A New Laccase of Lac 2 from the White Rot Fungus Cerrena unicolor 6884 and Lac 2-Mediated Degradation of Aflatoxin B1
Abstract
:1. Introduction
2. Results
2.1. Species Identification
2.2. Preparation and Purification
2.3. Laccase Identification
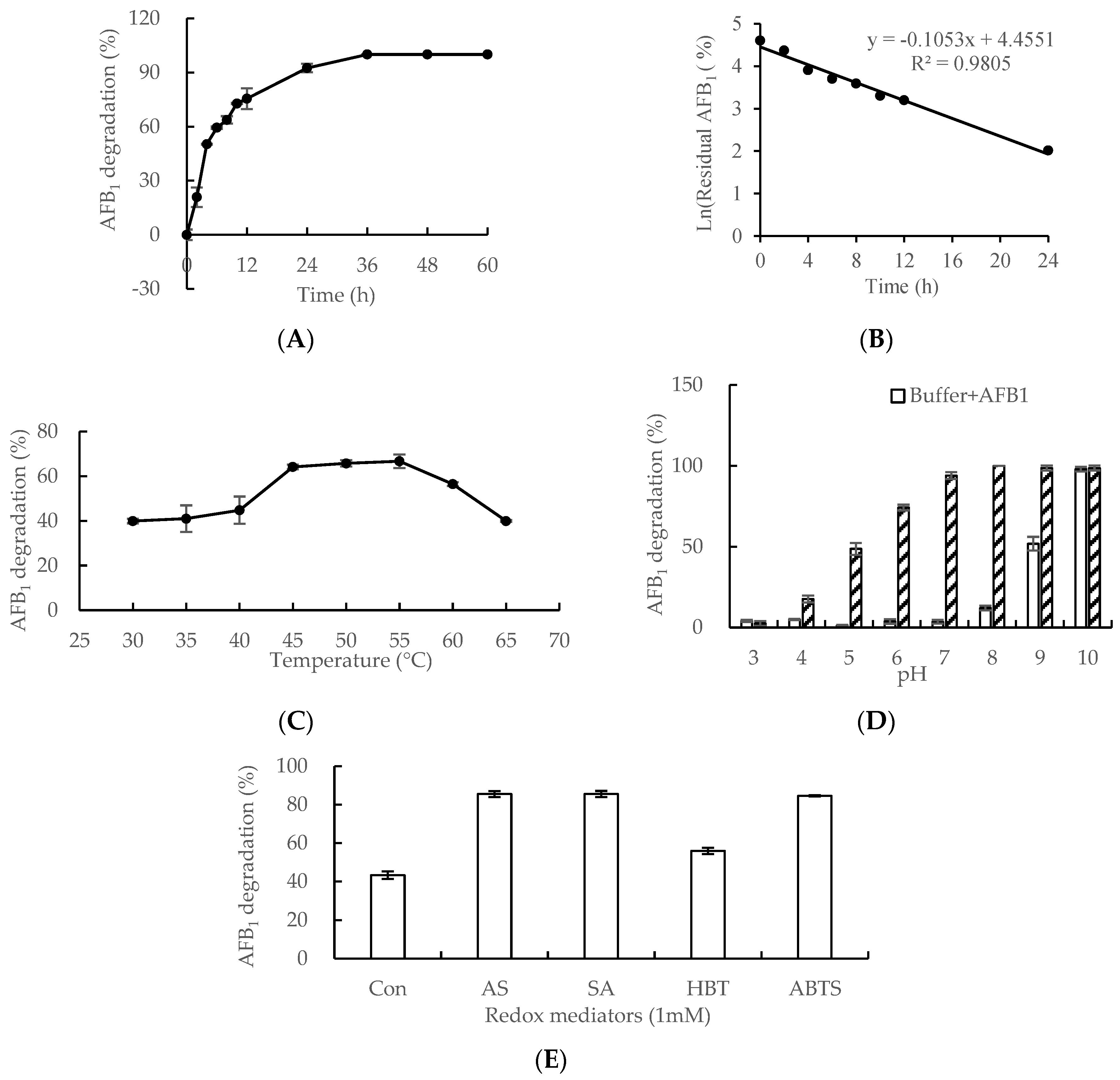
2.4. AFB1 Degradation by Lac 2 from Cerrena Unicolor 6884
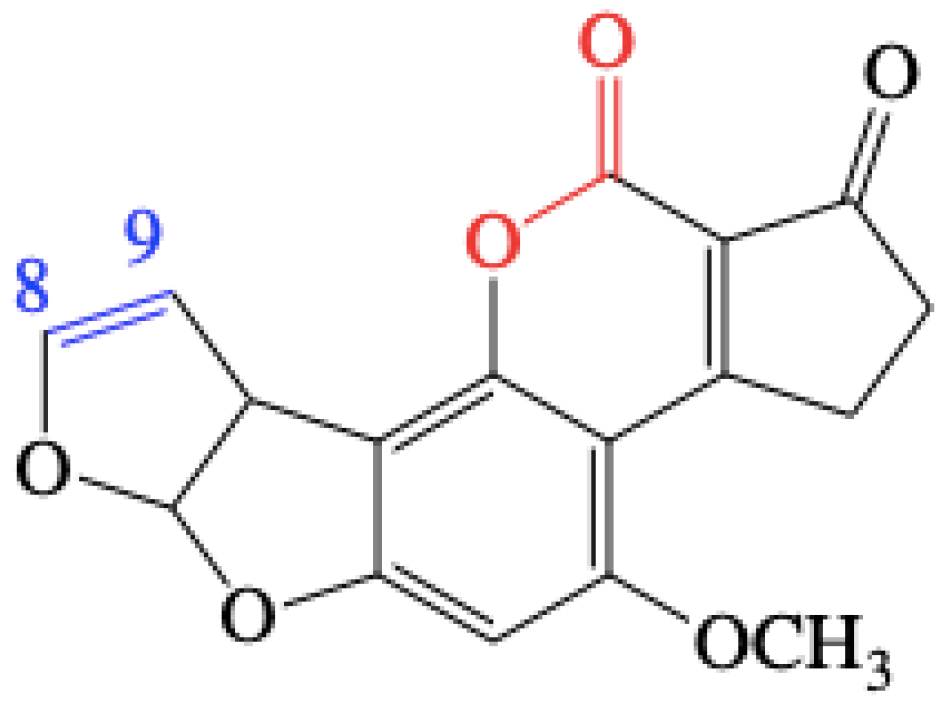
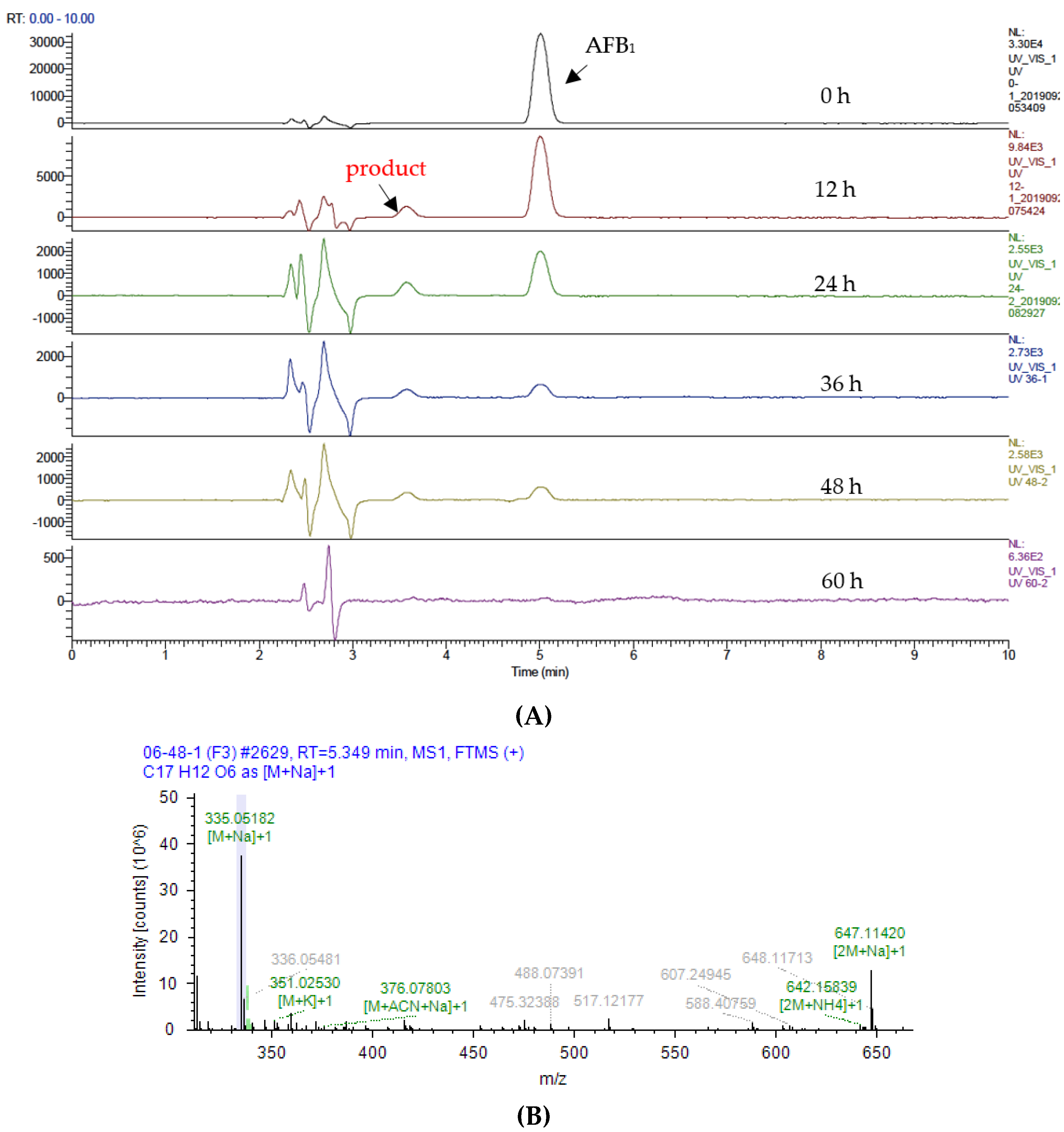
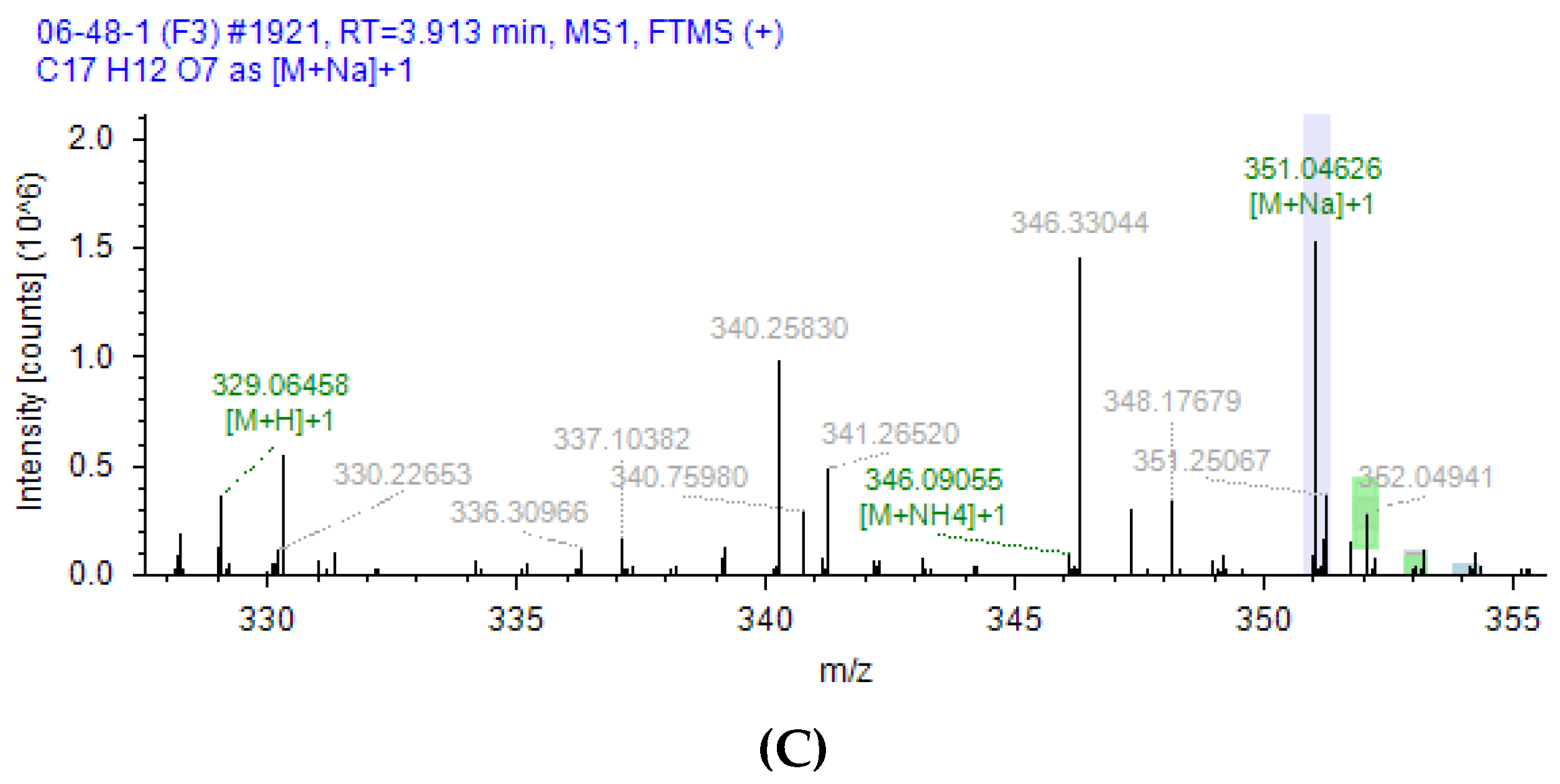
2.5. Analysis of Products of AFB1 Degradation Catalyzed by Lac 2
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Organism, Chemicals and Other Materials
5.2. Phylogenetic Analysis
5.3. Illumina Transcriptome Analysis of Cerrena Unicolor 6884
5.4. Production of Laccase
5.5. Enzyme Assay
5.6. Purification of Laccase
5.7. Identification of Laccase by UHPLC-MS/MS
5.8. Cloning of the Laccase cDNA from Cerrena Unicolor 6884
5.9. In Vitro Degradation of AFB1 with Lac 2
5.10. AFB1 Assay
5.11. UPLC-MS/MS Analysis of AFB1 Degradation Products
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABTS | [2,2′ -azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid)] |
| ACN | Acetonitrile |
| AFB1 | aflatoxin B1 |
| AFG1 | aflatoxin G1 |
| AFM1 | aflatoxin M1 |
| AFQ1 | aflatoxin Q1 |
| AS | Acetosyringone |
| DAD | Diode array detector |
| DEAE | diethylamino ethyl |
| FLD | Fluorescent detector |
| HBT | Hydroxy benzotriazole |
| Lac 2 | laccase 2 |
| LMS | laccase mediator system |
| SA | Syrinagaldehyde |
References
- Loi, M.; Fanelli, F.; Liuzzi, V.C.; Logrieco, A.; Mulè, G. Mycotoxin Biotransformation by Native and Commercial Enzymes: Present and Future Perspectives. Toxins 2017, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, T.; Zweifel, U.; Sagelsdorff, P.; Friederich, U.; Luethy, J.; Schlatter, C. Ammoniation of aflatoxin-containing corn: Distribution, in vivo covalent deoxyribonucleic acid binding, and mutagenicity of reaction products. J. Agric. Food Chem. 1985, 33, 311–316. [Google Scholar] [CrossRef]
- Hussein, H. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology 2001, 167, 101–134. [Google Scholar] [CrossRef]
- Strosnider, H.; Azziz-Baumgartner, E.; Banziger, M.; Bhat, R.V.; Breiman, R.; Brune, M.-N.; Decock, K.; Dilley, A.; Groopman, J.; Hell, K.; et al. Workgroup Report: Public Health Strategies for Reducing Aflatoxin Exposure in Developing Countries. Environ. Health Perspect. 2006, 114, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; He, B.; Zhang, L.; Li, P.; Zhang, Q.; Ding, X.; Zhang, W. A Structure Identification and Toxicity Assessment of the Degradation Products of Aflatoxin B1 in Peanut Oil under UV Irradiation. Toxins 2016, 8, 332. [Google Scholar] [CrossRef]
- Abrar, M.; Anjum, F.M.; Butt, M.S.; Pasha, I.; Randhawa, M.A.; Saeed, F.; Waqas, K. Aflatoxins: Biosynthesis, Occurrence, Toxicity, and Remedies. Crit. Rev. Food Sci. Nutr. 2013, 53, 862–874. [Google Scholar] [CrossRef]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar] [CrossRef]
- Luo, X.; Wang, R.; Wang, L.; Li, Y.; Bian, Y.; Chen, Z. Effect of ozone treatment on aflatoxin B1 and safety evaluation of ozonized corn. Food Control. 2014, 37, 171–176. [Google Scholar] [CrossRef]
- Ji, N.; Diao, E.; Li, X.; Zhang, Z.; Dong, H. Detoxification and safety evaluation of aflatoxin B1 in peanut oil using alkali refining. J. Sci. Food Agric. 2016, 96, 4009–4014. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, C.; Zhang, D.; Zhao, M.; Zheng, D.; Lyu, Y.; Cheng, W.; Guo, P.; Cui, Z. Effective degradation of aflatoxin B1 using a novel thermophilic microbial consortium TADC7. Bioresour. Technol. 2017, 224, 166–173. [Google Scholar] [CrossRef]
- Wang, J.; Ogata, M.; Hirai, H.; Kawagishi, H. Detoxification of aflatoxin B1 by manganese peroxidase from the white-rot fungus Phanerochaete sordida YK-624. FEMS Microbiol. Lett. 2010, 314, 164–169. [Google Scholar] [CrossRef]
- Jard, G.; Liboz, T.; Mathieu, F.; Guyonvarc’H, A.; Lebrihi, A. Review of mycotoxin reduction in food and feed: From prevention in the field to detoxification by adsorption or transformation. Food Addit. Contam. Part A 2011, 28, 1590–1609. [Google Scholar] [CrossRef] [PubMed]
- Adebo, O.A.; Njobeh, P.B.; Gbashi, S.; Nwinyi, O.C.; Mavumengwana, V. Review on microbial degradation of aflatoxins. Crit. Rev. Food Sci. Nutr. 2015, 57, 3208–3217. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yao, D.; Liang, R.; Ma, L.; Cheng, W.; Gu, L. Detoxfication of aflatoxin B1 by Enzymes Isolated from Armillariella tabescens. Food Chem. Toxicol. 1998, 36, 563–574. [Google Scholar] [CrossRef]
- Das, C.; Mishra, H. In vitro Degradation of Aflatoxin B1 in Groundnut (Arachis hypogea) Meal by Horse Radish Peroxidase. LWT Food Sci. Technol. 2000, 33, 308–312. [Google Scholar] [CrossRef]
- Taylor, M.C.; Jackson, C.J.; Tattersall, D.B.; French, N.; Peat, T.S.; Newman, J.; Briggs, L.J.; Lapalikar, G.V.; Campbell, P.M.; Scott, C.; et al. Identification and characterization of two families of F420H2-dependent reductases from Mycobacteria that catalyse aflatoxin degradation. Mol. Microbiol. 2010, 78, 561–575. [Google Scholar] [CrossRef] [Green Version]
- Yehia, R.S. Aflatoxin detoxification by manganese peroxidase purified from Pleurotus ostreatus. Braz. J. Microbiol. 2014, 45, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.H.; Guan, S.; Gao, X.; Ma, Q.G.; Lei, Y.P.; Bai, X.M.; Ji, C. Preparation, purification and characteristics of an aflatoxin degradation enzyme from Myxococcus fulvus ANSM068. J. Appl. Microbiol. 2010, 110, 147–155. [Google Scholar] [CrossRef]
- Xu, L.; Eisa Ahmed, M.F.; Sangare, L.; Liu, Y.; Selvaraj, J.N.; Xing, F.; Wang, Y.; Yang, H.; Liu, Y. Novel Aflatoxin-Degrading Enzyme from Bacillus shackletonii L7. Toxins 2017, 9, 36. [Google Scholar] [CrossRef]
- Wang, X.; Bai, Y.; Huang, H.; Tu, T.; Wang, Y.; Wang, Y.; Luo, H.; Yao, B.; Su, X. Degradation of aflatoxin B1 and zearalenone by bacterial and fungal laccases in presence of structurally defined chemicals and complex natural mediators. Toxins 2019, 11, 609. [Google Scholar] [CrossRef] [Green Version]
- Alberts, J.; Gelderblom, W.; Botha, A.; van Zyl, W. Degradation of aflatoxin B1 by fungal laccase enzymes. Int. J. Food Microbiol. 2009, 135, 47–52. [Google Scholar] [CrossRef]
- Loi, M.; Fanelli, F.; Zucca, P.; Liuzzi, V.C.; Quintieri, L.; Cimmarusti, M.T.; Monaci, L.; Haidukowski, E.M.; Logrieco, A.F.; Sanjust, E.; et al. Aflatoxin B1 and M1 Degradation by Lac2 from Pleurotus pulmonarius and Redox Mediators. Toxins 2016, 8, 245. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Liu, D.; Mo, X.; Xie, C.; Yao, D. A fungal enzyme with the ability of aflatoxin B1 conversion: Purification and ESI-MS/MS identification. Microbiol. Res. 2011, 166, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-Z.; Lu, F.-P.; Jiang, H.-L.; Tan, C.-P.; Yao, D.-S.; Xie, C.-F.; Liu, D.-L.; Chu, M.-Q. The furofuran-ring selectivity, hydrogen peroxide-production and low Km value are the three elements for highly effective detoxification of aflatoxin oxidase. Food Chem. Toxicol. 2015, 76, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Giardina, P.; Faraco, V.; Pezzella, C.; Piscitelli, A.; Vanhulle, S.; Sannia, G. Laccases: A never-ending story. Cell. Mol. Life Sci. 2010, 67, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Zeinvand-Lorestani, H.; Sabzevari, O.; Setayesh, N.; Amini, M.; Nili-Ahmadabadi, A.; Faramarzi, M.A. Comparative study of in vitro prooxidative properties and genotoxicity induced by aflatoxin B1 and its laccase-mediated detoxification products. Chemosphere 2015, 135, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dellafiora, L.; Galaverna, G.; Reverberi, M.; Dall’Asta, C. Degradation of Aflatoxins by Means of Laccases from Trametes versicolor: An in Silico Insight. Toxins 2017, 9, 17. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Mao, H.; Hu, C.; Tron, T.; Lin, J.; Wang, J.; Sun, B. Molecular docking studies and in vitro degradation of four aflatoxins (AFB1, AFB2, AFG1, and AFG2) by a recombinant laccase from Saccharomyces cerevisiae. J. Food Sci. 2020, 85, 1353–1360. [Google Scholar] [CrossRef]
- Loi, M.; Fanelli, F.; Cimmarusti, M.T.; Mirabelli, V.; Haidukowski, E.M.; Logrieco, A.F.; Caliandro, R.; Mulè, G. In vitro single and combined mycotoxins degradation by Ery4 laccase from Pleurotus eryngii and redox mediators. Food Control. 2018, 90, 401–406. [Google Scholar] [CrossRef]
- Guo, Y.; Qin, X.; Tang, Y.; Ma, Q.; Zhang, J.; Zhao, L. CotA laccase, a novel aflatoxin oxidase from Bacillus licheniformis, transforms aflatoxin B1 to aflatoxin Q1 and epi-aflatoxin Q1. Food Chem. 2020, 325, 126877. [Google Scholar] [CrossRef]
- Yang, J.; Lin, Q.; Ng, T.B.; Ye, X.; Lin, J. Purification and Characterization of a Novel Laccase from Cerrena sp. HYB07 with Dye Decolorizing Ability. PLoS ONE 2014, 9, e110834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogalski, J.; Janusz, G. Purification of Extracellular Laccase from Cerrena unicolor. Prep. Biochem. Biotechnol. 2010, 40, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Bekker, E.G.; Petrova, S.D.; Ermolova, O.V.; Elisashvili, V.I.; Sinitsyn, A.P. Extraction, purification and some properties of laccase from Cerrena unicolor. Biokhimiya 1990, 55, 2019–2024. [Google Scholar]
- Michniewicz, A.; Ullrich, R.; Ledakowicz, S.; Hofrichter, M. The white-rot fungus Cerrena unicolor strain 137 produces two laccase isoforms with different physico-chemical and catalytic properties. Appl. Microbiol. Biotechnol. 2006, 69, 682–688. [Google Scholar] [CrossRef]
- Janusz, G.; Mazur, A.; Checinska Sielaff, A.; Małek, W.; Rogalski, J.; Ohga, S. Cloning and characterization of a laccase gene from biotechnologically important basidiomycete Cerrena Unicolor. J. Fac. Agric. Kyushu Univ. 2012, 57, 41–49. [Google Scholar]
- Kachlishvili, E.; Metreveli, E.; Elisashvili, V. Modulation of Cerrena unicolor laccase and manganese peroxidase production. SpringerPlus 2014, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pawlik, A.; Mazur, A.; Wielbo, J.; Koper, P.; Żebracki, K.; Kubik-Komar, A.; Janusz, G. RNA Sequencing Reveals Differential Gene Expression of Cerrena Unicolor in Response to Variable Lighting Conditions. Int. J. Mol. Sci. 2019, 20, 290. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Ng, T.B.; Lin, J.; Ye, X. A novel laccase from basidiomycete Cerrena sp.: Cloning, heterologous expression, and characterization. Int. J. Biol. Macromol. 2015, 77, 344–349. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, L.; Zhang, H.; Wang, S.; Zhang, X.; Geng, A. A novel homodimer laccase from Cerrena unicolor BBP6: Purification, characterization, and potential in dye decolorization and denim bleaching. PLoS ONE 2018, 13, e0202440. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-C.; Wu, P.-H.; Su, Y.-C.; Wen, T.-N.; Wei, Y.-S.; Wang, N.-C.; Hsu, C.-A.; Wang, A.H.-J.; Shyur, L.-F. Biochemical characterization of a novel laccase from the basidiomycete fungus Cerrena sp. WR1. Protein Eng. Des. Sel. 2012, 25, 761–769. [Google Scholar] [CrossRef]
- Wang, S.-S.; Ning, Y.-J.; Wang, S.-N.; Zhang, J.; Zhang, X.; Chen, Q.-J. Purification, characterization, and cloning of an extracellular laccase with potent dye decolorizing ability from white rot fungus Cerrena unicolor GSM-01. Int. J. Biol. Macromol. 2017, 95, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhang, G.; Meng, L.; Wang, H.; Gao, K.; Ng, T.B. Purification and Characterization of a White Laccase with Pronounced Dye Decolorizing Ability and HIV-1 Reverse Transcriptase Inhibitory Activity from Lepista nuda. Molecules 2016, 21, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolás-Vázquez, M.I.; Méndez-Albores, A.; Moreno-Martínez, E.; Miranda, R.; Castro, M. Role of Lactone Ring in Structural, Electronic, and Reactivity Properties of Aflatoxin B1: A Theoretical Study. Arch. Environ. Contam. Toxicol. 2010, 59, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Raters, M.; Matissek, R. Thermal stability of aflatoxin B1 and ochratoxin A. Mycotoxin Res. 2008, 24, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.P.; Marth, E.H. Aflatoxin is degraded at different temperatures and pH values by mycella of Aspergillus parasiticus. Eur. J. Appl. Microbiol. Biotechnol. 1978, 6, 95–100. [Google Scholar] [CrossRef]
- Kiermeier, F.; Ruffer, L. Veränderung von Aflatoxin B1 in Alkalischer Lösung. Z. Lebensm. Unters. Forch. 1974, 155, 129–141. [Google Scholar] [CrossRef]
- Baiocco, P.; Barreca, A.M.; Fabbrini, M.; Galli, C.; Gentili, P. Promoting laccase activity towards non-phenolic substrates: A mechanistic investigation with some laccase-mediator systems. Org. Biomol. Chem. 2003, 1, 191–197. [Google Scholar] [CrossRef]
- Loi, M.; Renaud, J.B.; Rosini, E.; Pollegioni, L.; Vignali, E.; Haidukowski, M.; Sumarah, M.W.; Logrieco, A.F.; Mulè, G. Enzymatic transformation of aflatoxin B1 by Rh_DypB peroxidase and characterization of the reaction products. Chemosphere 2020, 250, 126296. [Google Scholar] [CrossRef]
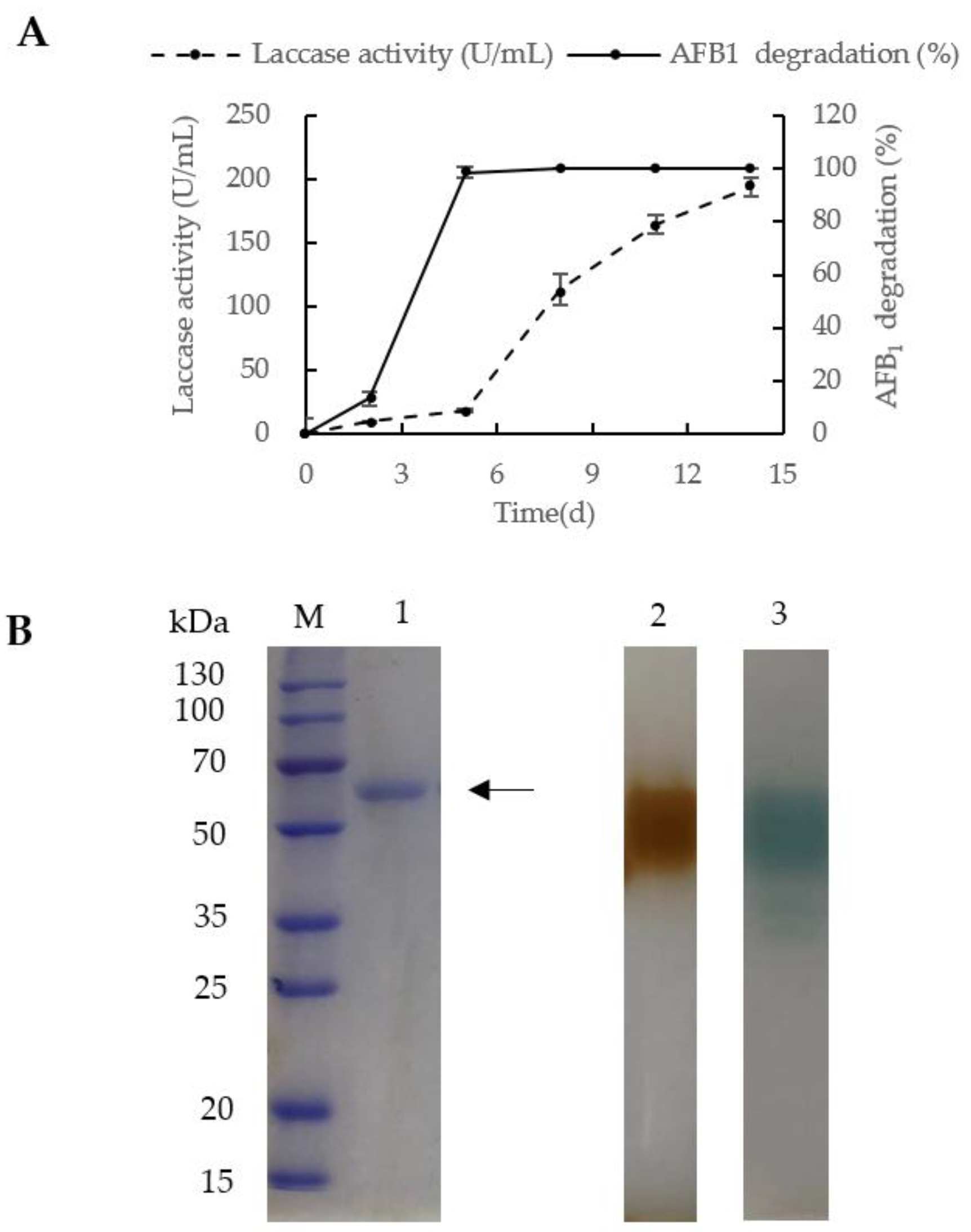
| Purification Step | Total Activity (U) | Total Protein (mg) | Specific Activity (U/mg) | Purification Fold | Recovery (%) |
|---|---|---|---|---|---|
| Crude extract | 44,641.67 | 655.86 | 68.07 | 1.00 | 100 |
| NH4(SO4)2 | 33,653.33 | 163.49 | 205.84 | 3.02 | 75.39 |
| DEAE cellulose | 6592.00 | 4.01 | 1643.70 | 24.15 | 14.77 |
| Assigned Protein | Coverage [%] | Peptides | Sequence | Confidence | Theo. MH+ [Da] |
|---|---|---|---|---|---|
| Lac 2 from Cerrena. unicolor 6884 | 48 | 13 | VVELVIPPLAVGGPHPFHLHGHNFWVVR | High | 3123.71556 |
| TVGGPAQSPLNEADLRPLVPAPVPGNAVPGGADINHR | 3653.91467 | ||||
| SQTGPADAELAVISVEHNKR | 2122.08872 | ||||
| SQTGPADAELAVISVEHNK | 1965.98761 | ||||
| SAGSDEYNFDDAILRDVVSIGAGTDEVTIR | 3185.52331 | ||||
| SAGSDEYNFDDAILR | 1672.74492 | ||||
| NASVEEPK | 873.43124 | ||||
| NAAILR | 657.40423 | ||||
| MLTPTSIHWHGFFQK | 1845.91049 | ||||
| YSFVLNANQPDDNYWIR | 2114.99303 | ||||
| MLTPTSIHWHGFFQK | 1829.91557 | ||||
| GAFVVYDPNDPHK | 1458.70120 | ||||
| DVVSIGAGTDEVTIR | 1531.79623 | ||||
| DLYDVDDESTVITLADWYHVLAQTVVGAATPDSTLINGLGR | 4404.18816 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Li, R.; Ng, T.B.; Lai, Y.; Yang, J.; Ye, X. A New Laccase of Lac 2 from the White Rot Fungus Cerrena unicolor 6884 and Lac 2-Mediated Degradation of Aflatoxin B1. Toxins 2020, 12, 476. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12080476
Zhou Z, Li R, Ng TB, Lai Y, Yang J, Ye X. A New Laccase of Lac 2 from the White Rot Fungus Cerrena unicolor 6884 and Lac 2-Mediated Degradation of Aflatoxin B1. Toxins. 2020; 12(8):476. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12080476
Chicago/Turabian StyleZhou, Zhimin, Renkuan Li, Tzi Bun Ng, Yunyun Lai, Jie Yang, and Xiuyun Ye. 2020. "A New Laccase of Lac 2 from the White Rot Fungus Cerrena unicolor 6884 and Lac 2-Mediated Degradation of Aflatoxin B1" Toxins 12, no. 8: 476. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12080476




