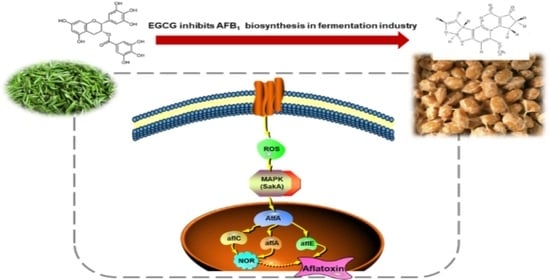
EGCG Alleviates Oxidative Stress and Inhibits Aflatoxin B1 Biosynthesis via MAPK Signaling Pathway
Abstract
:1. Introduction
2. Results
2.1. EGCG Inhibiting AFB1 Synthesis, but Not Mycelial Growth
2.2. EGCG Blocking ROS Accumulation
2.3. Transcriptome Analysis and qRT-PCR
2.4. EGCG Arresting SakA Phosphorylation in MAPK Signaling Pathway
2.5. EGCG Inhibiting NOR Production
2.6. Preventing AFB1 Production in Fermented Soybean Products with EGCG
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Strain
5.2. Determination of Fungal Growth and AFB1 Biosynthesis
5.3. Intracellular ROS Determination
5.4. RNA-Seq and RT-qPCR Validation
5.5. Western Blot Analysis
5.6. Anti-Aflatoxigenic Evaluation of EGCG in Fermented Soybean Products
5.7. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adeyeye, S.A.O. Aflatoxigenic fungi and mycotoxins in food: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 709–721. [Google Scholar] [CrossRef]
- Roze, L.V.; Hong, S.-Y.; Linz, J.E. Aflatoxin Biosynthesis: Current Frontiers. Annu. Rev. Food Sci. Technol. 2013, 4, 293–311. [Google Scholar] [CrossRef]
- Tian, F.; Lee, S.Y.; Woo, S.Y.; Choi, H.Y.; Park, S.B.; Chun, H.S. Effect of plant-based compounds on the antifungal and antiaflatoxigenic efficiency of strobilurins against Aspergillus flavus. J. Hazard. Mater. 2021, 415, 125663. [Google Scholar] [CrossRef] [PubMed]
- Moral, J.; Garcia-Lopez, M.T.; Camiletti, B.X.; Jaime, R.; Michailides, T.J.; Bandyopadhyay, R.; Ortega-Beltran, A. Present Status and Perspective on the Future Use of Aflatoxin Biocontrol Products. Agronomy 2020, 10, 491. [Google Scholar] [CrossRef] [Green Version]
- Amaike, S.; Keller, N.P. Aspergillus flavus. Annu. Rev. Phytopathol. 2011, 49, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ma, L.; Jin, J.; Zheng, M.; Pan, L.; Zhao, Y.; Sun, X.; Liu, Y.; Xing, F. The anti-aflatoxigenic mechanism of cinnamaldehyde in Aspergillus flavus. Sci. Rep. 2019, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Caceres, I.; Al Khoury, A.; El Khoury, R.; Lorber, S.; Oswald, I.P.; El Khoury, A.; Atoui, A.; Puel, O.; Bailly, J.-D. Aflatoxin Biosynthesis and Genetic Regulation: A Review. Toxins 2020, 12, 150. [Google Scholar] [CrossRef] [Green Version]
- WHO; IARC. Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins. IARC Monogr. Eval. Carcinog. Risks Hum. 1993, 56, 489–521. [Google Scholar]
- Wu, F. Perspective: Time to face the fungal threat. Nature 2014, 516, S7. [Google Scholar] [CrossRef]
- Li, X.; Ren, Y.; Jing, J.; Jiang, Y.; Yang, Q.; Luo, S.; Xing, F. The inhibitory mechanism of methyl jasmonate on Aspergillus flavus growth and aflatoxin biosynthesis and two novel transcription factors are involved in this action. Food Res. Int. 2021, 140, 110051. [Google Scholar] [CrossRef]
- Grintzalis, K.; Vernardis, S.; Klapa, M.I.; Georgiou, C.D. Role of Oxidative Stress in Sclerotial Differentiation and Aflatoxin B1 Biosynthesis in Aspergillus flavus. Appl. Environ. Microbiol. 2014, 80, 5561–5571. [Google Scholar] [CrossRef] [Green Version]
- Fountain, J.C.; Bajaj, P.; Pandey, M.; Nayak, S.N.; Yang, L.; Kumar, V.; Jayale, A.S.; Chitikineni, A.; Zhuang, W.; Scully, B.T.; et al. Oxidative stress and carbon metabolism influence Aspergillus flavus transcriptome composition and secondary metabolite production. Sci. Rep. 2016, 6, 38747. [Google Scholar] [CrossRef] [Green Version]
- Guan, X.; Zhao, Y.; Liu, X.; Shang, B.; Xing, F.; Zhou, L.; Wang, Y.; Zhang, C.; Bhatnagar, D.; Liu, Y. The bZIP transcription factor Afap1 mediates the oxidative stress response and aflatoxin biosynthesis in Aspergillus flavus. Rev. Argent. Microbiol. 2019, 51, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Jayashree, T.; Subramanyam, C. Oxidative stress as a prerequisite for aflatoxin production by Aspergillus parasiticus. Free Radic. Biol. Med. 2000, 29, 981–985. [Google Scholar] [CrossRef]
- Sun, Q.; Shang, B.; Wang, L.; Lu, Z.; Liu, Y. Cinnamaldehyde inhibits fungal growth and aflatoxin B1 biosynthesis by modulating the oxidative stress response of Aspergillus flavus. Appl. Microbiol. Biotechnol. 2016, 100, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Jin, J.; Zheng, M.; Yang, Q.; Xing, F. Ethanol Inhibits Aflatoxin B1 Biosynthesis in Aspergillus flavus by Up-Regulating Oxidative Stress-Related Genes. Front. Microbiol. 2020, 10, 2946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.-Q.; Jiang, H.-F.; Zhou, Y.-Q.; Lei, Y.; Wang, S.-Y.; Liao, B.-S. Ethylene inhibited aflatoxin biosynthesis is due to oxidative stress alleviation and related to glutathione redox state changes in Aspergillus flavus. Int. J. Food Microbiol. 2009, 130, 17–21. [Google Scholar] [CrossRef]
- Zhao, X.; Zhi, Q.-Q.; Li, J.-Y.; Keller, N.P.; He, Z.-M. The Antioxidant Gallic Acid Inhibits Aflatoxin Formation in Aspergillus flavus by Modulating Transcription Factors FarB and CreA. Toxins 2018, 10, 270. [Google Scholar] [CrossRef] [Green Version]
- Caceres, I.; El Khoury, R.; Bailly, S.; Oswald, I.P.; Puel, O.; Bailly, J.-D. Piperine inhibits aflatoxin B1 production in Aspergillus flavus by modulating fungal oxidative stress response. Fungal Genet. Biol. 2017, 107, 77–85. [Google Scholar] [CrossRef]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef] [Green Version]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef]
- Mo, H.Z.; Zhang, H.; Wu, Q.H.; Bin Hu, L. Inhibitory effects of tea extract on aflatoxin production by Aspergillus flavus. Lett. Appl. Microbiol. 2013, 56, 462–466. [Google Scholar] [CrossRef]
- Qin, G.; Gopalan-Kriczky, P.; Su, J.; Ning, Y.; Lotlikar, P.D. Inhibition of aflatoxin B1-induced initiation of hepatocarcinogenesis in the rat by green tea. Cancer Lett. 1997, 112, 149–154. [Google Scholar] [CrossRef]
- Pakshir, K.; Mirshekari, Z.; Nouraei, H.; Zareshahrabadi, Z.; Zomorodian, K.; Khodadadi, H.; Hadaegh, A. Mycotoxins Detection and Fungal Contamination in Black and Green Tea by HPLC-Based Method. J. Toxicol. 2020, 2020, 2456210. [Google Scholar] [CrossRef]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- Lara-Rojas, F.; Sánchez, O.; Kawasaki, L.; Aguirre, J. Aspergillus nidulans transcription factor AtfA interacts with the MAPK SakA to regulate general stress responses, development and spore functions. Mol. Microbiol. 2011, 80, 436–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, J.W.; Lee, L.S.; Vinnett, C. The correlation of aflatoxin and norsolorinic acid production. J. Am. Oil Chem. Soc. 1971, 48, 368–370. [Google Scholar] [CrossRef]
- Liu, B.; Yan, W. Lipophilization of EGCG and effects on antioxidant activities. Food Chem. 2019, 272, 663–669. [Google Scholar] [CrossRef]
- Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial effects of green tea: A literature review. Chin. Med. 2010, 5, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Yu, J.; Mahoney, N.; Chan, K.L.; Molyneux, R.J.; Varga, J.; Bhatnagar, D.; Cleveland, T.E.; Nierman, W.C.; Campbell, B.C. Elucidation of the functional genomics of antioxidant-based inhibition of aflatoxin biosynthesis. Int. J. Food Microbiol. 2008, 122, 49–60. [Google Scholar] [CrossRef]
- Reverberi, M.; Punelli, M.; Smith, C.A.; Zjalic, S.; Scarpari, M.; Scala, V.; Cardinali, G.; Aspite, N.; Pinzari, F.; Payne, G.A.; et al. How Peroxisomes Affect Aflatoxin Biosynthesis in Aspergillus Flavus. PLoS ONE 2012, 7, e48097. [Google Scholar] [CrossRef]
- Dröge-Laser, W.; Snoek, B.L.; Snel, B.; Weiste, C. The Arabidopsis bZIP transcription factor family—An update. Curr. Opin. Plant. Biol. 2018, 45, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Wee, J.; Hong, S.-Y.; Roze, L.V.; Day, D.M.; Chanda, A.; Linz, J.E. The Fungal bZIP Transcription Factor AtfB Controls Virulence-Associated Processes in Aspergillus parasiticus. Toxins 2017, 9, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roze, L.V.; Chanda, A.; Wee, J.; Awad, D.; Linz, J.E. Stress-related Transcription Factor AtfB Integrates Secondary Metabolism with Oxidative Stress Response in Aspergilli. J. Biol. Chem. 2011, 286, 35137–35148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cano, A.; Ettcheto, M.; Chang, J.-H.; Barroso, E.; Espina, M.; Kühne, B.A.; Barenys, M.; Auladell, C.; Folch, J.; Souto, E.B.; et al. Dual-drug loaded nanoparticles of Epigallocatechin-3-gallate (EGCG)/Ascorbic acid enhance therapeutic efficacy of EGCG in a APPswe/PS1dE9 Alzheimer’s disease mice model. J. Control. Release 2019, 301, 62–75. [Google Scholar] [CrossRef]
- Yang, G.; Cao, X.; Ma, G.; Qin, L.; Wu, Y.; Lin, J.; Ye, P.; Yuan, J.; Wang, S. MAPK pathway-related tyrosine phosphatases regulate development, secondary metabolism and pathogenicity in fungus Aspergillus flavus. Environ. Microbiol. 2020, 22, 5232–5247. [Google Scholar] [CrossRef]
- Manfiolli, A.O.; Siqueira, F.S.; Reis, T.; Van Dijck, P.; Schrevens, S.; Hoefgen, S.; Föge, M.; Straßburger, M.; de Assis, L.J.; Heinekamp, T.; et al. Mitogen-Activated Protein Kinase Cross-Talk Interaction Modulates the Production of Melanins in Aspergillus fumigatus. mBio 2019, 10, e00215-19. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Soto, D.; Ruiz-Herrera, J. Functional analysis of the MAPK pathways in fungi. Rev. Iberoam. Micol. 2017, 34, 192–202. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [Green Version]
- Garrido-Bazán, V.; Jaimes-Arroyo, R.; Sánchez, O.; Lara-Rojas, F.; Aguirre, J. SakA and MpkC Stress MAPKs Show Opposite and Common Functions During Stress Responses and Development in Aspergillus nidulans. Front. Microbiol. 2018, 9, 2518. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Wang, H.; Zhang, Y.; Yang, Z.; Sun, X. Inhibition of non-toxigenic Aspergillus niger FS10 isolated from Chinese fermented soybean on growth and aflatoxin B1 production by Aspergillus flavus. Food Control. 2013, 32, 359–365. [Google Scholar] [CrossRef]
- Keller, N.P.; Nesbitt, C.; Sarr, B.; Phillips, T.D.; Burow, G.B. pH Regulation of Sterigmatocystin and Aflatoxin Biosynthesis in Aspergillus spp. Phytopathology 1997, 87, 643–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.F.; Mylona, P.; Miedema, H.; Torres, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jones, J.; et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003, 422, 442–446. [Google Scholar] [CrossRef]
- Paul, S.; Diekema, D.; Moye-Rowley, W.S. Contributions of both ATP-Binding Cassette Transporter and Cyp51A Proteins Are Essential for Azole Resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2017, 61, e02748-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Dong, M.; Bo, L.; Li, C.; Liu, Q.; Li, Y.; Ma, L.; Xie, Y.; Fu, E.; Mu, D.; et al. Epigallocatechin-3-gallate Ameliorates Seawater Aspiration-Induced Acute Lung Injury via Regulating Inflammatory Cytokines and Inhibiting JAK/STAT1 Pathway in Rats. Mediat. Inflamm. 2015, 2014, 612593. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| GOID | GOTerm | QueryID | Description | p-Value | Enrichment |
|---|---|---|---|---|---|
| GO:0033674 | positive regulation of kinase activity | AFLA_034120 | vacuole-associated enzyme activator complex component Vac14 | 0.001 | 0.09 |
| GO:0033674 | positive regulation of kinase activity | AFLA_046890 | phospholipid metabolism enzyme regulator | 0.001 | 24.59 |
| GO:0006970 | response to osmotic stress | AFLA_094490 | protein phosphatase 2C | 0.01 | 4.28 |
| GO:0006970 | response to osmotic stress | AFLA_136040 | chitin synthase | 0.01 | 4.28 |
| GO:0006970 | response to osmotic stress | AFLA_136030 | chitin synthase ChsE | 0.01 | 4.28 |
| GO:0006970 | response to osmotic stress | AFLA_013690 | chitin synthase C | 0.01 | 4.28 |
| GO:0050850 | positive regulation of calcium-mediated signaling | AFLA_051240 | MAP kinase kinase (Mkk2) MAP | 0.02 | 9.84 |
| GO:0050850 | positive regulation of calcium-mediated signaling | AFLA_068440 | calcium channel subunit Cch1 | 0.02 | 9.84 |
| GO:0043619 | regulation of transcription from RNA polymerase II promoter in response to oxidative stress | AFLA_106830 | sensor histidine kinase/response regulator Fos-1/TcsA | 0.02 | 8.20 |
| GO:0030437 | ascospore formation | AFLA_137060 | plasma membrane SNARE protein (Sec9) | 0.04 | 2.67 |
| GO:0030437 | ascospore formation | AFLA_052430 | G protein complex alpha subunit GpaB | 0.04 | 2.67 |
| GO:0030437 | ascospore formation | AFLA_030580 | C2H2 transcription factor PacC | 0.04 | 2.67 |
| GO:0007186 | G-protein coupled receptor signaling pathway | AFLA_018540 | G protein complex alpha subunit GpaB | 0.04 | 6.15 |
| GO:0007186 | G-protein coupled receptor signaling pathway | AFLA_061620 | a-pheromone receptor PreA | 0.04 | 3.33 |
| GO:0006979 | response to oxidative stress | AFLA_106830 | sensor histidine kinase/response regulator Fos-1/TcsA | 0.04 | 3.07 |
| GO:0006979 | response to oxidative stress | AFLA_034380 | spore-specific catalase CatA | 0.04 | 3.07 |
| GO:0006979 | response to oxidative stress | AFLA_056170 | mycelial catalase Cat1 | 0.04 | 3.07 |
| GO:0000173 | inactivation of MAPK activity involved in osmosensory signaling pathway | AFLA_094490 | protein phosphatase 2C | 0.04 | 24.59 |
| GO:0010619 | adenylate cyclase-activating glucose-activated G-protein coupled receptor signaling pathway | AFLA_018540 | G protein complex alpha subunit GpaB | 0.04 | 24.59 |
| GO:0042992 | negative regulation of transcription factor import into nucleus | AFLA_032170 | protein serine/threonine kinase (Ran1) | 0.04 | 24.59 |
| GO:0043550 | regulation of lipid kinase activity | AFLA_034120 | vacuole-associated enzyme activator complex component Vac14 | 0.04 | 24.59 |
| GO:0035970 | peptidyl-threonine dephosphorylation | AFLA_094490 | protein phosphatase 2C | 0.04 | 24.59 |
| GO:0071619 | phosphorylation of RNA polymerase II C-terminal domain serine 2 residues | AFLA_005900 | protein kinase | 0.04 | 24.59 |
| GO:0034486 | vacuolar transmembrane transport | AFLA_112050 | cation chloride cotransporter | 0.04 | 24.59 |
| Pathway ID | Regulated | Pathway Term | Gene Number |
|---|---|---|---|
| PATH:04011 | Down | MAPK signaling pathway-yeast | 9 |
| PATH:00520 | Down | Amino sugar and nucleotide sugar metabolism | 9 |
| PATH:00380 | Down | Tryptophan metabolism | 7 |
| PATH:00500 | Down | Starch and sucrose metabolism | 8 |
| PATH:00340 | Down | Histidine metabolism | 3 |
| PATH:00630 | Down | Glyoxylate and dicarboxylate metabolism | 5 |
| PATH:00280 | Up | Valine, leucine and isoleucine degradation | 7 |
| PATH:00350 | Up | Tyrosine metabolism | 8 |
| PATH:01040 | Up | Biosynthesis of unsaturated fatty acids | 4 |
| PATH:01100 | Up | Metabolic pathways | 53 |
| PATH:00071 | Up | Fatty acid degradation | 5 |
| PATH:00410 | Up | beta-Alanine metabolism | 4 |
| PATH:00340 | Up | Histidine metabolism | 3 |
| PATH:00592 | Up | alpha-Linolenic acid metabolism | 2 |
| PATH:00053 | Up | Ascorbate and aldarate metabolism | 2 |
| Gene ID | Gene Name and Product | Style |
|---|---|---|
| AFLA_139310 | aflE/norA/aad/adh2/NOR (norsolorinic acid reductase) | down a |
| AFLA_139320 | aflJ/estA/esterase | down |
| AFLA_139360 | aflR/apa2/afl2/C6 transcription factor | down |
| AFLA_139380 | aflA/fas2/hexA/fatty acid synthase alpha subunit | down a |
| AFLA_139410 | aflC/pksA/pksL1/polyketide synthase | down a |
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| aflA | CGTCACGCTCTATACAATTTGCT | ATCCGATAAAGTTGCCTAGTTCC |
| aflC | TGTCAGACCACAAACGCACCT | CATCTCACAGAACGCCCTCAA |
| aflE | CTATCATCTAGCGCCGGTGT | CCATCTTTCGCTATCGCCTCC |
| aflJ | CTGCGTTGCTACACTCCCC | ATCACGCGGCAGAAACCATC |
| aflR | GCAGTCAATGGAACACGGAAAC | CCTGAAACGGTGGTAGTGGG |
| atfA | AAACTGAAGACTCCCAGGCGC | AAACTGAAGACTCCCAGGCGC |
| actin | AGGACTCTTATGTCGGTGATG | CGGTTGGACTTAGGGTTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Peng, S.; Guo, R.; Yao, L.; Mo, H.; Li, H.; Song, H.; Hu, L. EGCG Alleviates Oxidative Stress and Inhibits Aflatoxin B1 Biosynthesis via MAPK Signaling Pathway. Toxins 2021, 13, 693. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13100693
Xu D, Peng S, Guo R, Yao L, Mo H, Li H, Song H, Hu L. EGCG Alleviates Oxidative Stress and Inhibits Aflatoxin B1 Biosynthesis via MAPK Signaling Pathway. Toxins. 2021; 13(10):693. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13100693
Chicago/Turabian StyleXu, Dan, Shurui Peng, Rui Guo, Lishan Yao, Haizhen Mo, Hongbo Li, Hongxin Song, and Liangbin Hu. 2021. "EGCG Alleviates Oxidative Stress and Inhibits Aflatoxin B1 Biosynthesis via MAPK Signaling Pathway" Toxins 13, no. 10: 693. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13100693