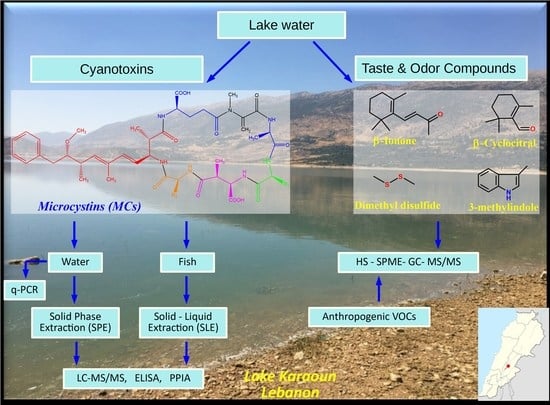
Investigation of the Occurrence of Cyanotoxins in Lake Karaoun (Lebanon) by Mass Spectrometry, Bioassays and Molecular Methods
Abstract
:1. Introduction
2. Results
2.1. Water Quality and Diversity of Cyanobacteria in Lake Karaoun
2.2. Occurrence of Cyanotoxins (CTs) in Lake Karaoun
2.2.1. LC-MS/MS Analysis of Water Samples
2.2.2. ELISA and PPIA Analysis of Water Samples
2.2.3. Molecular Detection of Cyanobacteria and Cyanotoxin Genes with qPCR
2.2.4. LC-MS/MS Analysis of MCs in Fish Samples
2.3. Taste and Odor (T&O) and Volatile Organic Compounds (VOCs)
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Site and Sampling
5.2. Chemicals and Reagents
5.3. Microscopic Examination
5.4. LC-MS/MS Analysis of Cyanotoxins
5.4.1. Sample Preparation of Water Samples
5.4.2. Sample Preparation of Fish Samples
5.4.3. Determination by LC-MS/MS
5.5. ELISA for Microcystins
5.6. Protein Phosphatase Inhibition Assay (PPIA) for Microcystins
5.7. qPCR Assay for Total Cyanobacteria and Cyanotoxin Genes
5.8. GC-MS Analysis of Volatile Compounds
5.8.1. Untargeted Screening of Cyanobacterial Volatiles and T&O Compounds
5.8.2. Quantitative Determination of VOCs
5.9. Method Validation and Quality Control Procedures
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouaïcha, N.; Miles, C.; Beach, D.; Labidi, Z.; Djabri, A.; Benayache, N.; Nguyen-Quang, T. Structural diversity, characterization and toxicology of microcystins. Toxins 2019, 11, 714. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Shen, D.; Fang, D. Nodularins in poisoning. Clin. Chim. Acta 2013, 425, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Van Apeldoorn, M.E.; Van Egmond, H.P.; Speijers, G.J.A.; Bakker, G.J.I. Toxins of cyanobacteria. Mol. Nutr. Food Res. 2007, 51, 7–60. [Google Scholar] [CrossRef] [PubMed]
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef]
- De La Cruz, A.A.; Hiskia, A.; Kaloudis, T.; Chernoff, N.; Hill, D.; Antoniou, M.G.; He, X.; Loftin, K.; O’Shea, K.; Zhao, C.; et al. A review on cylindrospermopsin: The global occurrence, detection, toxicity and degradation of a potent cyanotoxin. Environ. Sci. Process Impacts 2013, 15, 1979–2003. [Google Scholar] [CrossRef]
- Osswald, J.; Rellán, S.; Gago, A.; Vasconcelos, V. Toxicology and detection methods of the alkaloid neurotoxin produced by cyanobacteria, anatoxin-a. Environ. Int. 2007, 33, 1070–1089. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Antoniou, M.G.; He, X.; Dionysiou, D.D.; de la Cruz, A.A.; Tsimeli, K.; Triantis, T.; Hiskia, A.; Kaloudis, T.; Williams, C.; et al. Sources and Occurrence of Cyanotoxins Worldwide. In Xenobiotics in the Urban Water Cycle: Mass Flows, Environmental Processes, Mitigation and Treatment Strategies; Fatta-Kassinos, D., Bester, K., Kümmerer, K., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 101–127. [Google Scholar]
- Sivonen, K.; Jones, G. Cyanobacterial toxins. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring, and Management; Chorus, I., Bartram, J., Eds.; E & FN Spon: London, UK, 1999; pp. 41–111. [Google Scholar]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Genet. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Svirčev, Z.; Lalić, D.; Savić, G.B.; Tokodi, N.; Backović, D.D.; Chen, L.; Meriluoto, J.; Codd, G.A. Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Arch. Toxicol. 2019, 93, 2429–2481. [Google Scholar] [CrossRef] [PubMed]
- Chorus, I.; Welker, M. Exposure to cyanotoxins: Understanding it and short-term interventions to prevent it. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management, 2nd ed.; Chorus, I., Welker, M., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 295–400. [Google Scholar]
- Osseiran, K.; Kabakian, V. Hydropower in Lebanon; History and Prospects; United Nations Development Pogramme CEDRO (UNDP/CEDRO). 2013. Available online: http://www.cedro-undp.org/content/uploads/Publication/141009092113199~Exchange%204.pdf (accessed on 7 July 2021).
- Yazbek, H.; Fadel, A.; Slim, K. Facts about the degradation of Lake Qaraoun, Lebanon, and cyanobacterial harmful algal blooms (HABS). J. Environ. Hydrol. 2019, 27, 1. [Google Scholar]
- Atoui, A.; Hafez, H.; Slim, K. Occurrence of toxic cyanobacterial blooms for the first time in Lake Karaoun, Lebanon. Water Environ. J. 2012, 27, 42–49. [Google Scholar] [CrossRef]
- Fadel, A.; Lemaire, B.J.; Atoui, A.; Leite, B.V.; Amacha, N.; Slim, K.; Tassin, B. First assessment of the ecological status of Karaoun reservoir, Lebanon. Lakes Reserv. Res. Manag. 2014, 19, 142–157. [Google Scholar] [CrossRef] [Green Version]
- Fadel, A.; Slim, K. Evaluation of the Physicochemical and Environmental Status of Qaraaoun Reservoir. In The Litani River, Lebanon: An Assessment and Current Challenges; Shaban, A., Hamzé, M., Eds.; Springer: Cham, Switzerland, 2018; pp. 71–86. [Google Scholar]
- Fadel, A.; Atoui, A.; Lemaire, B.J.; Vinçon-Leite, B.; Slim, K. Dynamics of the toxin cylindrospermopsin and the cyanobacterium chrysosporum (aphanizomenon) ovalisporum in a mediterranean eutrophic reservoir. Toxins 2014, 6, 3041–3057. [Google Scholar] [CrossRef] [Green Version]
- Watson, S.B. Aquatic taste and odor: A primary signal of drinking-water integrity. J. Toxicol. Environ. Health Part A 2004, 67, 1779–1795. [Google Scholar] [CrossRef]
- McGuire, M.J. Off-flavor as the consumer’s measure of drinking water safety. Water Sci. Technol. 1995, 31, 1–8. [Google Scholar] [CrossRef]
- Stefan, M.I. (Ed.) Advanced Oxidation Processes for Water Treatment—Fundamentals and Applications; IWA Publishing: London, UK, 2018; pp. 1–686. [Google Scholar]
- Sukenik, A.; Carmeli, S.; Hadas, O.; Leibovici, E.; Malinsky-Rushansky, N.; Parparov, R.; Pinkas, R.; Viner-Mozzini, Y.; Wynne, D. Water pollutants. In Lake Kinneret: Ecology and Management; Zohary, T., Sukenik, A., Berman, T., Nishri, A., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 577–606. [Google Scholar]
- Zervou, S.-K.; Christophoridis, C.; Kaloudis, T.; Triantis, T.; Hiskia, A. New SPE-LC-MS/MS method for simultaneous determination of multi-class cyanobacterial and algal toxins. J. Hazard. Mater. 2017, 323, 56–66. [Google Scholar] [CrossRef]
- Christophoridis, C.; Zervou, S.-K.; Manolidi, K.; Katsiapi, M.; Moustaka-Gouni, M.; Kaloudis, T.; Triantis, T.M.; Hiskia, A. Occurrence and diversity of cyanotoxins in Greek lakes. Sci. Rep. 2018, 8, 17877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Rai, P.K.; Jeon, Y.J.; Kim, K.-H.; Kwon, E.E. The role of algae and cyanobacteria in the production and release of odorants in water. Environ. Pollut. 2017, 227, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R.; Pinto, E.; Torres, M.A.; Dörr, F.; Mazur-Marzec, H.; Szubert, K.; Tartaglione, L.; Dell’Aversano, C.; Miles, C.O.; Beach, D.G.; et al. CyanoMetDB, a comprehensive public database of secondary metabolites from cyanobacteria. Water Res. 2021, 196, 117017. [Google Scholar] [CrossRef] [PubMed]
- Birbeck, J.A.; Westrick, J.A.; O’Neill, G.M.; Spies, B.; Szlag, D.C. Comparative analysis of microcystin prevalence in Michigan lakes by online concentration LC/MS/MS and ELISA. Toxins 2019, 11, 13. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Rautela, A.; Kesari, V.; Szlag, D.; Westrick, J.; Kumar, S. Recent developments in the methods of quantitative analysis of microcystins. J. Biochem. Mol. Toxicol. 2020, 34, e22582. [Google Scholar] [CrossRef]
- Sanseverino, I.; Conduto António, D.; Loos, R.; Lettieri, T. Cyanotoxins: Methods and Approaches for Their Analysis and Detection; EUR 28624; Joint Research Centre (JRC), European Union. 2017. Available online: https://0-doi-org.brum.beds.ac.uk/10.2760/36186 (accessed on 7 July 2021).
- Gaget, V.; Lau, M.; Sendall, B.; Froscio, S.; Humpage, A.R. Cyanotoxins: Which detection technique for an optimum risk assessment? Water Res. 2017, 118, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Hardy, F.J.; Johnson, A.; Hamel, K.; Preece, E. Cyanotoxin bioaccumulation in freshwater fish, Washington State, USA. Environ. Monit. Assess. 2015, 187. [Google Scholar] [CrossRef] [PubMed]
- Haddad, S.P.; Bobbitt, J.M.; Taylor, R.; Lovin, L.; Conkle, J.L.; Chambliss, C.K.; Brooks, B.W. Determination of microcystins, nodularin, anatoxin-a, cylindrospermopsin, and saxitoxin in water and fish tissue using isotope dilution liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2019, 1599, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Franklin, R.; Garman, G.; McIninch, S.; Porter, A.; Bukaveckas, P. Exposure to the cyanotoxin microcystin arising from interspecific differences in feeding habits among fish and shellfish in the James River Estuary, Virginia. Environ. Sci. Technol. 2014, 48, 5194–5202. [Google Scholar] [CrossRef]
- Wituszynski, D.M.; Hu, C.; Zhang, F.; Chaffin, J.; Lee, J.; Ludsin, S.A.; Martin, J.F. Microcystin in Lake Erie fish: Risk to human health and relationship to cyanobacterial blooms. J. Great Lakes Res. 2017, 43, 1084–1090. [Google Scholar] [CrossRef] [Green Version]
- Wynne, T.T.; Stumpf, R.P. Spatial and temporal patterns in the seasonal distribution of toxic cyanobacteria in western Lake Erie from 2002–2014. Toxins 2015, 7, 1649–1663. [Google Scholar] [CrossRef] [Green Version]
- Kozlowsky-Suzuki, B.; Wilson, A.E.; Ferrão-Filho, A.D.S. Biomagnification or biodilution of microcystins in aquatic foodwebs? Meta-analyses of laboratory and field studies. Harmful Algae 2012, 18, 47–55. [Google Scholar] [CrossRef]
- Adamovský, O.; Kopp, R.; Hilscherová, K.; Babica, P.; Palíková, M.; Pašková, V.; Navrátil, S.; Maršálek, B.; Bláha, L. Microcystin kinetics (bioaccumulation and elimination) and biochemical responses in common carp (Cyprinus carpio) and silver carp (hypophthalmichthys molitrix) exposed to toxic cyanobacterial blooms. Environ. Toxicol. Chem. 2007, 26, 2687–2693. [Google Scholar] [CrossRef] [Green Version]
- Pollingher, U. Phytoplankton periodicity in a subtropical lake (Lake Kinneret, Israel). Hydrobiologia 1986, 138, 127–138. [Google Scholar] [CrossRef]
- Berman, T.; Shteinman, B. Phytoplankton development and turbulent mixing in Lake Kinneret (1992–1996). J. Plankton Res. 1998, 20, 709–726. [Google Scholar] [CrossRef] [Green Version]
- Pollingher, U.; Zohary, T.; Hadas, O.; Yacobi, Y.Z.; Berman, T. Aphanizomenon ovalisporum (Forti) in Lake Kinneret, Israel. J. Plankton Res. 1998, 20, 1321–1339. [Google Scholar] [CrossRef]
- Du, X.; Liu, H.; Yuan, L.; Wang, Y.; Ma, Y.; Wang, R.; Chen, X.; Losiewicz, M.D.; Guo, H.; Zhang, H. The diversity of cyanobacterial Toxins on structural characterization, distribution and identification: A systematic review. Toxins 2019, 11, 530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triantis, T.; Tsimeli, K.; Kaloudis, T.; Thanassoulias, N.; Lytras, E.; Hiskia, A. Development of an integrated laboratory system for the monitoring of cyanotoxins in surface and drinking waters. Toxicon 2010, 55, 979–989. [Google Scholar] [CrossRef]
- Jüttner, F. Dynamics of the volatile organic substances associated with cyanobacteria and algae in a eutrophic shallow lake. Appl. Environ. Microbiol. 1984, 47, 814–820. [Google Scholar] [CrossRef] [Green Version]
- Jüttner, F.; Watson, S.B.; Von Elert, E.; Köster, O. β-Cyclocitral, a grazer defence signal unique to the cyanobacterium microcystis. J. Chem. Ecol. 2010, 36, 1387–1397. [Google Scholar] [CrossRef]
- Buttery, R.G.; Teranishi, R.; Ling, L.C.; Turnbaugh, J.G. Quantitative and sensory studies on tomato paste volatiles. J. Agric. Food Chem. 1990, 38, 336–340. [Google Scholar] [CrossRef]
- Simkin, A.J.; Underwood, B.A.; Auldridge, M.; Loucas, H.M.; Shibuya, K.; Schmelz, E.; Clark, D.G.; Klee, H.J. Circadian regulation of the PhCCD1 carotenoid cleavage dioxygenase controls emission of β-ionone, a fragrance volatile of petunia flowers. Plant Physiol. 2004, 136, 3504–3514. [Google Scholar] [CrossRef] [Green Version]
- Paparella, A.; Shaltiel-Harpaza, L.; Ibdah, M. β-Ionone: Its occurrence and biological function and metabolic engineering. Plants 2021, 10, 754. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Cheng, B.; Liu, M.; Nie, Y. Spatial and temporal variations of taste and odor compounds in surface water, overlying water and sediment of the Western Lake Chaohu, China. Bull. Environ. Contam. Toxicol. 2015, 96, 186–191. [Google Scholar] [CrossRef]
- Leffingwell, J.C.; Leffingwell, D. GRAS Flavor chemicals—Detection thresholds. Perfum. Flavorist 1991, 16, 1–19. [Google Scholar]
- Wang, C.; Yu, J.; Guo, Q.; Sun, D.; Su, M.; An, W.; Zhang, Y.; Yang, M. Occurrence of swampy/septic odor and possible odorants in source and finished drinking water of major cities across China. Environ. Pollut. 2019, 249, 305–310. [Google Scholar] [CrossRef]
- Guo, Q.; Yu, J.; Yang, K.; Wen, X.; Zhang, H.; Yu, Z.; Li, H.; Zhang, D.; Yang, M. Identification of complex septic odorants in Huangpu River source water by combining the data from gas chromatography-olfactometry and comprehensive two-dimensional gas chromatography using retention indices. Sci. Total. Environ. 2016, 556, 36–44. [Google Scholar] [CrossRef]
- Ma, Z.; Niu, Y.; Xie, P.; Chen, J.; Tao, M.; Deng, X. Off-flavor compounds from decaying cyanobacterial blooms of Lake Taihu. J. Environ. Sci. 2013, 25, 495–501. [Google Scholar] [CrossRef]
- Watson, S.B.; Jüttner, F. Malodorous volatile organic sulfur compounds: Sources, sinks and significance in inland waters. Crit. Rev. Microbiol. 2016, 43, 210–237. [Google Scholar] [CrossRef]
- Khiari, D. Managing Cyanotoxins; Water Research Foundation: Denver, CO, USA, 2019; p. 8. Available online: https://www.waterrf.org/sites/default/files/file/2019-12/Cyanotoxins_StateOfTheScience.pdf (accessed on 7 July 2021).
- Chorus, I.; Welker, M. (Eds.) Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 1–839. [Google Scholar]
- ECHA. European Chemicals Agency, REACH Registered Substance Portal. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.033.401 (accessed on 8 July 2021).
- Valentine, D.; Reddy, C.M. Latent hydrocarbons from cyanobacteria. Proc. Natl. Acad. Sci. USA 2015, 112, 13434–13435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, F.Y.; Ong, S.L.; Hu, J. Recent Advances in the use of chemical markers for tracing wastewater contamination in aquatic environment: A review. Water 2017, 9, 143. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Codd, G.A. Co-occurrence of cyanobacteria and cyanotoxins with other environmental health hazards: Impacts and implications. Toxins 2020, 12, 629. [Google Scholar] [CrossRef]
- Slim, K.; Atoui, A.; Elzein, G.; Temsah, M. Effets des facteurs environnementaux sur la qualité de l’eau et la prolifération toxique des cyanobactéries du lac KARAOUN (Liban). LARHYSS J. 2012, 9, 29–43. [Google Scholar]
- International Organization for Standardization. ISO 17943: Water Quality—Determination of Volatile Organic Compounds in Water—Method Using Headspace Solid-Phase Micro-Extraction (HS-SPME) Followed by gas Chromatography-Mass Spectrometry (GC-MS); International Organization for Standardization: Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/61076.html (accessed on 4 October 2021).
- International Organization for Standardization. ISO 17025:General Requirements for the Competence of Testing and Calibration Laboratories; International Organization for Standardization: Geneva, Switzerland, 2017; Available online: https://www.iso.org/publication/PUB100424.html (accessed on 4 October 2021).




| Sample ID | Sample Details | CYN | ATX | dm MC-RR | MC-RR | NOD | MC-YR | MC- HtyR | dm MC-LR | MC-LR | MC-HilR | MC-WR | MC-LA | MC-LY | MC-LW | MC-LF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | S1, 22/08/19 | ND 1/NA 2 | ND/NA | ND/NA | ND/NA | ND/NA | ND/NA | ND/NA | ND/NA | ND/NA | ND/NA | ND/NA | ND/NA | ND/NA | ND/NA | ND/NA |
| 2 | S2, 02/10/19 | ND/ND | ND/ND | ND/1.60 | 0.339/26.4 | ND/ND | ND/1.67 | ND/ND | ND/1.87 | 0.438/91.5 | ND/1.29 | <LOQ 3/ND | ND/ND | <LOQ/3.29 | 0.014/10.5 | <LOQ/7.48 |
| 3 | S4, 02/10/19 | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND |
| 4 | S1, 22/12/19 | ND/ND | ND/ND | ND/0.53 | 0.050/7.42 | ND/ND | 0.187/8.28 | ND/ND | 0.050/0.70 | 0.158/8.43 | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND |
| 5 | S1, 15/01/20 | ND/ND | ND/ND | ND/ND | 0.007/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND |
| 6 | S4, 04/02/20 | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND |
| 7 | S5, 15/02/20 | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND |
| 8 | S5, 17/02/20 | ND/ND | ND/ND | ND/ND | 0.005/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND |
| 9 | S1, 22/02/20 | ND/ND | ND/ND | ND/ND | 0.007/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND |
| 10 | S2, 22/02/20 | ND/ND | ND/ND | ND/ND | 0.008/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND |
| 11 | S1, 15/04/20 | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND |
| 12 | S2, 22/04/20 | ND/NA | ND/NA | ND/NA | 0.090/NA | ND/NA | ND/NA | ND/NA | ND/NA | ND/NA | ND/NA | ND/NA | ND/NA | ND/NA | ND/NA | ND/NA |
| 13 | S4, 22/04/20 | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND |
| 14 | S1, 14/08/20 | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND |
| 15 | S2, 14/08/20 | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND |
| 16 | S3, 09/10/20 | ND/ND | ND/ND | 0.069/ND | 7.0/0.086 | ND/ND | 197/1.94 | ND/ND | 6.9/<LOQ | 209/2.18 | 1.6/ND | ND/ND | 3.0/ND | <LOQ/ND | ND/ND | 0.021/ND |
| ID | SAMPLE DETAILS | Total Extracellular/Intracellular MCs | ||
|---|---|---|---|---|
| LC-MS/MS * sum of MCs, μg/L | ELISA ** μg/L eq.MC-LR | PPIA ** μg/L eq.MC-LR | ||
| 1 | S1, 22/08/2019 | ND/NA | ND/NA | ND/NA |
| 2 | S2, 02/10/2019 | 0.83/146 | NA/97.2 | NA/111 |
| 3 | S4, 02/10/2019 | ND/ND | ND/ND | ND/ND |
| 4 | S1, 22/12/2019 | 0.45/25.4 | 0.13/30.6 | 0.25/16.2 |
| 5 | S1, 15/01/2020 | 0.007/ND | ND/ND | ND/ND |
| 6 | S4, 04/02/2020 | ND/ND | ND/ND | ND/ND |
| 7 | S5, 15/02/2020 | ND/ND | ND/ND | ND/ND |
| 8 | S5, 17/02/2020 | 0.005/ND | 0.12/ND | ND/ND |
| 9 | S1, 22/02/2020 | 0.007/ND | ND/ND | ND/ND |
| 10 | S2, 22/02/2020 | 0.008/ND | ND/ND | ND/ND |
| 11 | S1, 15/04/2020 | ND/ND | 0.25/ND | 0.39/ND |
| 12 | S2, 22/04/2020 | 0.090/NA | 1.80/NA | 0.87/NA |
| 13 | S4, 22/04/2020 | ND/ND | 2.30/ND | 2.32/ND |
| 14 | S1, 14/08/2020 | ND/ND | ND/ND | ND/ND |
| 15 | S2, 14/08/2020 | ND/ND | ND/ND | ND/ND |
| 16 | S3, 9/10/2020 | 425/4.29 | 190/2.41 | 290/2.34 |
| ID | SAMPLE DETAILS | Gene Copies/mL | |||
|---|---|---|---|---|---|
| 16S rRNA (Cyanobacteria) | mcyE (MCs & NODs) | cyrA (CYN) | stxA (STX) | ||
| 1 | S1, 22/08/2019 | NA | NA | NA | NA |
| 2 | S2, 02/10/2019 | 6951375 | 69857 | ND | ND |
| 3 | S4, 02/10/2019 | NA | NA | NA | NA |
| 4 | S1, 22/12/2019 | 9765354 | 127870 | ND | ND |
| 5 | S1, 15/01/2020 | NA | NA | NA | NA |
| 6 | S4, 04/02/2020 | 194446 | ND | ND | ND |
| 7 | S5, 15/02/2020 | 9808 | ND | ND | ND |
| 8 | S5, 17/02/2020 | 54262 | ND | ND | ND |
| 9 | S1, 22/02/2020 | 62344 | ND | ND | ND |
| 10 | S2, 22/02/2020 | 5093 | ND | ND | ND |
| 11 | S1, 15/04/2020 | 40877 | ND | ND | ND |
| 12 | S2, 22/04/2020 | 186812 | ND | ND | ND |
| 13 | S4, 22/04/2020 | 15466 | ND | ND | ND |
| 14 | S1, 14/08/2020 | 112948 | ND | ND | ND |
| 15 | S2, 14/08/2020 | 34032 | ND | ND | ND |
| 16 | S3, 09/10/2020 | 171308 | 6921 | ND | ND |
| No | Compound | Identification Level | Chemical Group | tR (min) | Number of Samples | % Peak Area |
|---|---|---|---|---|---|---|
| 1 | α-Thujene | A | Terpenes/Terpenoids | 4.82 | 2 | 0.05–0.08 |
| 2 | 2,2,4,6,6-pentamethyl heptane | B | Hydrocarbons | 5.43 | 16 | 0.03- 0.80 |
| 3 | 2,2,6-trimethyl- cyclohexanone | A | Aldehydes/Ketones | 5.95 | 1 | 0.004 |
| 4 | p-Cresol | B | Phenols | 6.28 | 2 | 0.04–5.55 |
| 5 | 2,4-Dimethyldecane | A | Hydrocarbons | 6.59 | 1 | 0.17 |
| 6 | Nonanal | A | Aldehydes/Ketones | 6.64 | 9 | 0.06–0.43 |
| 7 | 4,7-Dimethylundecane | A | Hydrocarbons | 7.62 | 1 | 0.01 |
| 8 | β-Cyclocitral | B,C | Terpenes/Terpenoids | 7.90 | 3 | 0.05–0.16 |
| 9 | 2,6,6-Trimethyl-1- Cyclohexene- 1-acetaldehyde | A | Terpenes/Terpenoids | 8.25 | 1 | 0.007 |
| 10 | 6,10-Dimethyl-5,9- Undecadien-2-one | B | Aldehydes/Ketones | 9.92 | 1 | 0.35 |
| 11 | β-Ionone | B,C | Terpenes/Terpenoids | 10.25 | 3 | 0.04–0.38 |
| 12 | Pentadecane | B,C | Hydrocarbons | 10.35 | 1 | 0.14 |
| 13 | 2,4-di-tert-butylphenol | A,C | Phenols | 10.43 | 10 | 0.01–39 |
| 14 | Hexadecane | B,C | Hydrocarbons | 11.16 | 2 | 0.20–0.60 |
| 15 | Heptadecane | B,C | Hydrocarbons | 11.94 | 6 | 0.07–33 |
| 16 | Diisobutyl phthalate | B | Phthalates | 13.11 | 4 | 0.003–1.5 |
| 17 | Dimethyl disulfide | C,D | Alkyl sulfides | 5.81 | 1 | 0.02 |
| 18 | Dimethyl trisulfide | C,D | Alkyl sulfides | 12.35 | 1 | 0.64 |
| 19 | Dimethyl tetrasulfide | D | Alkyl sulfides | 17.68 | 1 | 0.78 |
| 20 | 3-methylindole | C,D | Indoles | 20.21 | 2 | 0.005–0.02 |
| Concentration (μg/L) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample ID | Sample Details | 1,2,3-Trichlorobenzene | 1,2,4-Trichlorobenzene | Dichloromethane | Toluene | 1,2-Dichloropropane | Benzene | Trichloromethane | o-Xylene | m+p-Xylene | Styrene |
| 1 | S1, 22/08/19 | ND | ND | 31.0 | 0.72 | ND | ND | ND | ND | ND | ND |
| 2 | S2, 02/10/19 | ND | ND | 28.8 | 0.65 | ND | ND | ND | ND | ND | ND |
| 3 | S4, 02/10/19 | ND | ND | 28.1 | 0.60 | ND | ND | ND | ND | ND | ND |
| 4 | S1, 22/12/19 | ND | ND | 29.1 | 0.25 | ND | ND | ND | ND | ND | ND |
| 5 | S1, 15/01/20 | ND | ND | 29.0 | <0.25 | ND | ND | ND | ND | ND | ND |
| 6 | S4, 04/02/20 | <0.25 | <0.25 | 27.2 | <0.25 | ND | ND | ND | ND | ND | ND |
| 7 | S5, 15/02/20 | ND | ND | 28.2 | ND | ND | ND | ND | ND | ND | ND |
| 8 | S5, 17/02/20 | ND | ND | 26.3 | <0.25 | ND | ND | ND | ND | ND | ND |
| 9 | S1, 22/02/20 | ND | ND | 34.0 | <0.25 | ND | ND | ND | ND | ND | ND |
| 10 | S2, 22/02/20 | ND | ND | 29.4 | <0.25 | ND | ND | ND | ND | ND | ND |
| 11 | S1, 15/04/20 | ND | ND | 27.2 | ND | ND | ND | 0.40 | ND | ND | ND |
| 13 | S4, 22/04/20 | ND | ND | 30.2 | ND | ND | ND | ND | ND | ND | ND |
| 14 | S1, 14/08/20 | ND | ND | 32.3 | 0.46 | ND | ND | ND | ND | ND | ND |
| 15 | S2, 14/08/20 | ND | ND | 33.6 | 0.35 | ND | ND | ND | ND | ND | ND |
| 16 | S3, 09/10/20 | ND | ND | 25.1 | 0.81 | <0.25 | <0.25 | 0.50 | <0.25 | <0.25 | <0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammoud, N.A.; Zervou, S.-K.; Kaloudis, T.; Christophoridis, C.; Paraskevopoulou, A.; Triantis, T.M.; Slim, K.; Szpunar, J.; Fadel, A.; Lobinski, R.; et al. Investigation of the Occurrence of Cyanotoxins in Lake Karaoun (Lebanon) by Mass Spectrometry, Bioassays and Molecular Methods. Toxins 2021, 13, 716. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13100716
Hammoud NA, Zervou S-K, Kaloudis T, Christophoridis C, Paraskevopoulou A, Triantis TM, Slim K, Szpunar J, Fadel A, Lobinski R, et al. Investigation of the Occurrence of Cyanotoxins in Lake Karaoun (Lebanon) by Mass Spectrometry, Bioassays and Molecular Methods. Toxins. 2021; 13(10):716. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13100716
Chicago/Turabian StyleHammoud, Noura Alice, Sevasti-Kiriaki Zervou, Triantafyllos Kaloudis, Christophoros Christophoridis, Aikaterina Paraskevopoulou, Theodoros M. Triantis, Kamal Slim, Joanna Szpunar, Ali Fadel, Ryszard Lobinski, and et al. 2021. "Investigation of the Occurrence of Cyanotoxins in Lake Karaoun (Lebanon) by Mass Spectrometry, Bioassays and Molecular Methods" Toxins 13, no. 10: 716. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13100716