Investigating Useful Properties of Four Streptomyces Strains Active against Fusarium graminearum Growth and Deoxynivalenol Production on Wheat Grains by qPCR
Abstract
:1. Introduction
2. Results
2.1. Streptomyces Influence on Fungal Growth
2.2. Fitness of the Streptomyces Strains on Wheat Grains
2.3. Influence of the BCA Treatment on Mycotoxin Production on Detached Wheat Grains
3. Discussion
4. Materials and Methods
4.1. Microorganisms
4.2. BCA treatments on Wheat Grains
4.3. Chemicals
4.4. Ergosterol Extraction and Determination
4.5. DON Extraction and Determination
4.6. DNA Extraction
4.7. Primers and PCR Analysis
4.8. qPCR Analysis
4.9. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Osborne, L.E.; Stein, J.M. Epidemiology of Fusarium Head Blight on small-grain cereals. Int. J. Food Microbiol. 2007, 103–108. [Google Scholar] [CrossRef]
- Pasquali, M.; Beyer, M.; Logrieco, A.; Audenaert, K.; Balmas, V.; Basler, R.; Boutigny, A.L.; Chrpová, J.; Czembor, E.; Gagkaeva, T.; et al. A European database of Fusarium graminearum and F. culmorum trichothecene genotypes. Front. Mic robiol. 2016, 7, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salgado, J.D.; Madden, L.V.; Paul, P.A. Quantifying the effects of Fusarium Head Blight on grain yield and test weight in soft red winter wheat. Phytopathology 2015, 105, 295–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beattie, S.; Schwarz, P.B.; Horsley, R.; Barr, J.; Casper, H.H. The effect of grain storage conditions on the viability of Fusarium and deoxynivalenol production in infested malting barley. J. Food Prot. 1998, 61, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.-S.; Yang, P.; Wu, A.-B.; Zuo, D.-Y.; He, W.-J.; Guo, M.-W.; Huang, T.; Li, H.-P.; Liao, Y.-C.; Yuan, Q.-S.; et al. Variation in the microbiome, trichothecenes, and aflatoxins in stored wheat grains in Wuhan, China. Toxins 2018, 10, 171. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Ryu, D. Worldwide occurrence of mycotoxins in cereals and cereal-derived food products: Public health perspectives of their co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef] [PubMed]
- Maresca, M. From the gut to the brain: Journey and pathophysiological effects of the food-associated trichothecene mycotoxin deoxynivalenol. Toxins 2013, 5, 784–820. [Google Scholar] [CrossRef] [PubMed]
- Miedaner, T.; Gwiazdowska, D.; Waśkiewicz, A. Management of Fusarium species and their mycotoxins in cereal food and feed. Front. Microbiol. 2017, 8, 1543. [Google Scholar] [CrossRef] [Green Version]
- Vogelgsang, S.; Beyer, M.; Pasquali, M.; Jenny, E.; Musa, T.; Bucheli, T.D.; Wettstein, F.E.; Forrer, H.R. An eight-year survey of wheat shows distinctive effects of cropping factors on different Fusarium species and associated mycotoxins. Eur. J. Agron. 2019, 105, 62–77. [Google Scholar] [CrossRef]
- Gilbert, J.; Haber, S. Overview of some recent research developments in Fusarium Head Blight of wheat. Can. J. Plant Pathol. 2013, 35, 149–174. [Google Scholar] [CrossRef]
- Legrand, F.; Picot, A.; Cobo-Díaz, J.F.; Chen, W.; Le Floch, G. Challenges facing the biological control strategies for the management of Fusarium Head Blight of cereals caused by F. graminearum. Biol. Control 2017, 113, 26–38. [Google Scholar] [CrossRef]
- Magan, N.; Aldred, D.; Mylona, K.; Lambert, R.J.W. Limiting mycotoxins in stored wheat. Food Addit. Contam. Part A 2010, 27, 644–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliot, M.A.; Buttner, M.J.; Nodwell, J.R. Multicellular development in Streptomyces. In Myxobacteria: Multicellularity and Differentiation; Whitworth, D.E., Ed.; ASM Press: Washington, DC, USA, 2008; pp. 419–437. [Google Scholar]
- Flärdh, K.; Buttner, M.J. Streptomyces morphogenetics: Dissecting differentiation in a filamentous bacterium. Nat. Rev. Microbiol. 2009, 7, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Chater, K.F.; Biró, S.; Lee, K.J.; Palmer, T.; Schrempf, H. The complex extracellular biology of Streptomyces. FEMS Microbiol. Rev. 2010, 34, 171–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seipke, R.F.; Kaltenpoth, M.; Hutchings, M.I. Streptomyces as symbionts: An emerging and widespread theme? FEMS Microbiol. Rev. 2012, 36, 862–876. [Google Scholar] [CrossRef] [Green Version]
- Rey, T.; Dumas, B. Plenty is no plague: Streptomyces symbiosis with crops. Trends Plant Sci. 2017, 22, 30–37. [Google Scholar] [CrossRef]
- Nourozian, J.; Etebarian, H.R.; Khodakaramian, G. Biological control of Fusarium graminearum on wheat by antagonistic bacteria. Songklanakarin J. Sci. Technol. 2006, 28, 29–38. [Google Scholar]
- Jung, B.; Park, S.Y.; Lee, Y.W.; Lee, J. Biological efficacy of Streptomyces sp. strain BN1 against the cereal head blight pathogen Fusarium graminearum. Plant Pathol. J. 2013, 29, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Winter, M.; Samuels, P.L.; Otto-Hanson, L.K.; Dill-Macky, R.; Linda, L. Biocontrol of Fusarium crown and root rot of wheat by Streptomyces isolates—it’s complicated. Phytobiomes 2019, 3, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Palazzini, J.M.; Ramirez, M.L.; Torres, A.M.; Chulze, S.N. Potential biocontrol agents for Fusarium Head Blight and deoxynivalenol production in wheat. Crop Prot. 2007, 26, 1702–1710. [Google Scholar] [CrossRef]
- Palazzini, J.M.; Yerkovich, N.; Alberione, E.; Chiotta, M.; Chulze, S.N. An integrated dual strategy to control Fusarium graminearum sensu stricto by the biocontrol agent Streptomyces sp. RC 87B under field conditions. Plant Gene 2017, 9, 13–18. [Google Scholar] [CrossRef]
- Palazzini, J.; Roncallo, P.; Cantoro, R.; Chiotta, M.; Yerkovich, N.; Palacios, S.; Echenique, V.; Torres, A.; Ramirez, M.; Karlovsky, P.; et al. Biocontrol of Fusarium graminearum sensu stricto, reduction of deoxynivalenol accumulation and phytohormone induction by two selected antagonists. Toxins 2018, 10, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newitt, J.T.; Prudence, S.M.M.; Hutchings, M.I.; Worsley, S.F.; Newitt, J.T.; Prudence, S.M.M.; Hutchings, M.I.; Worsley, S.F. Biocontrol of cereal crop diseases using Streptomycetes. Pathogens 2019, 8, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellan, L.; Durand, N.; Martinez, V.; Fontana, A.; Schorr-Galindo, S.; Strub, C. Commercial biocontrol agents reveal contrasting comportments against two mycotoxigenic fungi in cereals: Fusarium graminearum and Fusarium verticillioides. Toxins 2020, 12, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombo, E.M.; Kunova, A.; Cortesi, P.; Saracchi, M.; Pasquali, M. Critical assessment of Streptomyces spp. able to control toxigenic fusaria in cereals: A literature and patent review. Int. J. Mol. Sci. 2019, 20, 6119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essarioui, A.; LeBlanc, N.; Otto-Hanson, L.; Schlatter, D.C.; Kistler, H.C.; Kinkel, L.L. Inhibitory and nutrient use phenotypes among coexisting Fusarium and Streptomyces populations suggest local coevolutionary interactions in soil. Environ. Microbiol. 2020, 22, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.E.; Raj, S.S.A.; Wong, S.H.; Tey, D.; Tan, H.M. Estimation of fungal growth using the ergosterol assay: A rapid tool in assessing the microbiological status of grains and feeds. Lett. Appl. Microbiol. 2008, 46, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Tangni, E.K.; Pussemier, L. Ochratoxin A and citrinin loads in stored wheat grains: Impact of grain dust and possible prediction using ergosterol measurement. Food Addit. Contam. 2006, 23, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, B.S.; Rao, V.S.; Ramakrishna, Y.; Bhat, R.V. Rapid and specific method for screening ergosterol as an index of fungal contamination in cereal grains. Food Chem. 1989, 31, 51–56. [Google Scholar] [CrossRef]
- Da Silva Bomfim, N.; Nakassugi, L.P.; Faggion Pinheiro Oliveira, J.; Kohiyama, C.Y.; Mossini, S.A.G.; Grespan, R.; Nerilo, S.B.; Mallmann, C.A.; Alves Abreu Filho, B.; Machinski, M. Antifungal activity and inhibition of fumonisin production by Rosmarinus officinalis L. essential oil in Fusarium verticillioides (Sacc.) Nirenberg. Food Chem. 2015, 166, 330–336. [Google Scholar] [CrossRef] [Green Version]
- Shah, J.J.; Khedkar, V.; Coutinho, E.C.; Mohanraj, K. Design, synthesis and evaluation of benzotriazole derivatives as novel antifungal agents. Bioorg. Med. Chem. Lett. 2015, 25, 3730–3737. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Selvaraj, J.N.; Xing, F.; Zhou, L.; Wang, Y.; Song, H.; Tan, X.; Sun, L.; Sangare, L.; Minnie, Y.; et al. Antagonistic action of Bacillus subtilis strain SG6 on Fusarium graminearum. PLoS ONE 2014, 9, e92486. [Google Scholar] [CrossRef] [PubMed]
- Bluhm, B.H.; Woloshuk, C.P. Amylopectin induces fumonisin B1 production by Fusarium verticillioides during colonization of maize kernels. Mol. Plant-Microbe Interact. 2005, 18, 1333–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tellenbach, C.; Grünig, C.R.; Sieber, T.N. Suitability of quantitative real-time PCR to estimate the biomass of fungal root endophytes. Appl. Environ. Microbiol. 2010, 76, 5764–5772. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.J.; Fan, P.S.; Zhang, X.; Chen, C.J.; Zhou, M.G. Quantification of Fusarium graminearum in harvested grain by real-time polymerase chain reaction to assess efficacies of fungicides on Fusarium Head Blight, deoxynivalenol contamination, and yield of winter wheat. Phytopathology 2009, 99, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Bilska, K.; Kulik, T.; Ostrowska-Kołodziejczak, A.; Buśko, M.; Pasquali, M.; Beyer, M.; Baturo-Cieśniewska, A.; Juda, M.; Załuski, D.; Treder, K.; et al. Development of a highly sensitive FcMito qPCR assay for the quantification of the toxigenic fungal plant pathogen Fusarium culmorum. Toxins 2018, 10, 211. [Google Scholar] [CrossRef] [Green Version]
- Gimeno, A.; Sohlberg, E.; Pakula, T.; Limnell, J.; Keller, B.; Laitila, A.; Vogelgsang, S. TaqMan qPCR for quantification of Clonostachys rosea used as a biological control agent against Fusarium graminearum. Front. Microbiol. 2019, 10, 1627. [Google Scholar] [CrossRef] [Green Version]
- Sanzani, S.M.; Li Destri Nicosia, M.G.; Faedda, R.; Cacciola, S.O.; Schena, L. Use of quantitative PCR detection methods to study biocontrol agents and phytopathogenic fungi and oomycetes in environmental samples. J. Phytopathol. 2014, 162, 1–13. [Google Scholar] [CrossRef]
- Colombo, E.M.; Pizzatti, C.; Kunova, A.; Gardana, C.; Saracchi, M.; Cortesi, P.; Pasquali, M. Evaluation of in-vitro methods to select effective Streptomycetes against toxigenic Fusaria. PeerJ 2019, 7, e6905. [Google Scholar] [CrossRef]
- Colombo, E.M.; Pizzatti, C.; Kunova, A.; Saracchi, M.; Cortesi, P.; Pasquali, M.; Pizzatti, C.; Saracchi, M.; Cortesi, P.; Pasquali, M. Selection of an endophytic Streptomyces sp. strain DEF09 from wheat roots as a biocontrol agent against Fusarium graminearum. Front. Microbiol. 2019, 10, 2356. [Google Scholar] [CrossRef] [Green Version]
- Seong, K.Y.; Pasquali, M.; Zhou, X.; Song, J.; Hilburn, K.; McCormick, S.; Dong, Y.; Xu, J.R.; Kistler, H.C. Global gene regulation by Fusarium transcription factors Tri6 and Tri10 reveals adaptations for toxin biosynthesis. Mol. Microbiol. 2009, 72, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, T.D.; De Moraes, L.A.B.; De Melo, I.S. Streptomyces sp. ASBV-1 reduces aflatoxin accumulation by Aspergillus parasiticus in peanut grains. J. Appl. Microbiol. 2008, 105, 2153–2160. [Google Scholar] [CrossRef]
- Bonaldi, M.; Kunova, A.; Saracchi, M.; Sardi, P.; Cortesi, P. Streptomycetes as biological control agents against basal drop. Acta Horticulturae 2014, 1044, 313–318. [Google Scholar] [CrossRef]
- Mille-Lindblom, C.; Fischer, H.J.; Tranvik, L. Antagonism between bacteria and fungi: Substrate competition and a possible tradeoff between fungal growth and tolerance towards bacteria. Oikos 2006, 113, 233–242. [Google Scholar] [CrossRef]
- Jones, S.E.; Ho, L.; Rees, C.A.; Hill, J.E.; Nodwell, J.R.; Elliot, M.A. Streptomyces exploration is triggered by fungal interactions and volatile signals. Elife 2017, 6, e21738. [Google Scholar] [CrossRef]
- Fguira, L.F.B.; Smaoui, S.; Karray-Rebai, I.; Bejar, S.; Mellouli, L. The antifungal activity of the terrestrial Streptomyces US80 strain is induced by heat-killed fungi. Biotechnol. J. 2008, 3, 1058–1066. [Google Scholar] [CrossRef]
- Elleuch, L.; Smaoui, S.; Najah, S.; Chakchouk, A.; Sellem, I.; Karray-Rebai, I.; Mellouli, L. Production of diketopiperazine derivative cyclo (L-Leu-L-Arg) by Streptomyces sp. TN262 after exposure to heat-killed fungus Fusarium sp. J. Chem. Soc. Pakistan 2013, 35, 1530–1534. [Google Scholar]
- Zhao, J.; Xue, Q.H.; Niu, G.G.; Xue, L.; Shen, G.H.; Du, J.Z. Extracellular enzyme production and fungal mycelia degradation of antagonistic Streptomyces induced by fungal mycelia preparation of cucurbit plant pathogens. Ann. Microbiol. 2013, 63, 809–812. [Google Scholar] [CrossRef]
- Pasquali, M.; Migheli, Q. Genetic approaches to chemotype determination in type B-trichothecene producing Fusaria. Int. J. Food Microbiol. 2014, 189, 164–182. [Google Scholar] [CrossRef]
- Martinez Tuppia, C.; Atanasova-Penichon, V.; Chéreau, S.; Ferrer, N.; Marchegay, G.; Savoie, J.-M.; Richard-Forget, F. Yeast and bacteria from ensiled high moisture maize grains as potential mitigation agents of fumonisin B 1. J. Sci. Food Agric. 2017, 97, 2443–2452. [Google Scholar] [CrossRef]
- He, J.; Boland, G.J.; Zhou, T. Concurrent selection for microbial suppression of Fusarium graminearum, Fusarium Head Blight and deoxynivalenol in wheat. J. Appl. Microbiol. 2009, 106, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Dalié, D.; Pinson-Gadais, L.; Atanasova-Penichon, V.; Marchegay, G.; Barreau, C.; Deschamps, A.; Richard-Forget, F. Impact of Pediococcus pentosaceus strain L006 and its metabolites on fumonisin biosynthesis by Fusarium verticillioides. Food Control 2012, 23, 405–411. [Google Scholar] [CrossRef]
- Palaniyandi, S.A.; Yang, S.H.; Zhang, L.; Suh, J.W. Effects of Actinobacteria on plant disease suppression and growth promotion. Appl. Microbiol. Biotechnol. 2013, 97, 9621–9636. [Google Scholar] [CrossRef] [PubMed]
- Sakuda, S.; Ono, M.; Ikeda, H. Blasticidin A as an inhibitor of aflatoxin production by Aspergillus parasiticus of aflatoxin. J. Antibiot. 2000, 53, 1265–1271. [Google Scholar] [CrossRef] [Green Version]
- Kondo, T.; Sakurada, M.; Okamoto, S.; Ono, M.; Tsukigi, H.; Suzuki, A.; Nagasawa, H.; Sakuda, S. Effects of aflastatin A, an inhibitor of aflatoxin production, on aflatoxin biosynthetic pathway and glucose metabolism in Aspergillus parasiticus. J. Antibiot. 2001, 54, 650–657. [Google Scholar] [CrossRef] [Green Version]
- Yoshinari, T.; Noda, Y.; Yoda, K.; Sezaki, H.; Nagasawa, H.; Sakuda, S. Inhibitory activity of blasticidin A, a strong aflatoxin production inhibitor, on protein synthesis of yeast: Selective inhibition of aflatoxin production by protein synthesis inhibitors. J. Antibiot. 2010, 63, 309–314. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Bhatia, R.K.; Choi, Y.K.; Kan, E.; Kim, Y.G.; Yang, Y.H. Biotechnological potential of microbial consortia and future perspectives. Crit. Rev. Biotechnol. 2018, 38, 1209–1229. [Google Scholar] [CrossRef]
- Abbasi, S.; Safaie, N.; Sadeghi, A.; Shamsbakhsh, M. Tissue-specific synergistic bio-priming of pepper by two Streptomyces species against Phytophthora capsici. PLoS ONE 2020, 15, e0230531. [Google Scholar] [CrossRef] [Green Version]
- Pasquali, M.; Cocco, E.; Leclercq, C.C.; Planchon, S.; Guignard, C.; Renaut, J.; Hoffmann, L. A Fusarium graminearum strain-comparative proteomic approach identifies regulatory changes triggered by agmatine. J. Proteomics 2016, 137, 107–116. [Google Scholar] [CrossRef]
- Pani, G.; Dessì, A.; Dallocchio, R.; Scherm, B.; Azara, E.; Delogu, G.; Migheli, Q.M. Natural phenolic inhibitors of trichothecene biosynthesis by the wheat fungal pathogen Fusarium culmorum: A computational insight into the structure-activity relationship. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Sardi, P.; Saracchi, M.; Quaroni, S.; Petrolini, B.; Borgonovi, G.E.; Merli, S. Isolation of endophytic Streptomyces strains from surface-sterilized roots. Appl. Environ. Microbiol. 1992, 58, 2691–2693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardiner, D.M.; Stiller, J.; Kazan, K. Genome sequence of Fusarium graminearum isolate CS3005. Genome Announc. 2014, 2, e00227-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponts, N.; Pinson-Gadais, L.; Verdal-Bonnin, M.N.; Barreau, C.; Richard-Forget, F. Accumulation of deoxynivalenol and its 15-acetylated form is significantly modulated by oxidative stress in liquid cultures of Fusarium graminearum. FEMS Microbiol. Lett. 2006, 258, 102–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Huang, Y.; Wang, Y.; Zhao, Y.; Cui, Z. Potassium hydroxide-ethylene diamine tetraacetic acid method for the rapid preparation of small-scale PCR template DNA from Actinobacteria. Mol. Genet. Microbiol. Virol. 2014, 29, 42–46. [Google Scholar] [CrossRef]
- Guo, Y.P.; Zheng, W.; Rong, X.Y.; Huang, Y. A multilocus phylogeny of the Streptomyces griseus 16S rRNA gene clade: Use of multilocus sequence analysis for Streptomycete systematics. Int. J. Syst. Evol. Microbiol. 2008, 58, 149–159. [Google Scholar] [CrossRef]
- Nicolaisen, M.; Suproniene, S.; Nielsen, L.K.; Lazzaro, I.; Spliid, N.H.; Justesen, A.F. Real-time PCR for quantification of eleven individual Fusarium species in cereals. J. Microbiol. Methods 2009, 76, 234–240. [Google Scholar] [CrossRef]
- Pasquali, M.; Piatti, P.; Gullino, M.L.; Garibaldi, A. Development of a real-time polymerase chain reaction for the detection of Fusarium oxysporum f. sp. basilici from basil seed and roots. J. Phytopathol. 2006, 154, 632–636. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Cuomo, C.A.; Güldener, U.; Xu, J.-R.; Trail, F.; Turgeon, B.G.; Di Pietro, A.; Walton, J.D.; Ma, L.-J.; Baker, S.E.; Rep, M.; et al. The Fusarium graminearum genome reveals a link between localized polymorphism and pathogen specialization. Science 2007, 317, 1400–1402. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing, version R 3.5.3; R Core Team: Copenhagen, Denmark, 2019. [Google Scholar]
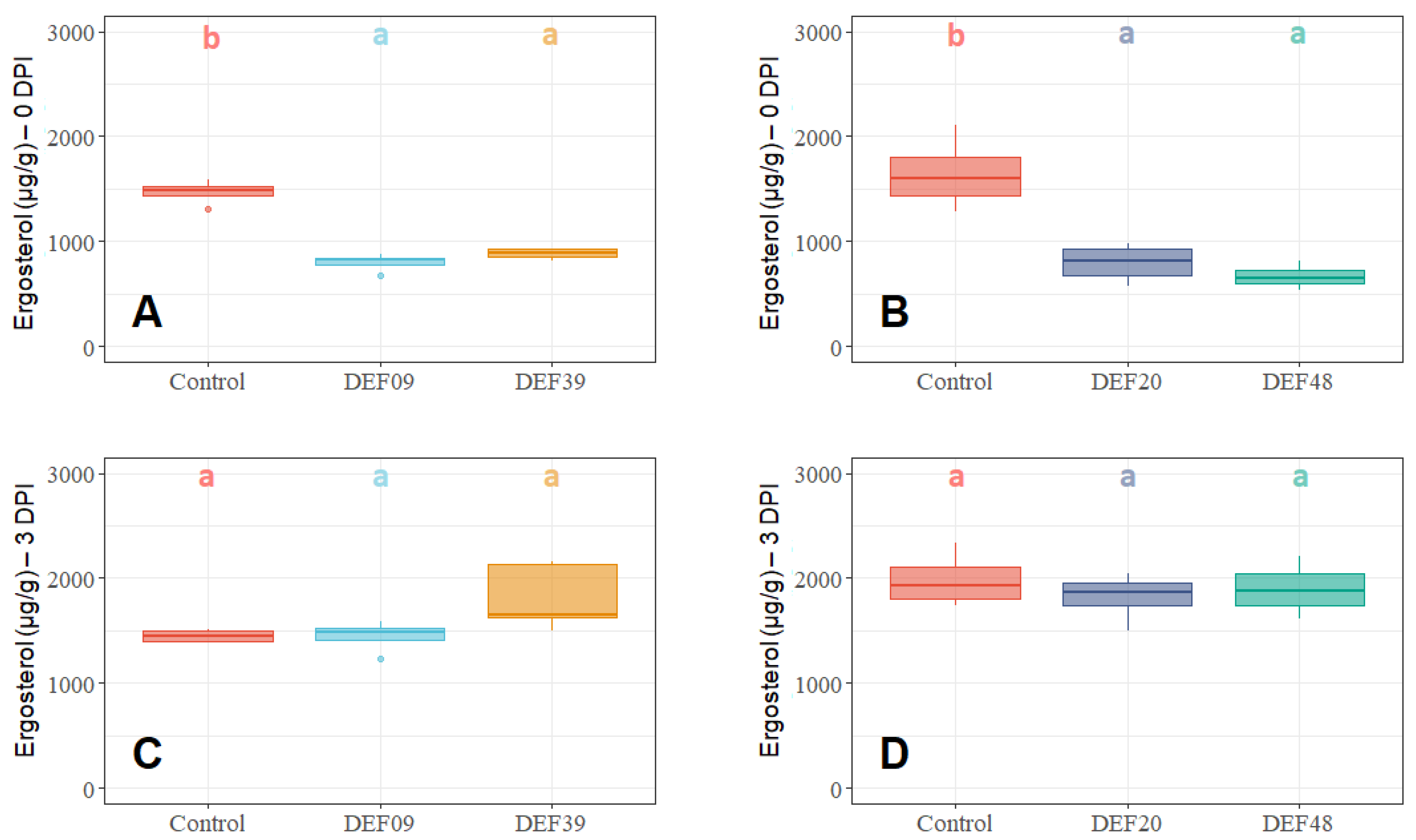
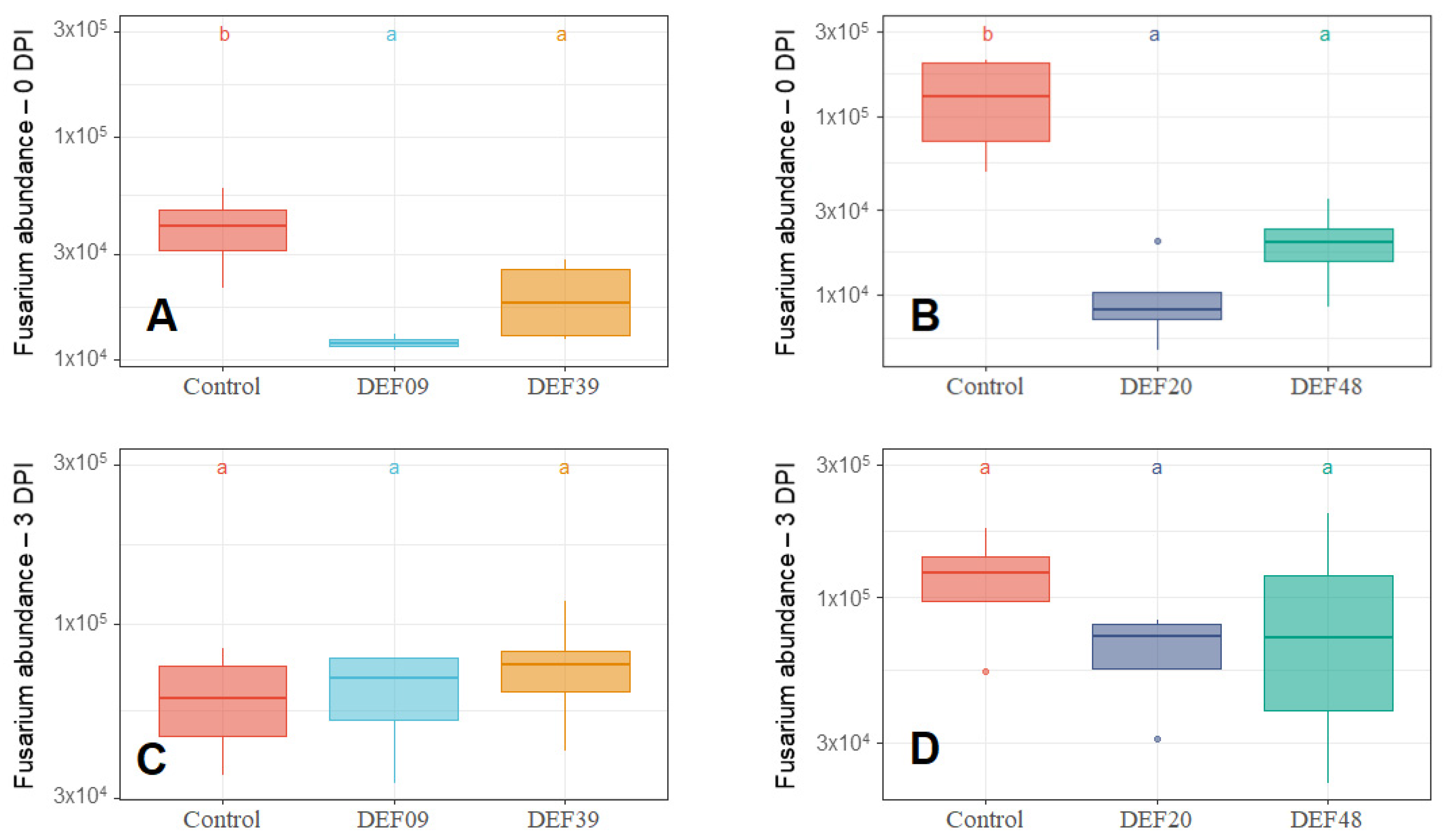
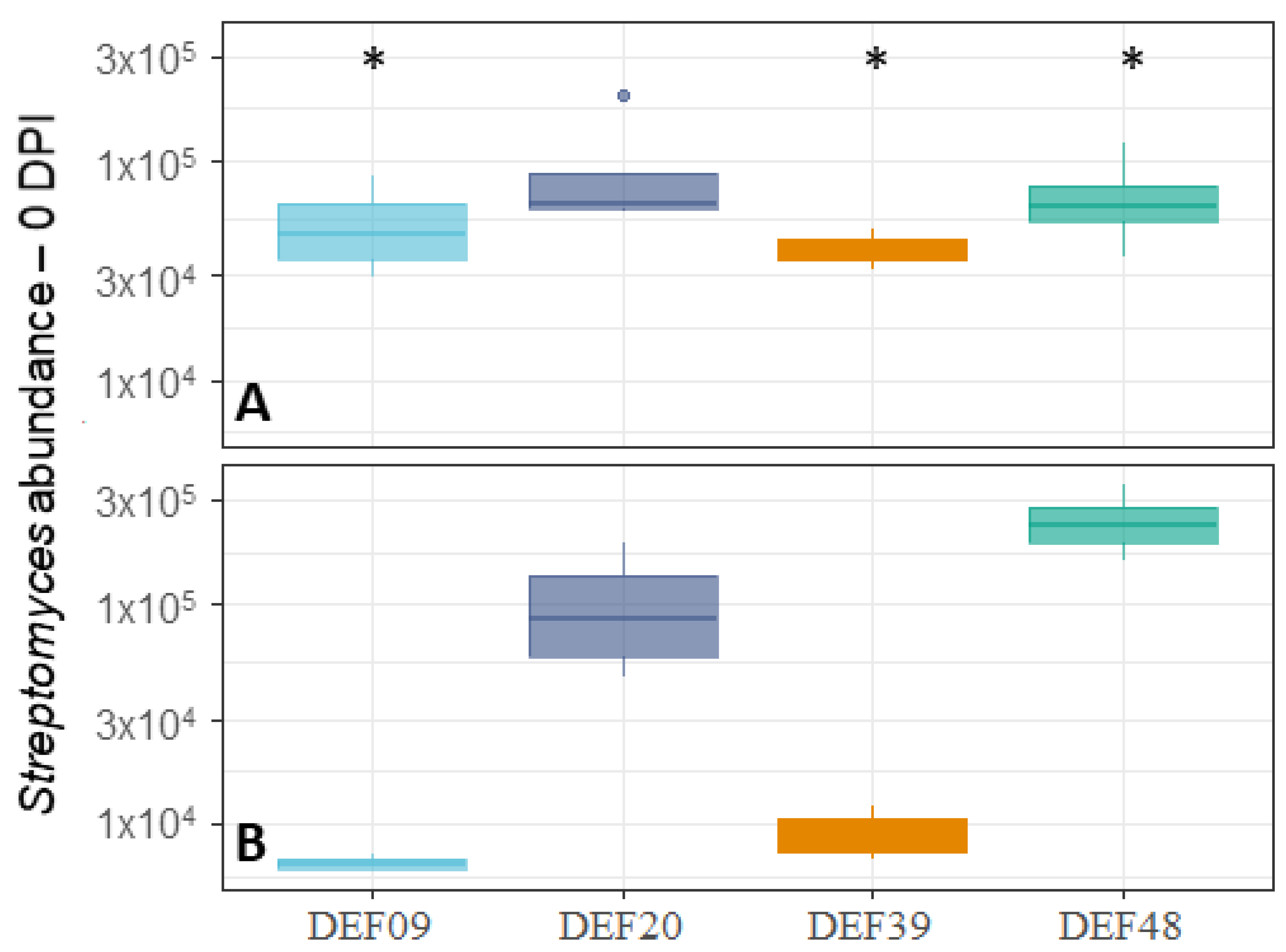
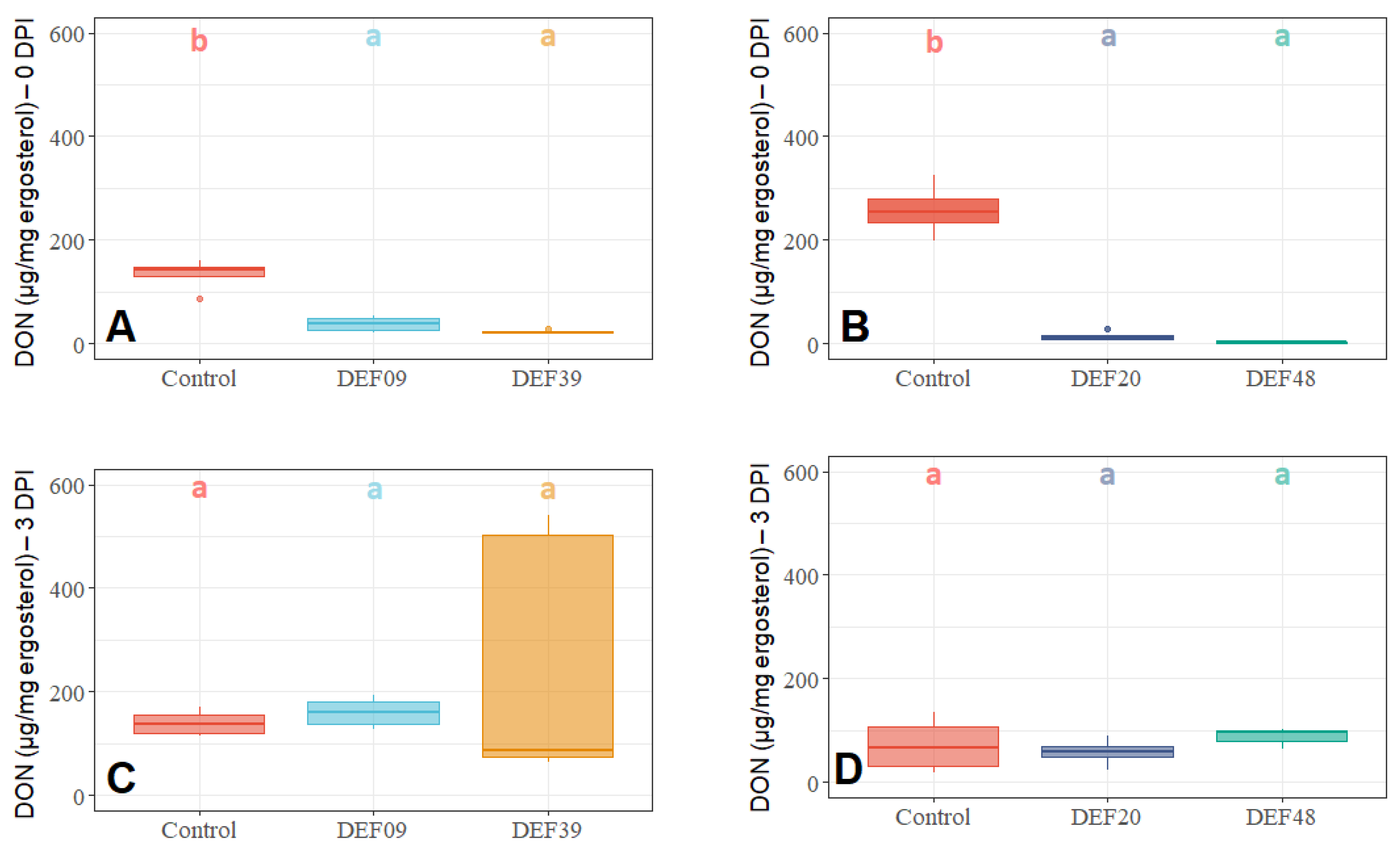
| Strain | Isolation Source | Fungal Growth Inhibition in vitro [40,41] | FFR Reduction on Plants (Greenhouse) [40,41] | Inhibition of F. graminearum Mycelium Growth on Grains at 0DPI | Toxin Inhibition in Grains Infected by F. graminearum 0 DPI | Streptomyces Growth in Grains Infected by F. graminearum 0 DPI | Possible Mechanism of DON Reduction | |
|---|---|---|---|---|---|---|---|---|
| qPCR | Ergosterol | |||||||
| DEF09 | Triticum aestivum | 59% | 46% | 70% | 45% | 71% | - | Mostly mycelium growth reduction |
| DEF20 | Carex sp. | 78% | 11% | 92% | 52% | 94% | = | Mostly mycelium growth reduction |
| DEF39 | Secale cereale | 64% | 0% | 50% | 40% | 83% | - | Specific toxin inhibition independent from inhibition of mycelium growth |
| EF48 | Zea mays | 70% | 29% | 85% | 60% | 99% | + | Mostly mycelium growth reduction |
| Primer Name | Assay Target | Primer Sequence | Melting Temp. | GC % | Reference |
|---|---|---|---|---|---|
| recAPF | Streptomyces spp. | CCGCRCTCGCACAGATTGAACGSCAATTC | 70.2 | 56.9 | [56] |
| recAPR | Streptomyces spp. | GCSAGGTCGGGGTTGTCCTTSAGGAAGTTGCG | 74.6 | 56.9 | [56] |
| recAF | Streptomyces spp. | ACAGATTGAACGGCAATTCG | 55.3 | 45 | [56] |
| recAR | Streptomyces spp. | ACCTTGTTCTTGACCACCTT | 55.3 | 45 | [56] |
| qstreptoREcAF | Streptomyces spp. | AAGATCACCAGTGCGCTCAA | 59.96 | 50 | This study |
| qstreptoREcAR | Streptomyces spp. | GAGCTGGTTGATGAAGATCGC | 59.40 | 52 | This study |
| TRI12QF | F. graminearum | ATCTCAGCCAGACGACAGGT | 59.87 | 55 | This study |
| TRI12DR | F. graminearum | CGAGGCGAGGTGTAATATCC | 59.55 | 55 | This study |
| Hor1f | Wheat | TCTCTGGGTTTGAGGGTGAC | 62 | 55 | [57] |
| Hor2r | Wheat | GGCCCTTGTACCAGTCAAGGT | 51 | 57 | [57] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, E.M.; Kunova, A.; Gardana, C.; Pizzatti, C.; Simonetti, P.; Cortesi, P.; Saracchi, M.; Pasquali, M. Investigating Useful Properties of Four Streptomyces Strains Active against Fusarium graminearum Growth and Deoxynivalenol Production on Wheat Grains by qPCR. Toxins 2020, 12, 560. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12090560
Colombo EM, Kunova A, Gardana C, Pizzatti C, Simonetti P, Cortesi P, Saracchi M, Pasquali M. Investigating Useful Properties of Four Streptomyces Strains Active against Fusarium graminearum Growth and Deoxynivalenol Production on Wheat Grains by qPCR. Toxins. 2020; 12(9):560. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12090560
Chicago/Turabian StyleColombo, Elena Maria, Andrea Kunova, Claudio Gardana, Cristina Pizzatti, Paolo Simonetti, Paolo Cortesi, Marco Saracchi, and Matias Pasquali. 2020. "Investigating Useful Properties of Four Streptomyces Strains Active against Fusarium graminearum Growth and Deoxynivalenol Production on Wheat Grains by qPCR" Toxins 12, no. 9: 560. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12090560





