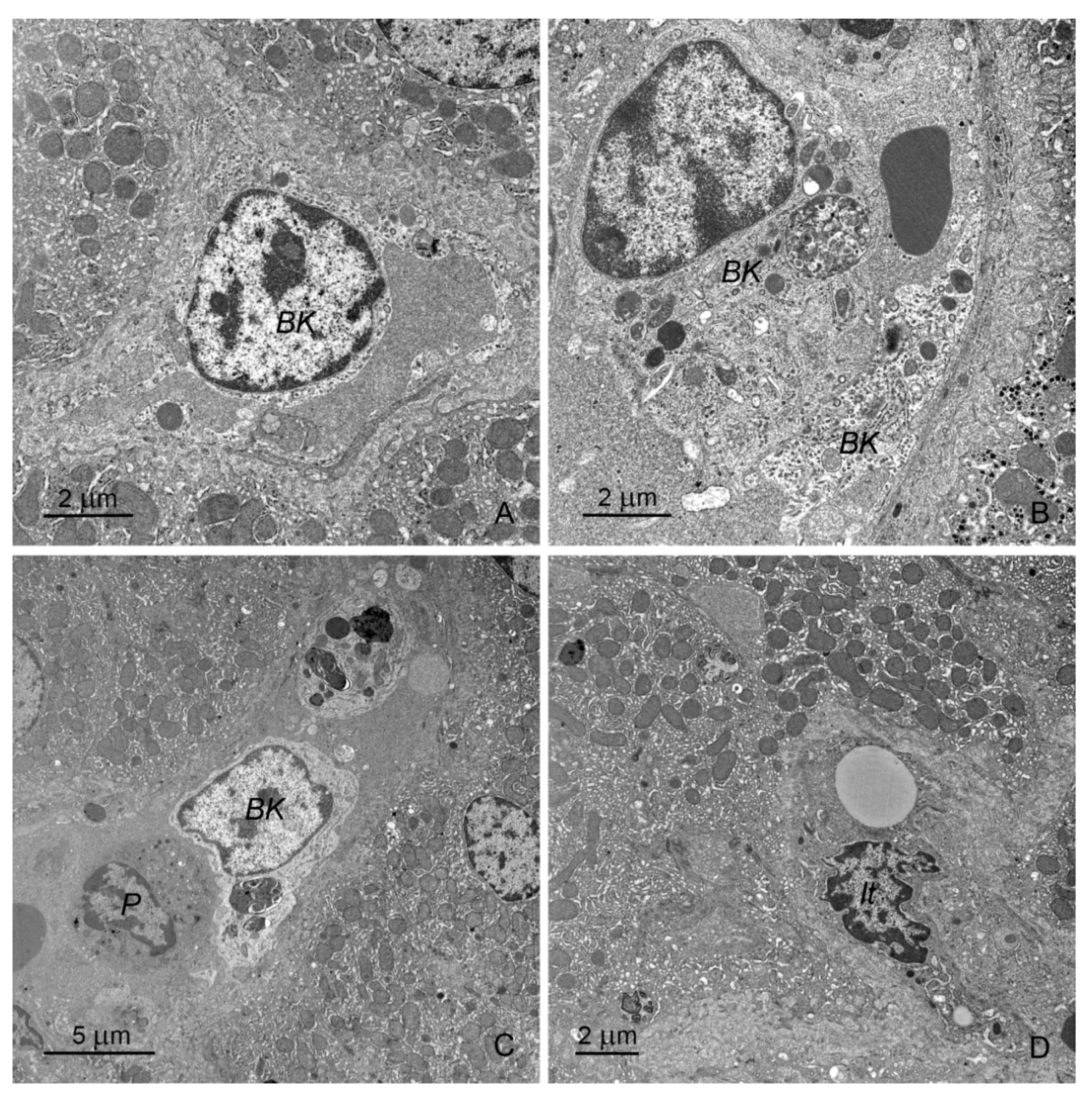
2.2.2. Sinusoids and Perivascular Species
3. Conclusions
4. Material and Methods
4.1. Animals, Toxins, and Experimental Design
4.2. Histological Examination
- The presence of lymphoid follicles, steatosis, hepatocellular dysplasia, karyomegaly;
- The dilatation of hepatic sinusoids using the semi-counted method.
- The cross-sectional area of lobules;
- The thickness of the interlobular connective tissue septa.
4.3. Ultrastructural Examination
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cavret, C.; Lecoeur, S. Fusariotoxin transfer in animals. Food Chem. Toxicol. 2006, 44, 444–453. [Google Scholar] [CrossRef]
- Buszewska-Forajta, M. Mycotoxins, invisible danger of feedstuff with toxic effect on animals. Toxicon 2020, 182, 34–53. [Google Scholar] [CrossRef]
- Nayakwadi, S.; Ramu, R.; Kumar Sharma, A.; Kumar Gupta, V.; Rajukumar, K.; Kumar, V.; Shirahatti, P.S.; Rashmi, L.; Basalingappa, K.M. Toxicopathological studies on the effects of T-2 mycotoxin and their interaction in juvenile goats. PLoS ONE 2020, 15, e229463. [Google Scholar] [CrossRef]
- Koselski, M.; Dziubińska, H.; Trębacz, K.; Sieprawska, A.; Filek, M. The Role of SV Ion Channels under the Stress of Mycotoxins Induced in Wheat Cells—Protective Action of Selenium Ions. J. Plant Growth Regul. 2019, 38, 1255–1259. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Wen, J.; Chen, R.; Deng, Y. Variable protein homeostasis in housekeeping and non-housekeeping pathways under mycotoxins stress. Sci. Rep. 2019, 9, 7819. [Google Scholar] [CrossRef]
- Hallsworth, J.E. Stress-free microbes lack vitality. Fungal Biol. 2018, 122, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, U.; Brüssow, K.P.; Dannenberger, D.; Jonas, L.; Pöhland, R.; Jäger, K.; Dänicke, S.; Hagemann, E. The effect of feeding a diet naturally contaminated with deoxynivalenol (DON) and zearalenone (ZON) on the spleen and liver of sow and fetus from day 35 to 70 of gestation. Toxicol. Lett. 2008, 179, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Z.; Yang, Z.B.; Gao, J.; Liu, F.X.; Broomhead, J.; Chi, F. Effects of purified zearalenone on growth performance, organ size, serum metabolities, and oxidative stress in postweaning gilts. J. Anim. Sci. 2011, 89, 3008–3015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conková, E.; Laciaková, A.; Kovác, A.; Seidel, H. Fusorial toxins and their role in animal disease. Vet. J. 2003, 165, 214–220. [Google Scholar] [CrossRef]
- Alm, H.; Brussow, K.P.; Vanselow, J.; Tomek, W.; Danicke, S.; Tiemann, U. Influence of Fusarium-toxins contaminated feed on initial quality and meiotic competence of gilt oocytes. Reprod. Toxicol. 2006, 22, 44–50. [Google Scholar] [CrossRef]
- Prelusky, D.B.; Hartin, K.E.; Trenholm, H.L.; Miller, J.D. Pharmacokinetic fate of 14C-labeled deoxynivalenol in swine. Fundam. Appl. Toxicol. 1988, 10, 276–286. [Google Scholar] [CrossRef]
- Dänicke, S.; Valenta, S.; Döll, S. On the toxicokinetics and the metabolism of deoxynivalenol (DON) in the pig. Arch. Anim. Nutr. 2004, 58, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Swiech, E.; Buraczewska, L.; Ueberschar, K.H. Kinetics and metabolism of zearalenone in young female pigs. J. Anim. Physiol. Anim. Nutr. 2005, 89, 268–276. [Google Scholar] [CrossRef]
- Pinton, P.; Oswald, I.P. Effects of deoxynivalenol and other Type B trichothecenes on the intestine: A review. Toxins 2014, 6, 1615–1643. [Google Scholar] [CrossRef]
- Gerez, J.R.; Pinton, P.; Callu, P.; Grosjean, F.; Oswald, I.P.; Bracarense, A.P. Deoxynivalenol alone or in combination with nivalenol and zearalenone induce systemic histological changes in pigs. Exp. Tox. Pathol. 2015, 67, 89–98. [Google Scholar] [CrossRef]
- Lewczuk, B.; Przybylska-Gornowicz, B.; Gajęcka, M.; Targońska, K.; Ziółkowska, N.; Prusik, M.; Gajęcki, M. Histological structure of duodenum in gilts receiving low doses of zearalenone and deoxynivalenol in feed. Exp. Toxicol. Pathol. 2016, 68, 157–166. [Google Scholar] [CrossRef]
- Przybylska-Gornowicz, B.; Tarasiuk, M.; Lewczuk, B.; Prusik, M.; Ziółkowska, N.; Zielonka, Ł.; Gajęcki, M.; Gajęcka, M. The effects of low doses of two Fusarium toxins, zearalenone and deoxynivalenol, on the pig jejunum. A light and electron microscopic study. Toxins 2015, 7, 4684–4705. [Google Scholar] [CrossRef] [Green Version]
- Przybylska-Gornowicz, B.; Lewczuk, B.; Prusik, M.; Hanuszewska, M.; Petrusewicz-Kosińska, M.; Gajęcka, M.; Zielonka, Ł.; Gajęcki, M. The effects of Deoxynivalenol and Zearalenone on the pig large intestine. A light and electron microscopy study. Toxins 2018, 10, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doll, S.; Danicke, S. The fusarium toxins deoxynivalenol (DON) and zearalenone (ZON) in animal feeding. Prev. Vet. Med. 2011, 102, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Gajęcki, M. Zearalenone—Ndesirable substances in feed. Pol. J. Vet. Sci. 2002, 5, 117–122. [Google Scholar]
- Malekinejad, H.; Maas-Bakker, R.F.; Fink-Gremmels, J. Bioactivation of zearalenone by porcine hepatic biotransformation. Vet. Res. 2005, 36, 799–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, M.; Kiessling, K.H. Species differences in zearalenone-reducing activity in subcellular fractions of liver from female domestic animals. Acta Pharmacol. Toxicol. 1983, 52, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Miles, C.O.; Alistair, L.W.; Wilkins, A.L.; Neal, R.T.; Barry, L.S.; Ian, G.; Bryan, G.S.; Richard, H.P. Ovine metabolism of zearalenone to alpha-zearalenol (Zeranol). J. Agric. Food Chem. 1996, 44, 3244–3250. [Google Scholar] [CrossRef]
- Malekinejad, H.; Maas-Bakker, R.F.; Fink-Gremmels, J. Species differences in the hepatic biotransformation of zearalenone. Vet. J. 2006, 172, 96–102. [Google Scholar] [CrossRef]
- Dong, M.; Tulayakul, P.; LI, J.Y.; Manabe, N.; Kumagai, S. Metabolic conversion of zearalenone to alpha-zearalenol by goat tissues. J. Vet. Med. Sci. 2010, 72, 307–312. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Song, G.; Lim, W. Effects of endocrine disrupting chemicals in pigs. Environ. Pollut. 2020, 114505. [Google Scholar] [CrossRef]
- Wan, L.Y.M.; Turner, P.C.; El-Nezami, H. Individual and combined cytotoxic effects of Fusarium toxins (deoxynivalenol, nivalenol, zearalenone and fumonisins B1) on swine jejunal epithelium cells. Food Chem. Tox. 2013, 57, 276–283. [Google Scholar] [CrossRef]
- Ji, J.; Cui, F.; Pi, F.; Zhang, Y.; Li, Y.; Wang, J.; Sun, X. The antagonistic effect of mycotoxins deoxynivalenol and zearalenone on metabolic profiling in serum and liver mice. Toxins 2017, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Van Le Thanh, B.; Lemay, M.; Bastien, A.; Lapointe, J.; Lessard, M.; Chorfi, Y.; Guay, F. The potential effects of antioxidant feed additives in mitigating the adverse effects of corn naturally contaminated with fusarium mycotoxins on antioxidant systems in the intestinal mucosa, plasma, and liver in weaned pigs. Mycotoxin Res. 2016, 32, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Alassane-Kpembi, I.; Kolf-Clauw, M.; Gauthier, T.; Abrami, R.; Abiola, F.A.; Oswald, P.; Puel, O. New insights into mycotoxin mixtures: The toxicity of low doses of Type B trichothecenes on intestinal epithelial cells is synergistic. Toxicol. Appl. Pharmacol. 2013, 272, 191–198. [Google Scholar] [CrossRef]
- Reddy, K.E.; Jeong, J.; Lee, Y.; Lee, H.-J.; Kim, M.S.; Kim, D.W.; Jung, H.-J.; Choe, C.; Oh, Y.K.; Lee, S.D. Deoxynivalenol- and zearalnone-contaminated feeds alter gene expression profiles in the livers of piglets. Asian-Australas. J. Anim. Sci. 2018, 31, 595–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zollner, P.; Jodlauber, J.; Kleinova, M.; Kahlbacher, H.; Kuhn, T.; Hochsteiner, W.; Lindner, W. Concentrations levels of zearalenone and its metabolities in urine, muscle tissue, and liver samples of pigs fed with mycotoxins-contaminated oats. J. Agric. Food Chem. 2002, 50, 2494–2501. [Google Scholar] [CrossRef]
- Mikami, O.; Yamaguchi, H.; Murata, H.; Nakajima, Y.; Miyazaki, S. Induction of apoptotic lesions in liver and lymphoid tissues and modulation of cytokine mRNA expression by acute exposure to deoxynivalenol in piglets. J. Vet. Sci. 2010, 12, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Renner, L.; Kahlert, S.; Tesch, T.; Bannert, E.; Frahm, J.; Barta-Böszörményi, A.; Kluess, J.; Kersten, S.; Schönfeld, P.; Rothkötter, H.J.; et al. Chronic DON exposure and acute LPS challenge: Effects on porcine liver morphology and function. Mycotoxin Res. 2017, 33, 207–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Povero, D.; Busletta, C.; Novo, E.; di Bonzo, L.V.; Cannito, S.; Paternostro, C.; Parola, M. Liver fibrosis: A dynamic and potentially reversible process. Histol. Histopathol. 2010, 25, 1075–1091. [Google Scholar] [CrossRef] [PubMed]
- Greaves, P. Histopathology of Preclinical Toxicity Studies: Interpretation and Relevance in Drug Safety Evaluation, 4th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; pp. 433–479. [Google Scholar]
- Weber, S.N.; Wasmuth, H.E. Liver fibrosis: From animal models to mapping of human risk variants. Best Pract. Res. Clin. Gastrooenterol. 2010, 24, 635–646. [Google Scholar] [CrossRef]
- Stanek, C.; Reinhardt, N.; Diesing, A.-K.; Nossol, C.; Kahlert, S.; Panther, P.; Kluess, J.; Rothkotter, H.-J.; Kuester, D.; Brosig, B.; et al. A chronic oral exposure of pigs with deoxynivalenol partially prevents the acute effects of lipopolisaccharides on hepatic histopathology and blood clinical chemistry. Toxicol. Res. 2012, 215, 193–200. [Google Scholar] [CrossRef]
- Friedman, S.L.; McQuaid, K.R.; Grendell, J.H. Current Diagnosis & Treatment in Gastroenterology, 2nd ed.; Lang Medical Books/McGraw-Hill: New York, NY, USA, 2003; pp. 664–679. [Google Scholar]
- Mills, S.E. Histology for Pathologists, 3rd ed.; W.B. Saunders Company: Sydney, Australia, 2007; pp. 685–703. [Google Scholar]
- Foster, J.R. Spontaneous and drug-induced hepatic pathology of the laboratory beagle dog, the cynomolgus macaque and the marmoset. Toxicol. Pathol. 2005, 33, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Van Amersfoort, E.S.; Van Berkel, T.J.C.; Kuiper, J. Receptors, Mediators, and Mechanisms Involved in Bacterial Sepsis and Septic Shock. Clin. Microbiol. Rev. 2003, 16, 379–414. [Google Scholar] [CrossRef] [Green Version]
- Coulombe, R.A., Jr. Pyrrolizidine alkaloids in foods. Adv. Food Nutr. Res. 2003, 45, 61–99. [Google Scholar] [CrossRef]
- Winkler, K.; Poulsen, H. Liver disease with periportal sinusoidal dilatation. A possible complication to contraceptive steroids. Scand. J. Gastroenterol. 1975, 10, 699–704. [Google Scholar]
- Ribero, D.; Wang, H.; Donadon, M.; Zorzi, D.; Thomas, M.B.; Eng, C.; Chang, D.Z.; Curley, S.A.; Abdalla, E.K.; Ellis, L.M.; et al. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer 2007, 110, 2761–2767. [Google Scholar] [CrossRef] [Green Version]
- Halliday, J.W.; Searle, J. Hepatic iron deposition in human disease and animal models. Biometals 1996, 9, 205–209. [Google Scholar] [CrossRef]
- Graham, R.M.; Chua, A.C.; Herbison, C.E.; Olynyk, J.K.; Trinder, D. Liver iron transport. World J. Gastroenterol. 2007, 13, 4725–4736. [Google Scholar] [CrossRef]
- Deugnier, Y.; Turlin, B. Pathology of hepatic iron overload. World J. Gastroenterol. 2007, 13, 475–4760. [Google Scholar] [CrossRef]
- Pietrangelo, A. Mechanisms of iron hepatotoxicity. J. Hepatol. 2016, 65, 226–227. [Google Scholar] [CrossRef] [PubMed]
- Yiannikourides, A.; Latunde-Dada, G.O. A Short Review of Iron Metabolism and Pathophysiology of Iron Disorders. Medicines 2019, 6, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheville, N.F. Ultrastructural pathology. In The Comparative Cellular Basis of Diseases, 2nd ed.; Willey-Blackwell: Hoboken, NJ, USA, 2009; pp. 1–1000. [Google Scholar]
- Hutterer, F.; Schaffner, F.; Klion, F.M.; Popper, H. Hypertrophic, Hypoactive Smooth Endoplasmic Reticulum: A Sensitive Indicator of Hepatotoxicity Exemplified by Dieldrin. Science 1968, 161, 1017–1019. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Yung, H.W. Endoplasmic reticulum stress in the pathogenesis of early-onset pre-eclampsia. Pregnancy Hypertens. 2011, 1, 72–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.Y.; Rho, H.S.; Kim, A.; Kim, T.H.; Jang, K.; Jun, D.W.; Kim, J.W.; Kim, B.; Kim, S.G. FXR inhibits endoplasmic reticulum stress-induced NLRP3 inflammasome in hepatocytes and ameliorates liver injury. Cell Rep. 2018, 24, 2985–2999. [Google Scholar] [CrossRef] [Green Version]
- Crawford, J.M.; Bioulac-Sage, P.; Hytiroglou, P. Structure, Function, and Responses to Injury. In Macsween’s Pathology of the Liver, 7th ed.; Burt, A.D., Ferrell, L.D., Hübscher, S.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–87. [Google Scholar] [CrossRef]
- Hasegawa, D.; Wallace, M.C.; Friedman, S.C. Chapter 4—Stellate Cells and Hepatic Fibrosis. In Stellate Cells in Health and Disease; Gandhi, C.R., Pinzani, M., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 41–62. [Google Scholar] [CrossRef]
- Peng, H.; Wisse, E.; Tian, Z. Liver natural killer cells: Subsets and roles in liver immunity. Cell Mol. Immunol. 2016, 13, 328–336. [Google Scholar] [CrossRef] [Green Version]
- Gajęcka, M.; Tarasiuk, M.; Zielonka, Ł.; Dąbrowski, M.; Gajęcki, M. Risk assessment for changes in the metabolic profile and body weights of pre-pubertal gilts during long-term monotonic exposure to low doses of zearalenone (ZEN). Res. Vet. Sci. 2016, 109, 169–180. [Google Scholar] [CrossRef]
- Nicpoń, J.; Sławuta, P.; Nicpoń, J. Effect of zearalenone toxicosis on the complete blood cell count and serum biochemical analysis in wild boars. Vet. Med. 2016, 72, 250–254. [Google Scholar]
- Holanda, D.M.; Kim, S.W. Efficacy of Mycotoxin Detoxifiers on Health and Growth of Newly-Weaned Pigs under Chronic Dietary Challenge of Deoxynivalenol. Toxins 2020, 12, 311. [Google Scholar] [CrossRef] [PubMed]
- Ishak, K.; Baptista, A.; Bianchi, L.; Callea, F.; De Groote, J.; Gudat, F.; Denk, H.; Desmet, V.; Korb, G.; MacSween, R.N.M.; et al. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995, 22, 696–699. [Google Scholar] [CrossRef]




| Duration of Mycotoxin Treatment | Group | Testing Results p < 0.05 | |||
|---|---|---|---|---|---|
| Control (C) | ZEN (Z) | DON (D) | DON + ZEN (M) | ||
| 1 week | 0 | 0 | 1.0 | 2.67 | C, Z < D; D < M |
| 3 weeks | 0 | 0 | 1.0 | 2.3 | C, Z < D; D < M |
| 6 weeks | 0 | 0 | 1.33 | 3.0 | C, Z < D; D < M |
| Duration of Mycotoxin Treatment | Group | Testing Results p < 0.05 | |||
|---|---|---|---|---|---|
| Control (C) | ZEN (Z) | DON (D) | DON + ZEN (M) | ||
| 1 week | 0 | 3.00 | 3.67 | 3.33 | C < Z, D, M |
| 3 weeks | 0.66 | 2.33 | 3.33 | 3.67 | C < Z, D, M; Z < M |
| 6 weeks | 0.33 | 2.0 | 2.33 | 3.33 | C < Z, D, M; Z, D < M |
| Time of Mycotoxin Treatment | Groups | Testing Results | |||
|---|---|---|---|---|---|
| Control (C) | ZEN (Z) | DON (D) | DON + ZEN (M) | ||
| Central part | |||||
| 1 week | 0 | 3.00 | 2.33 | 2.33 | C < Z, D, M |
| 3 weeks | 2.66 | 3.33 | 2.33 | 3.00 | − |
| 6 weeks | 3.00 | 2.66 | 3.00 | 3.33 | − |
| Peripheral part | |||||
| 1 week | 0 | 4.00 | 4.00 | 3.33 | C < Z, D, M |
| 3 weeks | 3.66 | 4.00 | 4.00 | 4.00 | - |
| 6 weeks | 4.00 | 3.66 | 4.00 | 444 | - |
| Time of Mycotoxin Treatment | Groups | Testing Results | |||
|---|---|---|---|---|---|
| Control (C) | ZEN (Z) | DON (D) | DON + ZEN (M) | ||
| One week | 1.00 | 1.00 | 1.00 | 1.00 | - |
| Three weeks | 1.00 | 1.66 | 1.00 | 2.00 | C, D < Z, M |
| Six weeks | 1.33 | 3.33 | 1.33 | 3.33 | C, D < Z, M |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skiepko, N.; Przybylska-Gornowicz, B.; Gajęcka, M.; Gajęcki, M.; Lewczuk, B. Effects of Deoxynivalenol and Zearalenone on the Histology and Ultrastructure of Pig Liver. Toxins 2020, 12, 463. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12070463
Skiepko N, Przybylska-Gornowicz B, Gajęcka M, Gajęcki M, Lewczuk B. Effects of Deoxynivalenol and Zearalenone on the Histology and Ultrastructure of Pig Liver. Toxins. 2020; 12(7):463. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12070463
Chicago/Turabian StyleSkiepko, Natalia, Barbara Przybylska-Gornowicz, Magdalena Gajęcka, Maciej Gajęcki, and Bogdan Lewczuk. 2020. "Effects of Deoxynivalenol and Zearalenone on the Histology and Ultrastructure of Pig Liver" Toxins 12, no. 7: 463. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12070463






