pH-Dependent Protein Binding Properties of Uremic Toxins In Vitro
Abstract
:1. Introduction
2. Results
2.1. Conformational Change of Albumin Depending on the pH of Solutions
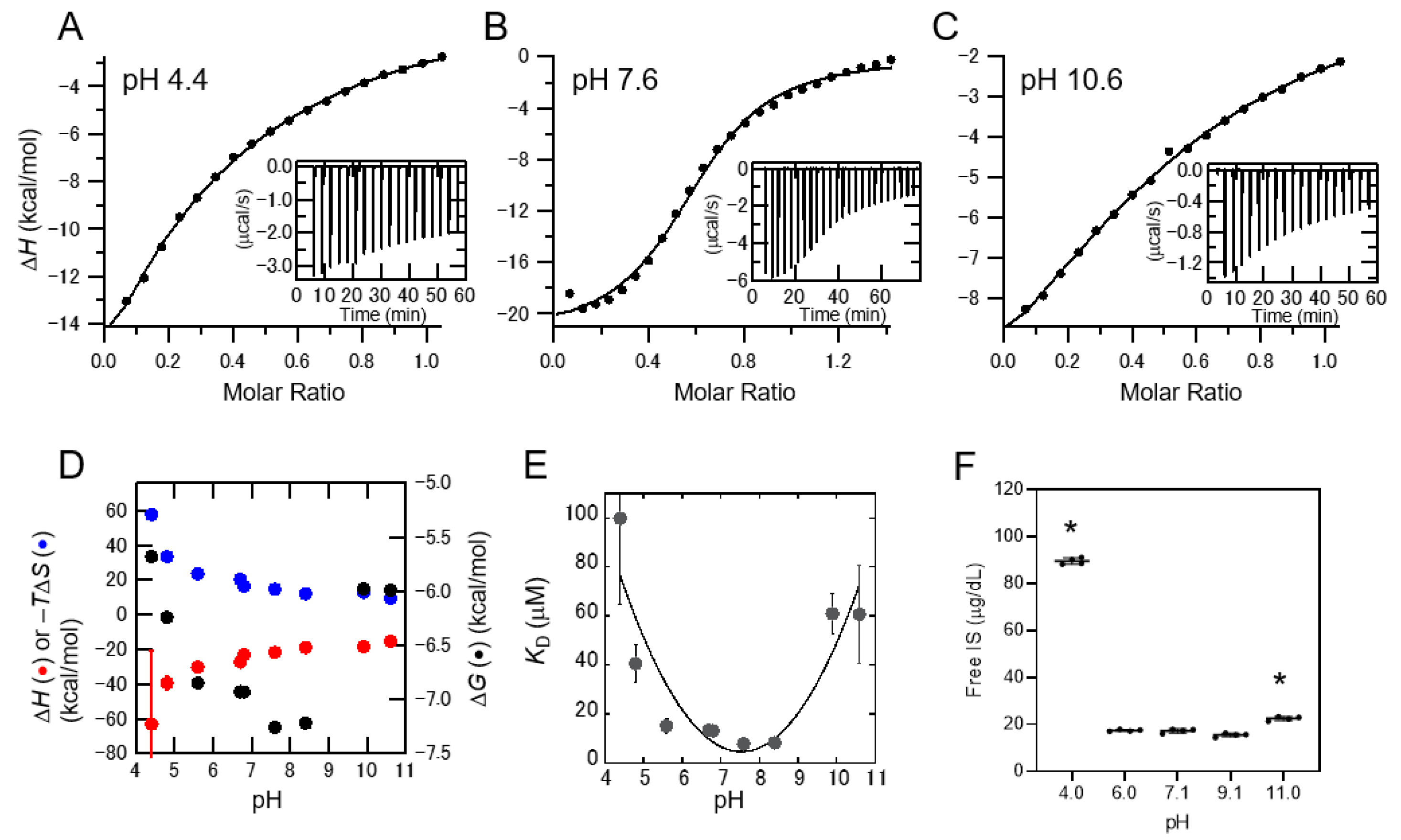
2.2. Interaction between IS and Albumin Depending on the pH of the Solution
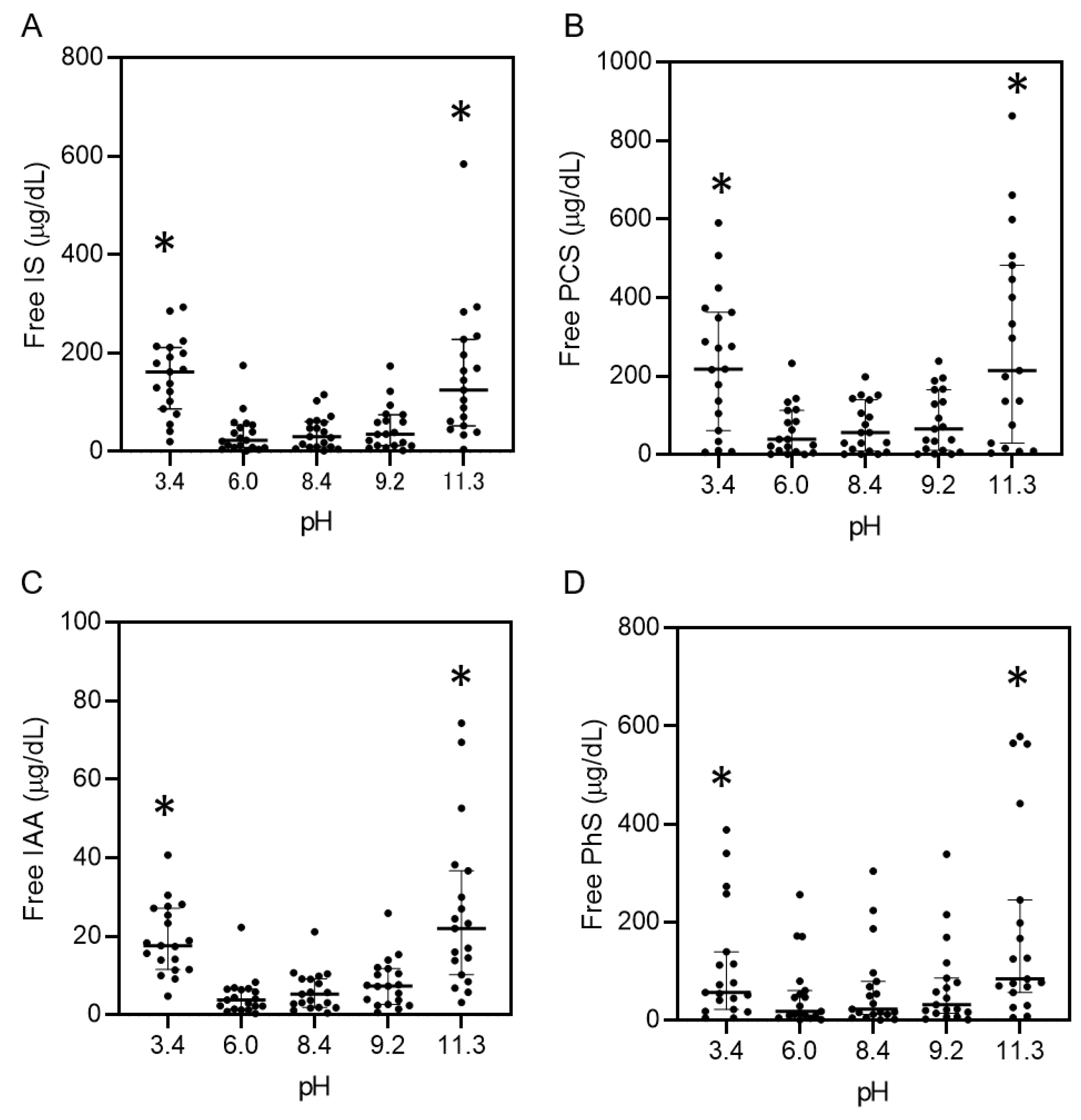
2.3. pH-Dependent Protein-Binding Property of Uremic Toxins in Serum
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. CD Measurement of Albumin
5.2. Interaction between Albumin and Indoxyl Sulfate Monitored by ITC
5.3. Reaction of Albumin with Indoxyl Sulfate under Changing pH
5.4. Reaction of Uremic Toxins and Serum from Normal Subjects with Changing pH
5.5. Uremic Serum from Patients with Changing pH
5.6. Measurement of Uremic Toxins
5.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lekawanvijit, S.; Kompa, A.R.; Wang, B.H.; Kelly, D.J.; Krum, H. Cardiorenal syndrome: The emerging role of protein-bound uremic toxins. Circ. Res. 2012, 111, 1470–1483. [Google Scholar] [CrossRef] [Green Version]
- Assem, M.; Lando, M.; Grissi, M.; Kamel, S.; Massy, Z.A.; Chillon, J.-M.; Hénaut, L. The impact of uremic toxins on cerebrovascular and cognitive disorders. Toxins 2018, 10, 303. [Google Scholar] [CrossRef] [Green Version]
- Barreto, F.C.; Barreto, D.V.; Liabeuf, S.; Meert, N.; Glorieux, G.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A. Serum Indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1551–1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bammens, B.; Evenepoel, P.; Keuleers, H.; Verbeke, K.; Vanrenterghem, Y. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int. 2006, 69, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, Y.; Ezawa, A.; Kikuchi, K.; Tsuruta, Y.; Niwa, T. Protein-bound uremic toxins in hemodialysis patients measured by liquid chromatography/tandem mass spectrometry and their effects on endothelial ROS production. Anal. Bioanal. Chem. 2012, 403, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Deltombe, O.; de Loor, H.; Glorieux, G.; Dhondt, A.; Van Biesen, W.; Meijers, B.; Eloot, S. Exploring binding characteristics and the related competition of different protein-bound uremic toxins. Biochimie 2017, 139, 20–26. [Google Scholar] [CrossRef]
- Ghuman, J.; Zunszain, P.A.; Petitpas, I.; Bhattacharya, A.A.; Otagiri, M.; Curry, S. Structural basis of the drug-binding specificity of human serum albumin. J. Mol. Biol. 2005, 353, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Takadate, A.; Otagiri, M. Characterization of binding site of uremic toxins on human serum albumin. Biol. Pharm. Bull. 1995, 18, 1755–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, H.; Noguchi, T.; Miyamoto, Y.; Kadowaki, D.; Kotani, S.; Nakajima, M.; Miyamura, S.; Ishima, Y.; Otagiri, M.; Maruyama, T. Interaction between two sulfate-conjugated uremic toxins, p-Cresyl sulfate and indoxyl sulfate, during binding with human serum albumin. Drug Metab. Dispos. 2012, 40, 1423–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabbani, G.; Ahmad, E.; Zaidi, N.; Khan, R.H. pH-dependent conformational transitions in conalbumin (ovotransferrin), a metalloproteinase from hen egg white. Cell Biochem. Biophys. 2011, 61, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Dockal, M.; Carter, D.C.; Rüker, F. Conformational transitions of the three recombinant domains of human serum albumin depending on pH. J. Biol. Chem. 2000, 275, 3042–3050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tijink, M.S.; Wester, M.; Glorieux, G.; Gerritsen, K.G.; Sun, J.; Swart, P.C.; Borneman, Z.; Wessling, M.; Vanholder, R.; Joles, J.A.; et al. Mixed matrix hollow fiber membranes for removal of protein-bound toxins from human plasma. Biomaterials 2013, 34, 7819–7828. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Kazama, J.J.; Omori, K.; Matsuo, K.; Takahashi, Y.; Kawamura, K.; Matsuto, T.; Watanabe, H.; Maruyama, T.; Narita, I. Continuous reduction of protein-bound uraemic toxins with improved oxidative stress by using the oral charcoal adsorbent AST-120 in haemodialysis patients. Sci. Rep. 2015, 5, 14381. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.W.; Peattie, J.W.; Miller, J.D.; Dinh, D.C.; Recht, N.S.; Walther, J.L.; Hostetter, T.H. Increasing the clearance of protein-bound solutes by addition of a sorbent to the dialysate. J. Am. Soc. Nephrol. 2007, 18, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Meijers, B.K.; Bammens, B.; Verbeke, K.; Evenepoel, P. A Review of albumin binding in CKD. Am. J. Kidney Dis. 2008, 51, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Mingrone, G.; De Smet, R.; Greco, A.; Bertuzzi, A.; Gandolfi, A.; Ringoir, S.; Vanholder, R. Serum uremic toxins from patients with chronic renal failure displace the binding of l-tryptophan to human serum albumin. Clin. Chim. Acta 1997, 260, 27–34. [Google Scholar] [CrossRef]
- Meijers, B.K.; De Loor, H.; Bammens, B.; Verbeke, K.; Vanrenterghem, Y.; Evenepoel, P. p-Cresyl Sulfate and indoxyl sulfate in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1932–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Chalabi, A.; Matevossian, E.; von Thaden, A.; Schreiber, C.; Radermacher, P.; Huber, W.; Perez Ruiz de Garibay, A.; Kreymann, B. Evaluation of an advanced organ support (ADVOS) system in a two-hit porcine model of liver failure plus endotoxemia. Intensive Care Med. Exp. 2017, 5, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavita, U.; Duo, J.; Crawford, S.M.; Liu, R.; Valcin, J.; Gleason, C.; Dong, H.; Gadkari, S.; Dodge, R.W.; Pillutla, R.C.; et al. A systematic study of the effect of low pH acid treatment on anti-drug antibodies specific for a domain antibody therapeutic: Impact on drug tolerance, assay sensitivity and post-validation method assessment of ADA in clinical serum samples. J. Immunol. Methods 2017, 448, 91–104. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, S.; Sasahara, K.; Domon, M.; Yamaguchi, K.; Ito, T.; Goto, S.; Goto, Y.; Narita, I. pH-Dependent Protein Binding Properties of Uremic Toxins In Vitro. Toxins 2021, 13, 116. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13020116
Yamamoto S, Sasahara K, Domon M, Yamaguchi K, Ito T, Goto S, Goto Y, Narita I. pH-Dependent Protein Binding Properties of Uremic Toxins In Vitro. Toxins. 2021; 13(2):116. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13020116
Chicago/Turabian StyleYamamoto, Suguru, Kenichi Sasahara, Mio Domon, Keiichi Yamaguchi, Toru Ito, Shin Goto, Yuji Goto, and Ichiei Narita. 2021. "pH-Dependent Protein Binding Properties of Uremic Toxins In Vitro" Toxins 13, no. 2: 116. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13020116




