First Detection of Tetrodotoxin in Bivalves and Gastropods from the French Mainland Coasts
Abstract
:1. Introduction
2. Results
2.1. In-House Method Validation
2.2. Sample Analysis
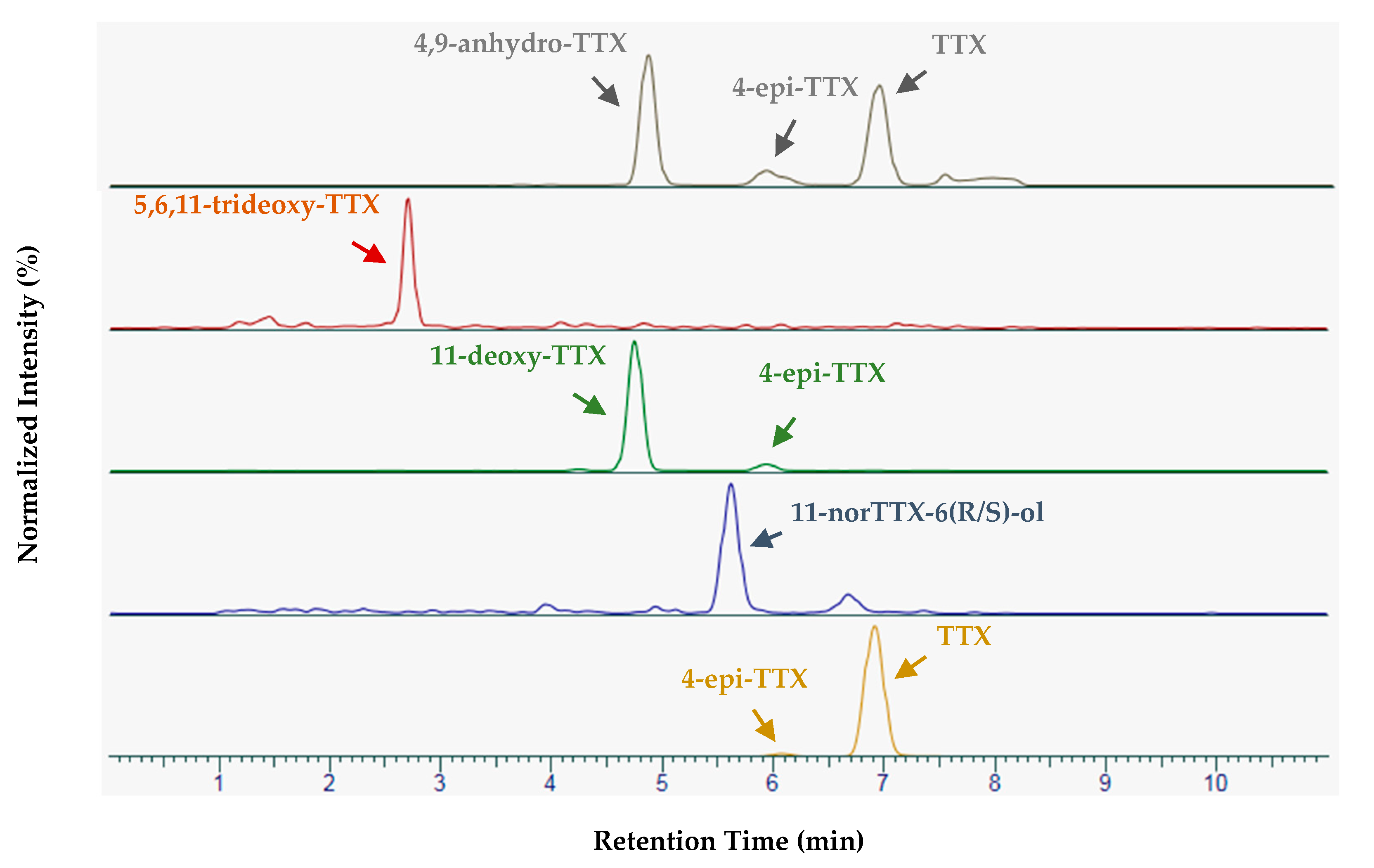
2.3. Complementary Investigations
2.3.1. In-House Validation of the Sensitive Variant of the Method
2.3.2. Analysis of Samples Containing Tetrodotoxin with the Sensitive Variant of the Method
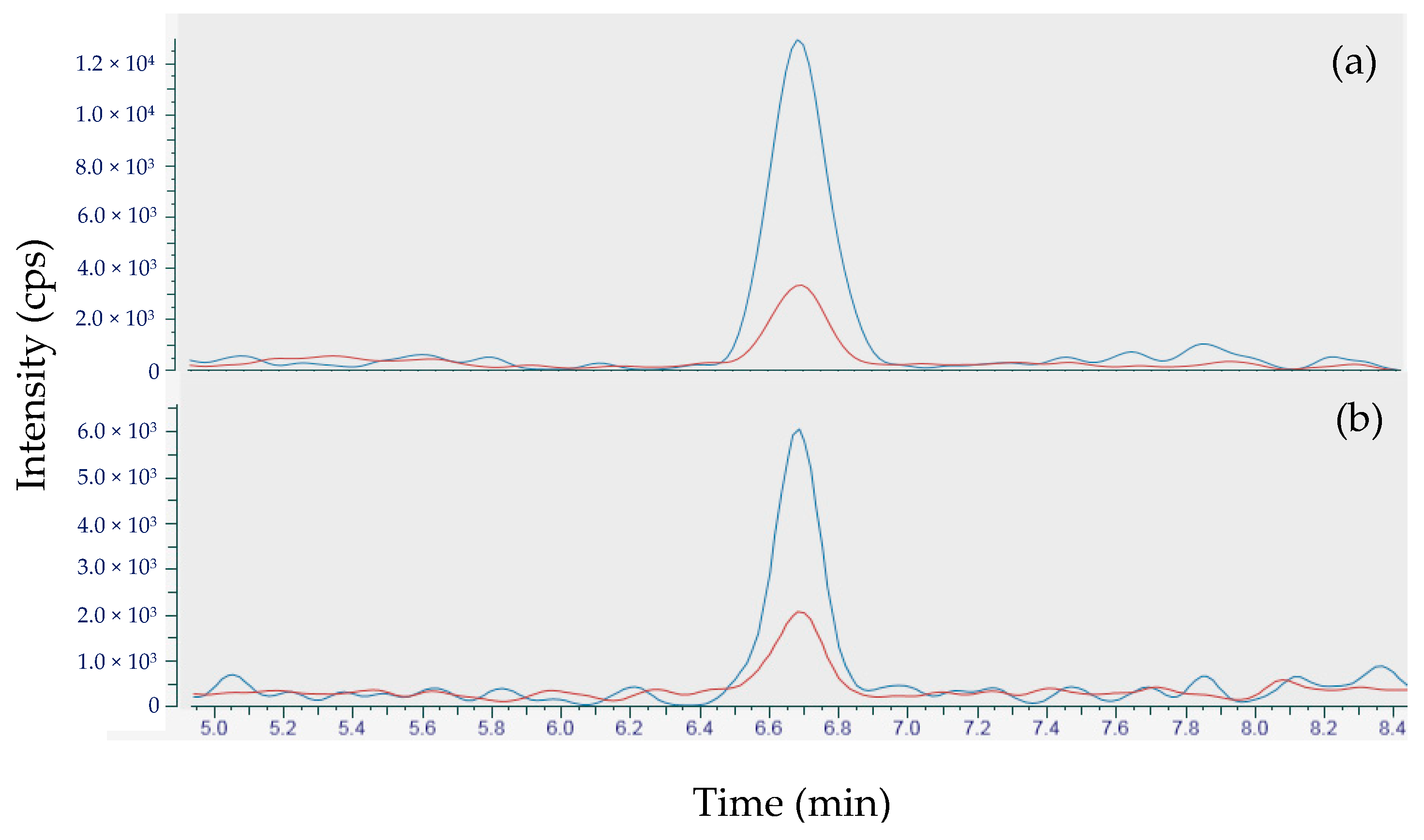
2.3.3. Distribution of TTX in the Whelk Sample 17 ET2M 773
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Reagents
5.2. Sampling Design
5.3. Sample Preparation
5.4. Tetrodotoxin Extraction
5.5. Liquid Chromatography Conditions
5.6. Tandem Mass Spectrometry Conditions
5.7. Method Validation Methodology
5.8. Quality Control
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bane, V.; Lehane, M.; Dikshit, M.; O’Riordan, A.; Furey, A. Tetrodotoxin: Chemistry, toxicity, source, distribution and detection. Toxins 2014, 6, 693–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, M.; Rodríguez, I.; Barreiro, A.; Kaufmann, M.; Neto, A.I.; Hassouani, M.; Sabour, B.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. Tetrodotoxins occurrence in non-traditional vectors of the north atlantic waters (Portuguese maritime territory, and morocco coast). Toxins 2019, 11, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guardone, L.; Maneschi, A.; Meucci, V.; Gasperetti, L.; Nucera, D.; Armani, A. A Global Retrospective Study on Human Cases of Tetrodotoxin (TTX) Poisoning after Seafood Consumption. Food Rev. Int. 2019, 1–23. [Google Scholar] [CrossRef]
- Rodriguez, P.; Alfonso, A.; Vale, C.; Alfonso, C.; Vale, P.; Tellez, A.; Botana, L.M. First toxicity report of tetrodotoxin and 5,6,11-trideoxyTTX in the trumpet shell Charonia lampas lampas in Europe. Anal. Chem. 2008, 80, 5622–5629. [Google Scholar] [CrossRef]
- Fernández-Ortega, J.F.; Morales-de los Santos, J.M.; Herrera-Gutiérrez, M.E.; Fernández-Sánchez, V.; Rodríguez Loureo, P.; Alfonso Rancaño, A.; Téllez-Andrade, A. Seafood intoxication by tetrodotoxin: First case in europe. J. Emerg. Med. 2010, 39, 612–617. [Google Scholar] [CrossRef]
- Silva, M.; Azevedo, J.; Rodriguez, P.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. New gastropod vectors and tetrodotoxin potential expansion in temperate waters of the Atlantic Ocean. Mar. Drugs 2012, 10, 712–726. [Google Scholar] [CrossRef] [Green Version]
- Turner, A.D.; Powell, A.; Schofield, A.; Lees, D.N.; Baker-Austin, C. Detection of the pufferfish toxin tetrodotoxin in European bivalves, England, 2013 to 2014. Eurosurveillance 2015, 20. [Google Scholar] [CrossRef]
- Vlamis, A.; Katikou, P.; Rodriguez, I.; Rey, V.; Alfonso, A.; Papazachariou, A.; Zacharaki, T.; Botana, A.M.; Botana, L.M. First detection of tetrodotoxin in greek shellfish by UPLC-MS/MS potentially linked to the presence of the dinoflagellate prorocentrum minimum. Toxins 2015, 7, 1779–1807. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Risks for public health related to the presence of tetrodotoxin (TTX) and TTX analogues in marine bivalves and gastropods. EFSA J. 2017, 15, 4752. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Notification Number: 2016/175/NL—Policy Guideline on Tetrodotoxin in Live Bivalve Molluscs; European Commission: Brussels, Belgium, 2016. [Google Scholar]
- Abal, P.; Louzao, M.C.; Antelo, A.; Alvarez, M.; Cagide, E.; Vilariño, N.; Vieytes, M.R.; Botana, L.M. Acute oral toxicity of tetrodotoxin in mice: Determination of Lethal Dose 50 (LD50) and no observed adverse effect level (NOAEL). Toxins 2017, 9, 75. [Google Scholar] [CrossRef] [Green Version]
- Gerssen, A.; Bovee, T.H.F.; Klijnstra, M.D.; Poelman, M.; Portier, L.; Hoogenboom, R.L.A.P. First Report on the occurrence of tetrodotoxins in bivalve mollusks in The Netherlands. Toxins 2018, 10, 450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leão, J.M.; Lozano-Leon, A.; Giráldez, J.; Vilariño, Ó.; Gago-Martínez, A. Preliminary results on the evaluation of the occurrence of tetrodotoxin associated to marine vibrio spp. in bivalves from the galician rias (Northwest of Spain). Mar. Drugs 2018, 16, 81. [Google Scholar] [CrossRef] [Green Version]
- Blanco, L.; Lago, J.; González, V.; Paz, B.; Rambla-Alegre, M.; Cabado, A.G. Occurrence of tetrodotoxin in bivalves and gastropods from harvesting areas and other natural spaces in Spain. Toxins 2019, 11, 331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dell’Aversano, C.; Tartaglione, L.; Polito, G.; Dean, K.; Giacobbe, M.; Casabianca, S.; Capellacci, S.; Penna, A.; Turner, A.D. First detection of tetrodotoxin and high levels of paralytic shellfish poisoning toxins in shellfish from Sicily (Italy) by three different analytical methods. Chemosphere 2019, 215, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.M.; Pinto, E.P.; Oliveira, P.; Pedro, S.; Costa, P.R. Evaluation of the occurrence of tetrodotoxin in bivalve mollusks from the Portuguese coast. J. Mar. Sci. Eng. 2019, 7, 232. [Google Scholar] [CrossRef] [Green Version]
- Katikou, P.; Vlamis, A. Tetrodotoxins: Recent advances in analysis methods and prevalence in European waters. Curr. Opin. Food Sci. 2017, 18, 1–6. [Google Scholar] [CrossRef]
- Biessy, L.; Boundy, M.J.; Smith, K.F.; Harwood, D.T.; Hawes, I.; Wood, S.A. Tetrodotoxin in marine bivalves and edible gastropods: A mini-review. Chemosphere 2019, 236, 124404. [Google Scholar] [CrossRef]
- Boundy, M.J.; Selwood, A.I.; Harwood, D.T.; McNabb, P.S.; Turner, A.D. Development of a sensitive and selective liquid chromatography-mass spectrometry method for high throughput analysis of paralytic shellfish toxins using graphitised carbon solid phase extraction. J. Chromatogr. A 2015, 1387, 1–12. [Google Scholar] [CrossRef]
- European Union Reference Laboratory for Marine Biotoxins (EURL MB). Determination of Tetrodotoxin by HILIC-MS/MS; EURL MB: Vigo, Spain, 2017. [Google Scholar]
- Turner, A.D.; Boundy, M.J.; Dhanji Rapkova, M. Development and single-laboratory validation of a liquid chromatography tandem mass spectrometry method for quantitation of tetrodotoxin in mussels and oysters. J. AOAC Int. 2017, 100, 1469–1482. [Google Scholar] [CrossRef]
- European Commitee for Standardization (CEN). Performance Criteria for Single Laboratory Validated Methods of Analysis for the Determination of Mycotoxins (FD CEN/TR 16059); CEN: Brussels, Belgium, 2011. [Google Scholar]
- Turner, A.D.; Dhanji-Rapkova, M.; Fong, S.Y.T.; Hungerford, J.; McNabb, P.S.; Boundy, M.J.; Harwood, D.C. Ultrahigh-performance hydrophilic interaction liquid chromatography with tandem mass spectrometry method for the determination of paralytic shellfish toxins and tetrodotoxin in mussels, oysters, clams, cockles, and scallops: Collaborative study. J. AOAC Int. 2020, 103, 1–30. [Google Scholar] [CrossRef]
- Rey, V.; Botana, A.M.; Antelo, A.; Alvarez, M.; Botana, L.M. Rapid analysis of paralytic shellfish toxins and tetrodotoxins by liquid chromatography-tandem mass spectrometry using a porous graphitic carbon column. Food Chem. 2018, 269, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Nzoughet, J.K.; Campbell, K.; Barnes, P.; Cooper, K.M.; Chevallier, O.P.; Elliott, C.T. Comparison of sample preparation methods, validation of an UPLC-MS/MS procedure for the quantification of tetrodotoxin present in marine gastropods and analysis of pufferfish. Food Chem. 2013, 136, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Salvitti, L.; Wood, S.A.; Taylor, D.I.; McNabb, P.; Cary, S.C. First identification of tetrodotoxin (TTX) in the flatworm Stylochoplana sp.; A source of TTX for the sea slug Pleurobranchaea maculata. Toxicon 2015, 95, 23–29. [Google Scholar] [CrossRef] [PubMed]
- McNabb, P.S.; Taylor, D.I.; Ogilvie, S.C.; Wilkinson, L.; Anderson, A.; Hamon, D.; Wood, S.A.; Peake, B.M. First detection of tetrodotoxin in the bivalve Paphies australis by liquid chromatography coupled to triple quadrupole mass spectrometry with and without precolumn reaction. J. AOAC Int. 2014, 97, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Bane, V.; Brosnan, B.; Barnes, P.; Lehane, M.; Furey, A. High-resolution mass spectrometry analysis of tetrodotoxin (TTX) and its analogues in puffer fish and shellfish. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2016, 33, 1468–1489. [Google Scholar] [CrossRef] [PubMed]
- Reverté, L.; Rambla-Alegre, M.; Leonardo, S.; Bellés, C.; Campbell, K.; Elliott, C.T.; Gerssen, A.; Klijnstra, M.D.; Diogène, J.; Campàs, M. Development and validation of a maleimide-based enzyme-linked immunosorbent assay for the detection of tetrodotoxin in oysters and mussels. Talanta 2018, 176, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Joint advice of the Aquaculture Advisory Council and the Market Advisory Council. In Presence of Tetrodotoxin in Shellfish; Aquaculture Advisory Council: Brussels, Belgium, 2018.
- Katikou, P. Public health risks associated with tetrodotoxin and its analogues in european waters: Recent advances after the EFSA scientific opinion. Toxins 2019, 11, 240. [Google Scholar] [CrossRef] [Green Version]
- Magarlamov, T.Y.; Melnikova, D.I.; Chernyshev, A.V. Tetrodotoxin-producing bacteria: Detection, distribution and migration of the toxin in aquatic systems. Toxins 2017, 9, 166. [Google Scholar] [CrossRef]
- Farabegoli, F.; Blanco, L.; Rodríguez, L.P.; Manuel Vieites, J.; García Cabado, A. Phycotoxins in marine shellfish: Origin, occurrence and effects on humans. Mar. Drugs 2018, 16, 188. [Google Scholar] [CrossRef] [Green Version]
- Durán-Riveroll, L.M.; Cembella, A.D. Guanidinium toxins and their interactions with voltage-gated sodium ion channels. Mar. Drugs 2017, 15, 303. [Google Scholar] [CrossRef] [Green Version]
- Turner, A.D.; Dhanji-Rapkova, M.; Coates, L.; Bickerstaff, L.; Milligan, S.; O’Neill, A.; Faulkner, D.; McEneny, H.; Baker-Austin, C.; Lees, D.N.; et al. Detection of Tetrodotoxin Shellfish Poisoning (TSP) toxins and causative factors in bivalve molluscs from the UK. Mar. Drugs 2017, 15, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- REPHY. French observation and monitoring program for phytoplankton and hydrology in coastal waters REPHY dataset—French observation and monitoring program for phytoplankton and hydrology in coastal waters. 1987–2018 metropolitan data. SEANOE 2019. [Google Scholar] [CrossRef]
- National Institute of Geographic and Forest Information (IGN). Carte littorale—Géoportail. Available online: https://www.geoportail.gouv.fr/donnees/carte-littorale (accessed on 6 May 2020).
- Biessy, L.; Smith, K.F.; Boundy, M.J.; Webb, S.C.; Hawes, I.; Wood, S.A. Distribution of tetrodotoxin in the New Zealand clam, Paphies australis, established using immunohistochemistry and liquid chromatography-tandem quadrupole mass spectrometry. Toxins 2018, 10, 282. [Google Scholar] [CrossRef] [Green Version]
- Biessy, L.; Smith, K.F.; Harwood, D.T.; Boundy, M.J.; Hawes, I.; Wood, S.A. Spatial variability and depuration of tetrodotoxin in the bivalve Paphies australis from New Zealand. Toxicon X 2019, 2, 100008. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.F.; Chueh, C.H.; Jeng, S.S. Occurrence of tetrodotoxin in the gastropod mollusk Natica lineata (lined moon shell). Toxicon 1990, 28, 21–27. [Google Scholar] [CrossRef]
- Hwang, D.F.; Tai, K.P.; Chueh, C.H.; Lin, L.C.; Jeng, S.S. Tetrodotoxin and Derivatives in Several Species of the gastropod Naticidae. Toxicon 1991, 29, 1019–1024. [Google Scholar] [CrossRef]
- Hwang, D.F.; Lin, L.C.; Jeng, S.S. Variation and secretion of toxins in gastropod mollusc Niotha clathrata. Toxicon 1992, 30, 1189–1194. [Google Scholar] [CrossRef]
- Yin, H.L.; Lin, H.S.; Huang, C.C.; Hwang, D.F.; Liu, J.S.; Chen, W.H. Tetrodotoxication with Nassauris glans: A possibility of tetrodotoxin spreading in marine products near Pratas Island. Am. J. Trop. Med. Hyg. 2005, 73, 985–990. [Google Scholar] [CrossRef] [Green Version]
- Jen, H.C.; Lin, S.J.; Lin, S.Y.; Huang, Y.W.; Liao, I.C.; Arakawa, O.; Hwang, D.F. Occurrence of tetrodotoxin and paralytic shellfish poisons in a gastropod implicated in food poisoning in southern Taiwan. Food Addit. Contam. 2007, 24, 902–909. [Google Scholar] [CrossRef]
- Taniyama, S.; Isami, Y.; Matsumoto, T.; Nagashima, Y.; Takatani, T.; Arakawa, O. Toxicity and toxin profile of tetrodotoxin detected in the scavenging gastropod nassarius (Alectrion) glans “kinshibai”. J. Food Hyg. Soc. Jpn. 2009, 50, 22–28. [Google Scholar] [CrossRef] [Green Version]
- European Commitee for Standardization (CEN). Foodstuffs—Determination of Saxitoxin-Group Toxins in Shellfish—HPLC Method Using Pre-Column Derivatization with Peroxide or Periodate Oxidation (EN 14526); European Commitee for Standardization: Brussels, Belgium, 2017. [Google Scholar]
- Turner, A.D.; McNabb, P.S.; Harwood, D.T.; Selwood, A.I.; Boundy, M.J. Single-laboratory validation of a multitoxin ultra- performance LC-hydrophilic interaction LC-MS/MS method for quantitation of paralytic shellfish toxins in bivalve shellfish. J. AOAC Int. 2015, 98, 609–621. [Google Scholar] [CrossRef] [PubMed]
 : mussels;
: mussels;  : oysters) and gastropod beds (
: oysters) and gastropod beds (  : whelks). Map generated using QGIS software (version 3.8.3) and map tiles by Stamen Design, under CC BY 3.0. Data by OpenStreetMap, under ODbL.
: whelks). Map generated using QGIS software (version 3.8.3) and map tiles by Stamen Design, under CC BY 3.0. Data by OpenStreetMap, under ODbL.
 : mussels;
: mussels;  : oysters) and gastropod beds (
: oysters) and gastropod beds (  : whelks). Map generated using QGIS software (version 3.8.3) and map tiles by Stamen Design, under CC BY 3.0. Data by OpenStreetMap, under ODbL.
: whelks). Map generated using QGIS software (version 3.8.3) and map tiles by Stamen Design, under CC BY 3.0. Data by OpenStreetMap, under ODbL.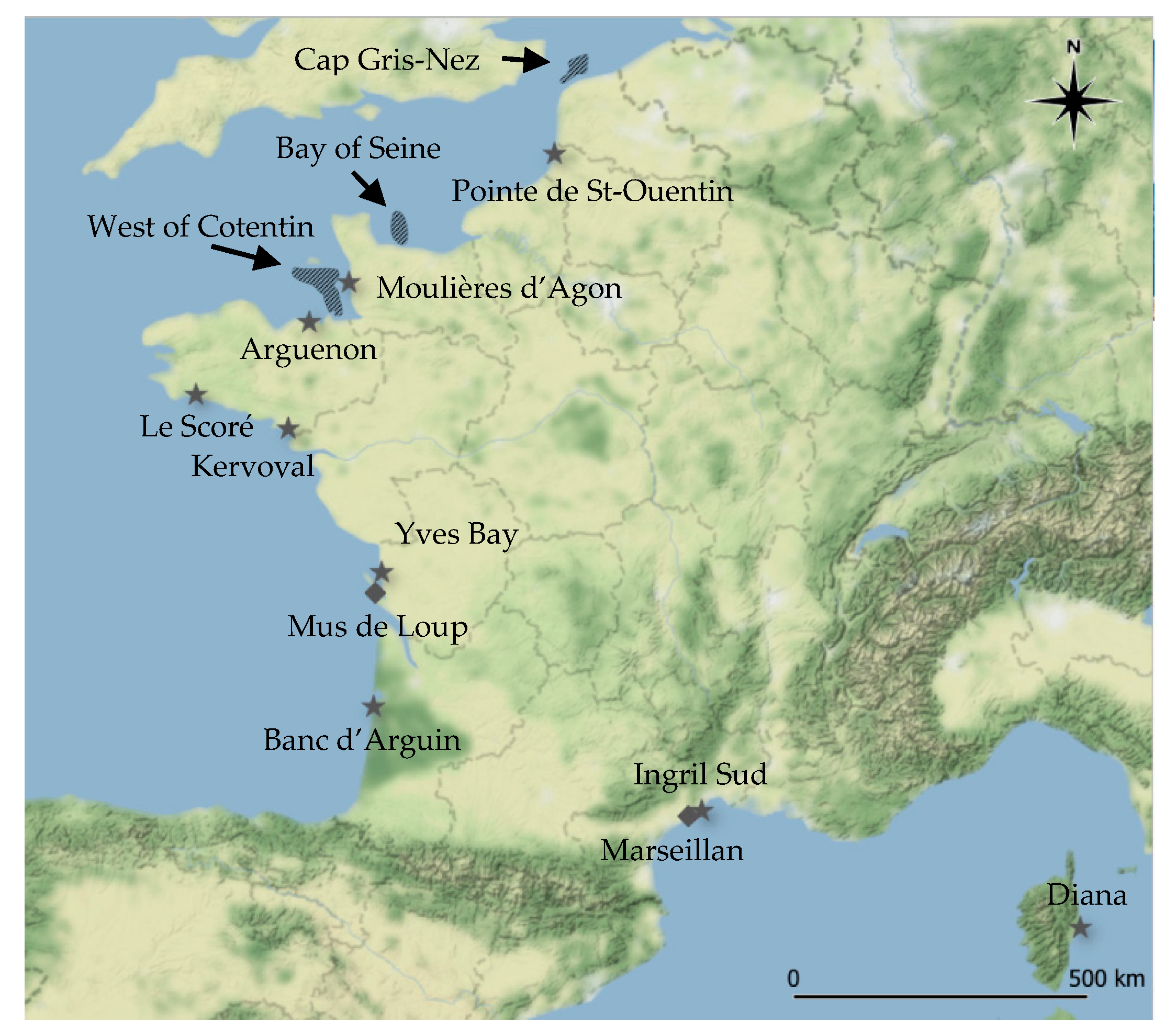
| Serial Number | Mussels | Oysters | Whelks |
|---|---|---|---|
| 1 | +6.0% | +8.9% | +11.6% |
| 2 | +5.2% | +0.7% | +4.5% |
| 3 | +6.7% | −2.8% | +4.9% |
| 4 | −0.8% | −5.7% | +3.3% |
| Mean | +4.3% | +0.3% | +6.1% |
| Shellfish | Method Characteristic | TTX Concentration (µg/kg) | |||
|---|---|---|---|---|---|
| 15.0 | 20.0 | 44.0 | 100.0 | ||
| Mussels | Recovery (%) | 101 | 98 | 97 | 93 |
| Repeatability (CVr, %) | 6.7 | 9.1 | 9.0 | 5.8 | |
| Intermediate precision (CVIP, %) | 8.3 | 9.1 | 9.0 | 7.2 | |
| Oysters | Recovery (%) | 111 | 101 | 101 | 99 |
| Repeatability (CVr, %) | 10.2 | 7.2 | 7.3 | 3.6 | |
| Intermediate precision (CVIP, %) | 10.2 | 7.2 | 8.7 | 7.7 | |
| Whelks | Recovery (%) | 79 | 79 | 80 | 74 |
| Repeatability (CVr, %) | 19.8 | 10.7 | 6.6 | 10.9 | |
| Intermediate precision (CVIP, %) | 19.8 | 15.1 | 13.9 | 12.2 | |
| Sample Number | Shellfish | Seaboard | Sampling Site | Sampling Month | Results | |
|---|---|---|---|---|---|---|
| Method | Sensitive Variant | |||||
| 18 BM 107 | Mussels (Bivalves) | Mediterranean Sea | Ingril Lagoon | May | < LOQ 1 | 11.2 µg TTX/kg |
| 18 BM 139 | Mussels (Bivalves) | Atlantic Ocean | Banc d’Arguin | July | < LOQ 1 | < LOQ 2 |
| 18 BM 154 | Mussels (Bivalves) | English Channel | Moulières d’Agon | July | < LOQ 1 | < LOQ 2 |
| 17 ET2M 773 | Whelks (Gastropods) | English Channel | West of Cotentin | November | < LOQ 1 | < LOQ 2 |
| Method Characteristic | Mussels | Oysters | Whelks |
|---|---|---|---|
| Recovery (%) | 91 | 100 | 93 |
| Repeatability (CVr, %) | 8.5 | 9.9 | 12.9 |
| Intermediate precision (CVIP, %) | 12.0 | 23.0 | 24.0 |
| Compounds | Precursor Ion (m/z) | Collision Energy (V) | Product Ion 1 (m/z) |
|---|---|---|---|
| TTX/4-epi-TTX | 320.1 | 23 | 302.1(Q) |
| 35 | 162.1 (q) | ||
| 5,6,11-trideoxy-TTX | 272.1 | 30 | 254.1 (Q) |
| 35 | 162.1 (q) | ||
| 11-norTTX-6(R/S)-ol | 290.1 | 30 | 272.1 (Q) |
| 35 | 162.1 (q) | ||
| 4,9-anhydro-TTX | 302.0 | 24 | 256.0 (Q) |
| 35 | 162.1 (q) | ||
| 5-deoxy-TTX/11-deoxy-TTX | 304.1 | 30 | 286.1 (Q) |
| 30 | 176.0 (q) | ||
| Arginine | 175.0 | 30 | 70.0 (Q) |
| 30 | 60.0 (q) | ||
| Hydroxy arginine | 191.0 | 30 | 86.0 (Q) |
| 30 | 68.0 (q) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hort, V.; Arnich, N.; Guérin, T.; Lavison-Bompard, G.; Nicolas, M. First Detection of Tetrodotoxin in Bivalves and Gastropods from the French Mainland Coasts. Toxins 2020, 12, 599. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12090599
Hort V, Arnich N, Guérin T, Lavison-Bompard G, Nicolas M. First Detection of Tetrodotoxin in Bivalves and Gastropods from the French Mainland Coasts. Toxins. 2020; 12(9):599. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12090599
Chicago/Turabian StyleHort, Vincent, Nathalie Arnich, Thierry Guérin, Gwenaëlle Lavison-Bompard, and Marina Nicolas. 2020. "First Detection of Tetrodotoxin in Bivalves and Gastropods from the French Mainland Coasts" Toxins 12, no. 9: 599. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12090599





