Toxic Animal-Based Medicinal Materials Can Be Effective in Treating Endometriosis: A Scoping Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Scope of Toxic Animal-Based Medicinal Materials
2.2. Literature Search Strategy
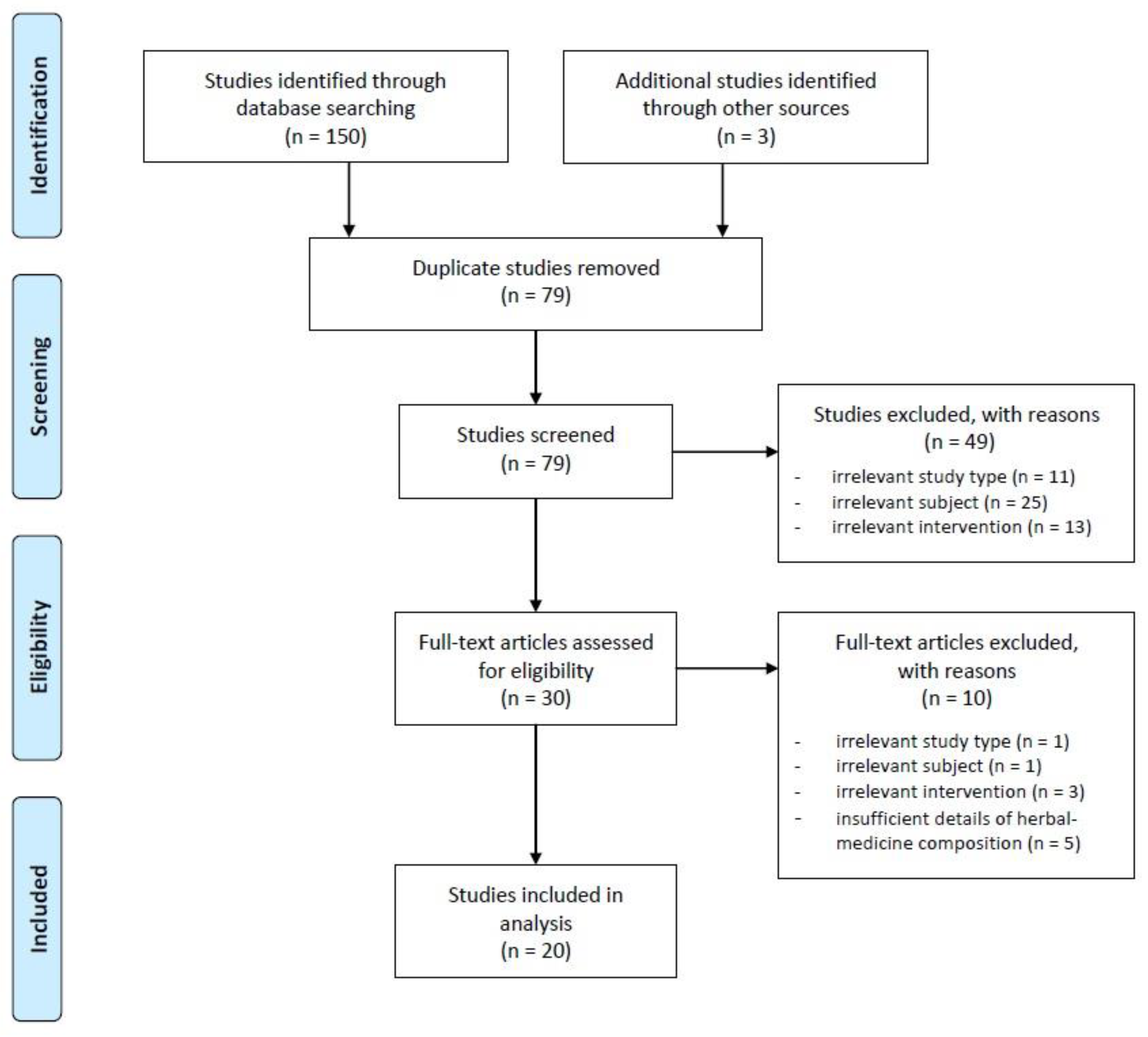
2.3. Inclusion and Exclusion Criteria
2.4. Study Selection and Data Extraction
3. Results
3.1. Preclinical Studies
3.2. Clinical Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat. Rev. Dis. 2018, 4, 9. [Google Scholar] [CrossRef]
- Shafrir, A.L.; Farland, L.V.; Shah, D.K.; Harris, H.R.; Kvaskoff, M.; Zondervan, K.; Missmer, S.A. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 1–15. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef]
- Falcone, T.; Flyckt, R. Clinical Management of Endometriosis. Obstet. Gynecol. 2018, 131, 557–571. [Google Scholar] [CrossRef] [Green Version]
- Peiris, A.N.; Chaljub, E.; Medlock, D. Endometriosis. JAMA 2018, 320, 2608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czyzyk, A.; Podfigurna, A.; Szeliga, A.; Meczekalski, B. Update on endometriosis pathogenesis. Minerva Ginecol. 2017, 69, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Flores, I.; Rivera, E.; Ruiz, L.A.; Santiago, O.I.; Vernon, M.W.; Appleyard, C.B. Molecular profiling of experimental endometriosis identified gene expression patterns in common with human disease. Fertil. Steril. 2007, 87, 1180–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Gorp, T.; Amant, F.; Neven, P.; Vergote, I.; Moerman, P. Endometriosis and the development of malignant tumours of the pelvis. A review of literature. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 349–371. [Google Scholar] [CrossRef] [PubMed]
- Krupa, A.; Padała, O.; Putowski, M.; Konopelko, M.; Piasek, E. Available treatment methods for endometriosis. J. Educ. Health Sport. 2019, 9, 178–184. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Treatment of pelvic pain associated with endometriosis: A committee opinion. Fertil. Steril. 2014, 101, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Bina, F.; Soleymani, S.; Toliat, T.; Hajimahmoodi, M.; Tabarrai, M.; Abdollahi, M.; Rahimi, R. Plant-derived medicines for treatment of endometriosis: A comprehensive review of molecular mechanisms. Pharmacol. Res. 2019, 139, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Meresman, G.F.; Götte, M.; Laschke, M.W. Plants as source of new therapies for endometriosis: A review of preclinical and clinical studies. Hum Reprod Update. 2020, dmaa039. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wu, J.; Gu, J.; Weng, H.; Wang, J.; Wang, T.; Liang, X.; Cao, L. Modular Characteristics and Mechanism of Action of Herbs for Endometriosis Treatment in Chinese Medicine: A Data Mining and Network Pharmacology–Based Identification. Front. Pharmacol. 2020, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.F.; Hou, L.H.; Zhou, Y.J.; Wu, X.K. A survey of TCM treatment for endometriosis. J. Tradit. Chin. Med. 2009, 29, 64–70. [Google Scholar] [CrossRef]
- Bae, K.Y.; Rhyu, M.R.; Roh, J.J.; Kim, D.I. Vasodilatory Activities and Safety of the Water Extracts of Three Medicinal Remedy in Species of Insects. J. Korean Obstet. Gynecol. 2007, 20, 114–124. [Google Scholar]
- Sha, B.; Zhu, W.H. On the Application of Insect Medicine in Rheumatism from the Inheritance of Menghe Medical School. Rheumatism Arthritis 2020, 8, 64–65. [Google Scholar]
- Wang, W.P.; Zou, J.; Wu, W. Case Records Illustration of Professor WU Wei Who has Used Insect Medicine to Treat Kidney Disease. J. Sichuan Tradit. Chin. Med. 2020, 7, 85–87. [Google Scholar]
- Chen, Y.; Ji, L.; Wang, M.; Li, M.; Cui, S. Research Progress in the Application of Insect Medicinals in Gynecological Diseases. Shandong J. Tradit. Chin. Med. 2020, 9, 1022–1025. [Google Scholar]
- Wang, J.H.; Wang, T.Z.; Jiang, D.Y. JIANG De-you’s experience in treating cardiovascular and cerebrovascular diseases with insect drug. China J. Tradit. Chin. Med. Pharm. 2020, 8, 3962–3965. [Google Scholar]
- Klupczynska, A.; Pawlak, M.; Kokot, Z.J.; Matysiak, J. Application of Metabolomic Tools for Studying Low Molecular-Weight Fraction of Animal Venoms and Poisons. Toxins 2018, 10, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bordon, K.C.F.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Júnior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A.; Cardoso, I.A.; et al. From Animal Poisons and Venoms to Medicines: Achievements, Challenges and Perspectives in Drug Discovery. Front. Pharmacol. 2020, 11, 1132. [Google Scholar] [CrossRef]
- Chatterjee, B. Animal Venoms have Potential to Treat Cancer. Curr. Top. Med. Chem. 2018, 18, 2555–2566. [Google Scholar] [CrossRef]
- Ravi, K.U. Use of Animal Venom Peptides/Toxins in Cancer Therapeutics. Nat. Biomed. Eng. 2018, 16, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Choi, G. Animal-based medicinal materials in the pharmacopeias of Northeast Asian countries. Korean Herb. Med. Inf. 2018, 6, 203–230. [Google Scholar] [CrossRef]
- Herbology editorial committee of Korean medicine schools. Herbology; Yeonglimsa: Seoul, Korea, 2012; pp. 469–471, 546–548. [Google Scholar]
- Lim, E.-M.; Kwon, K.-R.; Lee, Y.-H. Effects of Bee Venom Acupuncture on Surgically Induced Endometriosis Rats. J. Pharmacopunct. 2006, 9, 21–32. [Google Scholar]
- Nasu, K.; Nishida, M.; Ueda, T.; Takai, N.; Bing, S.; Narahara, H.; Miyakawa, I. Bufalin induces apoptosis and the G0/G1 cell cycle arrest of endometriotic stromal cells: A promising agent for the treatment of endometriosis. Mol. Hum. Reprod. 2005, 11, 817–823. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.J.; Lee, J.E.; Park, M.J.; O’Malley, B.W.; Han, S.J. Bufalin suppresses endometriosis progression by inducing pyroptosis and apoptosis. J. Endocrinol. 2018, 237, 255–269. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.L. Investigate the role of “Pingchongjiangnifang” of the in vitro culture of human endometrial stromal cells. Master’s Thesis, Jiangxi University of Traditional Chinese Medicine, Nanchang, China, 1 May 2014. [Google Scholar]
- Xu, Z. Data Mining and Experimental Research on the Treatment of Endometriosis Dysmenorrhea by Professor Si Tuyi, a Famous Chinese Medicine Practitioner. Ph.D. Thesis, Guangzhou University of Chinese Medicine, Guangzhou, China, 1 April 2018. [Google Scholar]
- Liu, H.; Sun, X.; Zhao, Y.; Xia, M.; Wang, C. Anti-angiogenesis effect and mechanism study of Huangzhi Neiyi capsule in a rat endometriosis model. J. Int. Med. Res. 2020, 48, 300060519899767. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.F. The Effect of Wudanwan Modified Formula on Rat Endometriosis Model. Master’s Thesis, Beijing University of Chinese Medicine, Beijing, China, 1 May 2005. [Google Scholar]
- Zhang, Q. Effects of Disintegrin of Gloydius Brevicaudus Venom on Endometriosis of Rat and Its Mechanism. Master’s Thesis, Fujian Medical University, Fuzhou, China, 1 June 2015. [Google Scholar]
- Wu, X. Treatment of 60 cases of endometriosis by dissolving blood and removing blood stasis. Shaanxi Tradit. Chin. Med. 1993, 14, 530. [Google Scholar]
- Zhang, L. Professor Wang Ziyu’s Experience in Treating Endometriosis Dysmenorrhea. Master’s Thesis, Beijing University of Chinese Medicine, Beijing, China, 1 May 2007. [Google Scholar]
- Xu, Y.; Su, J.; Cao, H. Shuizhi Tongluo Capsule in the treatment of 52 cases of endometriosis. Hebei Tradit. Chin. Med. 2007, 29, 797. [Google Scholar]
- Zhang, X.; Wang, W. Clinical analysis of 78 cases of endometriosis treated with Quyu Jiedu Xiaozheng Decoction. Mod. Chin. Med. 2007, 27, 18–19. [Google Scholar]
- Feng, D. Xiaoyi Zhuyun Decoction in the treatment of 35 cases of endometriotic infertility. Res. Chin. Med. 2014, 27, 36–37. [Google Scholar]
- Han, D.; Yu, F.; Wang, H. Clinical observation on 78 cases of endometriosis treated with traditional Chinese medicine leech. Chin. J. Coal Ind. Med. 2009, 12, 303–304. [Google Scholar]
- Zhang, X.; Dong, L.; Wang, Q. Treatment of 45 cases of endometriosis with Quyu Jiedu Xiaozheng Decoction. Shaanxi J. Tradit. Chin. Med. 2009, 30, 260–261. [Google Scholar]
- Lin, H.; Xiao, W.; He, X.; Peng, J. Observation on the clinical efficacy of Quanxie Foshou Powder in the treatment of endometriosis. Lishizhen Med. Mater. Med. 2011, 22, 2819–2820. [Google Scholar]
- Meng, W.; Xu, Y. Clinical observation of 156 cases of endometriosis treated with traditional Chinese medicine leech. J. Changchun Univ. Tradit. Chin. Med. 2012, 28, 128–129. [Google Scholar]
- Guo, M.; Qi, S.; Niu, G.; Liu, Z. Bushen Quyu Decoction combined with desogestrel and ethinyl estradiol tablets in the treatment of 48 cases of infertility with endometriosis after laparoscopic surgery. J. Tradit. Chin. Med. 2013, 26, 26–28. [Google Scholar]
- Liu, H. Self-Made Dysmenorrhea Particles in the Treatment of Dysmenorrhea Caused by Endometriosis (Qi and Blood Stasis Type) the Safety and Clinical Research. Master’s Thesis, Changchun University of Traditional Chinese Medicine, Changchun, China, 1 March 2017. [Google Scholar]
- Yi, S.; Liu, L. The clinical study of Xiaoyizhitong Decoction combined with gestrinone in the treatment of endometriosis. Chin. Med. Guide 2018, 16, 215–216. [Google Scholar]
- Ko, C.R.; Ku, N.P.; Seol, S.S. A Comparative Study on the Traditional Medicine Policies between Korea and China: Focused on the Second Korean Medicine Development Plan and the 12.5 Traditional Chinese Medicine Development Plan. J. Korea Technol. Innov. Soc. 2014, 17, 421–447. [Google Scholar]
- Wei, W.L.; Hou, J.J.; Wang, X.; Yu, Y.; Li, H.J.; Li, Z.W.; Feng, Z.J.; Qu, H.; Wu, W.Y.; Guo, D.A. Venenum bufonis: An overview of its traditional use, natural product chemistry, pharmacology, pharmacokinetics and toxicology. J. Ethnopharmacol. 2019, 237, 215–235. [Google Scholar] [CrossRef]
- Jang, S.; Kim, K.H. Clinical Effectiveness and Adverse Events of Bee Venom Therapy: A Systematic Review of Randomized Controlled Trials. Toxins 2020, 12, 558. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.M.; Zhu, M.W.; Zhang, Z.T.; Jia, Z.G.; He, X.D.; Wan, Y.L.; Wang, S.; Xiu, D.R.; Tang, Y.; Li, J.; et al. A multicenter, phase III trial of hemocoagulase Agkistrodon: Hemostasis, coagulation, and safety in patients undergoing abdominal surgery. Chin. Med. J. 2010, 123, 589–593. [Google Scholar] [PubMed]
- Xu, Y.Y.; Ma, X.H.; Zhang, S.J. Hemocoagulase agkistrodon-induced anaphylactic shock: A case report and literature review. Int. J. Clin. Pharmacol. Ther. 2016, 54, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Bi, L.L.; Li, X.; Miao, S.; Zhang, J.; Zhang, S.; Yang, Q.; Xie, Y.H.; Zhang, J.; Wang, S.W. Anticancer effects of bufalin on human hepatocellular carcinoma HepG2 cells: Roles of apoptosis and autophagy. Int. J. Mol. Sci. 2013, 14, 1370–1382. [Google Scholar] [CrossRef]
- Qi, F.; Li, A.; Inagaki, Y.; Kokudo, N.; Tamura, S.; Nakata, M.; Tang, W. Antitumor activity of extracts and compounds from the skin of the toad Bufo bufo gargarizans Cantor. Int. Immunopharmacol. 2011, 11, 342–349. [Google Scholar] [CrossRef]
- Liu, L.; Chen, B.A.; Qin, S.K. Anti-angiogenesis effect of arsenic trioxide plus cinobufacin on human hepatocarcinoma transplantation model nude mice. Chin. J. Integr. Tradit. West. Med. 2011, 31, 67–72. [Google Scholar]
- Lee, J.A.; Son, M.J.; Choi, J.; Jun, J.H.; Kim, J.I.; Lee, M.S. Bee venom acupuncture for rheumatoid arthritis: A systematic review of randomised clinical trials. BMJ Open 2014, 4, e006140. [Google Scholar] [CrossRef] [PubMed]
- Gazerani, P.; Cairns, B.E. Venom-based biotoxins as potential analgesics. Expert Rev. Neurother. 2014, 14, 1261–1274. [Google Scholar] [CrossRef]
- Xi, S.; Gong, Y. Essentials of Chinese Materia Medica and Medical Formulas: New Century Traditional Chinese Medicine; Elsevier (Academic Press): London, UK, 2017; pp. 246–319. [Google Scholar]
- Lu, J.; Chen, X.; Xu, X.; Liu, J.; Zhang, Z.; Wang, M.; Li, X.; Chen, H.; Zhao, D.; Wang, J.; et al. Active polypeptides from Hirudo inhibit endothelial cell inflammation and macrophage foam cell formation by regulating the LOX-1/LXR-α/ABCA1 pathway. Biomed. Pharmacother. 2019, 115, 108840. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Ren, J.X.; Wang, J.J.; Ding, L.S.; Zhao, J.J.; Liu, S.Y.; Gao, H.M. Chinese Medicinal Leech: Ethnopharmacology, Phytochemistry, and Pharmacological Activities. Evid.-Based Complement. Altern. Med. 2016, 2016, 7895935. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Guo, J.; Lu, H.; Kong, Q. Impacts of leech hirudo and blister beetle mylabris on the angiogenesis of chick chorioallantonic membrance. World J. Integr. Tradit. West. Med. 2010, 5, 855–857. [Google Scholar]
- Guo, Y.; Tian, X.; Xiao, Z. Study on inhibition effects of freeze-thawing leech extract on HepG2 cells. Chin. J. Inf. Tradit. Chin. Med. 2009, 16, 30–31. [Google Scholar]
- Xiao, Y.S.; Liao, F.S.; Zhao, Z.D.; Zuo, A.R.; Zhu, D.C. Study on inhibitory functions of leech extract on HL60 cells in vitro experiment. J. Jiangxi Univ. Tradit. Chin. Med. 2013, 25, 63–66. [Google Scholar]
- Sig, A.K.; Guney, M.; Uskudar Guclu, A.; Ozmen, E. Medicinal leech therapy-an overall perspective. Integr. Med. Res. 2017, 6, 337–343. [Google Scholar] [CrossRef]
- Lee, W.; Lee, J.; Kulkarni, R.; Kim, M.A.; Hwang, J.S.; Na, M.; Bae, J.S. Antithrombotic and antiplatelet activities of small-molecule alkaloids from Scolopendra subspinipes mutilans. Sci. Rep. 2016, 6, 21956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.J.; Lee, H.Y.; Jung, Y.S.; Park, J.S.; Hwang, J.S.; Bae, Y.S. Antimicrobial peptide scolopendrasin VII, derived from the centipede Scolopendra subspinipes mutilans, stimulates macrophage chemotaxis via formyl peptide receptor 1. BMB Rep. 2015, 48, 479–484. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Lee, S.R. Anti-inflammatory activities of Scolopendra subspinipes mutilans in RAW 264.7 cells. J. Nutr. Health 2018, 51, 323. [Google Scholar] [CrossRef]
- Zheng, L.; He, H.; Shen, X.; Sun, Y. Centipede Scolopendra suppresses cell growth in human epidermoid carcinoma cell A431. Bangladesh J. Pharmacol. 2017, 12, 299. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Liu, R.; Qi, J.; Zhang, Y. Extracts of centipede Scolopendra subspinipes mutilans induce cell cycle arrest and apoptosis in A375 human melanoma cells. Oncol. Lett. 2014, 8, 414–420. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Zhang, D.; Zheng, L.; Zhan, Y.; Zhang, Y. Potential roles of Centipede Scolopendra extracts as a strategy against EGFR-dependent cancers. Am. J. Transl. Res. 2015, 7, 39–52. [Google Scholar]
- Hwang, L.; Ko, I.G.; Jin, J.J.; Kim, S.H.; Kim, C.J.; Jeon, J.W.; Han, J.H. Scolopendra subspinipes mutilans Extract Suppresses Inflammatory and Neuropathic Pain In Vitro and In Vivo. Evid.-Based Complement. Altern. Med. 2018, 2018, 5057372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.X.; Wu, N.; Wei, J.T.; Liu, J.; Zhao, J.; Ji, A.G.; Lin, X.K. A novel protein from Eupolyphaga sinensis inhibits adhesion, migration, and invasion of human lung cancer A549 cells. Biochem. Cell Biol. 2013, 91, 244–251. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhan, Y.; Zhang, D.; Dai, B.; Ma, W.; Qi, J.; Liu, R.; He, L. Eupolyphaga sinensis Walker displays inhibition on hepatocellular carcinoma through regulating cell growth and metastasis signaling. Sci. Rep. 2014, 4, 5518. [Google Scholar] [CrossRef] [Green Version]
- Zhan, Y.; Zhang, H.; Liu, R.; Wang, W.; Qi, J.; Zhang, Y. Eupolyphaga sinensis Walker ethanol extract suppresses cell growth and invasion in human breast cancer cells. Integr. Cancer Ther. 2016, 15, 102–112. [Google Scholar] [CrossRef] [Green Version]
- Ge, G.F.; Yu, C.H.; Yu, B.; Shen, Z.H.; Zhang, D.L.; Wu, Q.F. Antitumor effects and chemical compositions of Eupolyphaga sinensis Walker ethanol extract. J. Ethnopharmacol. 2012, 141, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yan, Y.; Zhang, F.; Wu, Q. The immuno-enhancement effects of tubiechong (Eupolyphaga sinensis) lyophilized powder in cyclophosphamide-induced immunosuppressed mice. Immunol. Investig. 2019, 48, 844–859. [Google Scholar] [CrossRef] [PubMed]
- Shoukry, N.M.; Salem, M.L.; Teleb, W.K.; Abdel Daim, M.M.; Abdel-Rahman, M.A. Antinociceptive, antiinflammatory, and antipyretic effects induced by the venom of Egyptian scorpion Androctonus amoreuxi. J. Basic Appl. Zool. 2020, 81, 56. [Google Scholar] [CrossRef]
- Tran, T.V.; Hoang, A.N.; Nguyen, T.T.T.; Phung, T.V.; Nguyen, K.C.; Osipov, A.V.; Ivanov, I.A.; Tsetlin, V.I.; Utkin, Y.N. Anticoagulant activity of low-molecular weight compounds from Heterometrus laoticus scorpion venom. Toxins 2017, 9, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.Q.; Xie, J.; Wang, J.F.; Shi, Z.F.; Zhang, X.; Du, Y.P.; Zhao, X.C. Scorpion inhibits epithelial-mesenchymal transition and metastasis of hepatocellular carcinoma. Exp. Biol. Med. (Maywood) 2018, 243, 645–654. [Google Scholar] [CrossRef]
- Kwon, K.B.; Kim, E.K.; Lim, J.G.; Jeong, E.S.; Shin, B.C.; Jeon, Y.S.; Kim, K.S.; Seo, E.A.; Ryu, D.G. Molecular mechanisms of apoptosis induced by Scorpio water extract in human hepatoma HepG2 cells. World J. Gastroenterol. 2005, 11, 943–947. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Riyasdeen, A.; Islam, M. Scorpion Venom Causes Upregulation of p53 and Downregulation of Bcl-x(L) and BID Protein Expression by Modulating Signaling Proteins Erk(1/2) and STAT3, and DNA Damage in Breast and Colorectal Cancer Cell Lines. Integr. Cancer Ther. 2018, 17, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, K.; Han, S.; Tian, Y.H.; Hu, P.C.; Xu, X.L.; He, Y.Q.; Pan, W.T.; Gao, Y.; Zhang, Z.; et al. Chlorotoxin targets ERα/VASP signaling pathway to combat breast cancer. Cancer Med. 2019, 8, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- Lazarou, J.; Pomeranz, B.H.; Corey, P.N. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA 1998, 279, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.J.; Pontefract, S.K. Adverse drug reactions. Clin. Med. (Lond.) 2016, 16, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Tang, G.; Wang, J.; Zhong, J.; Xia, Z.; Li, J.; Chen, G.; Zhang, Y.; Luo, S.; Huang, G.; et al. Safety and efficacy of herbal medicine for acute intracerebral hemorrhage (CRRICH): A multicentre randomised controlled trial. BMJ Open 2019, 9, e024932. [Google Scholar] [CrossRef]
- Yuen, M.F.; Tam, S.; Fung, J.; Wong, D.K.; Wong, B.C.; Lai, C.L. Traditional Chinese medicine causing hepatotoxicity in patients with chronic hepatitis B infection: A 1-year prospective study. Aliment. Pharmacol. Ther. 2006, 24, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.A.; Sun, L.M.; Chen, Y.X. Effects of Ailing Granule on immuno-reconstruction in HIV/AIDS patients. Chin. J. Integr. Tradit. West. Med. 2006, 26, 319–321. [Google Scholar]
- Johnson, N.P.; Hummelshoj, L.; Adamson, G.D.; Keckstein, J.; Taylor, H.S.; Abrao, M.S.; Bush, D.; Kiesel, L.; Tamimi, R.; Sharpe-Timms, K.L.; et al. World Endometriosis Society consensus on the classification of endometriosis. Hum. Reprod. 2017, 32, 315–324. [Google Scholar] [CrossRef] [PubMed]


| First Author (year) | Herbal Medicine | Component |
|---|---|---|
| Liu (2014) [30] | Pingchongjiangnifang | Cinnamomi Ramulus 15 g, Draconis Sanguis 3 g, Trogopterorum Faeces 10 g, Typhae Pollen 6 g, Aquilariae Lignum 6 g, Hirudo 3 g, Liriopis seu Ophiopogonis Tuber 10 g, Glycyrrhizae Radix et Rhizoma 6 g |
| Xu (2018) [31] | Eleng Capsule | Curcumae Rhizoma 10 g, Sparganii Rhizoma 10 g, Salviae Miltiorrhizae Radix 15 g, Curcumae Radix 15 g, Paeoniae Radix Rubra 15 g, Pelodiscis Carapax 15 g, Hirudo 3 g |
| Liu (2020) [32] | Huangzhi Neiyi Capsule | Hirudo 3 g, Rhei Radix et Rhizoma 9 g, Cyperi Rhizoma 6 g |
| Wang (2005) [33] | Wudan Wan Modified Decoction | Salviae Miltiorrhizae Radix 10 g, Paeoniae Radix Rubra 10 g, Curcumae Rhizoma 10 g, Cinnamomi Cortex 6 g, Corydalis Tuber 10 g, Hirudo 10 g, Scolopendra two pieces, Pelodiscis Carapax 15 g, Sappan Lignum 10 g |
| Study ID | Author (Year) | Study Design | Herbal Medicine (Toxic Animal-Based Medicinal Materials) | Target Cells (In Vitro)/Animal Model (In Vivo) | Dosage | Treatment Period | Outcome Measures | Results |
|---|---|---|---|---|---|---|---|---|
| 1 | Lee (2006) [27] | In vivo | Bee venom (n.s.) | Female SD rats (autotransplantation method) | 0.1 mg/mL (0.1 mL, CV4) | 6 weeks (3 times/week) | 1) Histological analysis (H&E) 2) Serum progesterone 3) Serum estradiol 4) Serum cytokines (TNF-α, IL-2, -4, -6, -10) | 1) Inhibited proliferation of endometrial tissue 2) NSD 3) Decreased a 4) TNF-α, IL-4, NSDIL-2: Decreased a IL-6, IL-10: Increased a |
| 2 | Nasu (2005) [28] | In vitro | Bufalin (Bufonis Venenum) | Human endometrial cells | 0, 0.001, 0.01, 0.1, 1, 10 ng/mL | - | 1) Cell proliferation and cell viability 2) Apoptotic cells(%) 3) Cell cycle assay(flow cytometry) 4) Apoptosis-related proteins and cell cycle-related proteins | 1) Decreased e 2) Increased d 3) G0/G1 cell cycle arrest observed c 4) Downregulated(Cyclin A, Bcl-2, Bcl-XL proteins), Upregulated(Bax, p21, cleaved caspase-9 protein), Unchanged(Cyclin B, cyclin D3, Fas, Fas ligand, cleaved caspase-3, cleaved caspase-8 protein) |
| 3 | Cho (2018) [29] | In vivo | Bufalin (Bufonis Venenum) | Female C57BL/6 mice (Autotransplantation method) | 1 mg/kg | 21 days | 1) Volume of endometriotic lesions 2) Protein levels of the SRC-1 isoform 3) Protein levels of ERβ | 1) Decreased b 2) Increased b 3) Decreased a |
| In vitro | Human endometrial cells | 0, 10, 20, 50, 100, 200, 400, 800 nM | - | 1) Intrinsic transcriptional activity of the SRC-1 isoform 2) Intrinsic transcriptional activity of ERβ3) Levels of PSMD2 4) Levels of Ki-67 5) Levels of active caspase 3 6) Levels of active IL-1β 7) Levels of caspase 1 8) Levels of ER-stress markers(PERK, PDI, and BiP) | 1) Increased b 2) NSD 3) Increased (epithelium d, stroma d) 4) Decreased (stroma c) 5) Increased (epithelium a) 6) Increased (stroma d) 7) Increased (stroma c) 8) PERK: Increased (epithelium d, stroma d), PDI: Increased (epithelium c, stroma d), BiP: Increased (epithelium c, stroma a) | |||
| 4 | Liu (2014) [30] | In vitro | Pingchongjiangnifang(Hirudo) | Human endometrial cells | 5%, 10%, 15%, 20% of the drug in serum (1 mL) | - | 1) Cell proliferation 2) Number of adherent cells 3) Number of invading cells 4) Apoptosis rate | 1) Decreased a 2) Decreased a 3) Decreased a 4) Decreased b |
| 5 | Xu (2018) [31] | In vivo | Eleng Capsule(Hirudo) | Female SD rats (autotransplantation method) | 4 g/kg (high dose), 2 g/kg (medium dose), 1 g/kg (low dose) | 28 days | 1) Volume of endometriotic foci 2) Histological analysis (H&E) 3) Serum levels of VEGF 4) Serum levels of bFGF 5) Serum levels of PDGF 6) Expression of VEGF 7) Expression of bFGF 8) Expression of PDGF 9) Expression of NF-κB 10) Expression of TLR4 | 1) Decreased (high a/medium a/low a) 2) Improved 3) Decreased (high b/medium b/low b) 4) Decreased (high b/medium b/low b) 5) Decreased (high b/medium a/low a) 6) Decreased (high b/medium b/low b) 7) Decreased (high b/medium b/low b) 8) Decreased (high b/medium b/low b) 9) Decreased (high b/medium b/low b) 10) Decreased (high b/medium b/low b) |
| 6 | Liu (2020) [32] | In vivo | Huangzhi Neiyi Capsule (Hirudo) | Female SD rats (autotransplantation method) | 9 g/kg (high dose), 4.5 g/kg (low dose) | 28 days | 1) PCNA 2) CD31 3) VEGF in peritoneal fluid 4) mRNA expression of VEGF 5) mRNA expression of HIF-1α | 1) Decreased a 2) Decreased a 2) Decreased (high a/low b) 3) Decreased (high b/low b), 4) Decreased (high b/low a) |
| 7 | Wang (2005) [33] | In vivo | Wudan Wan Modified Decoction (Hirudo, Scolopendra) | Female SD rats (autotransplantation method) | 1 mL/100 g (26.56 g/kg) (high dose), 1/2 diluted (medium dose), 1/4 diluted (low dose) | 28 days | 1) TER for disappearance of ectopic cysts 2) Effects on abdominal inflammation 3) TER for ectopic endometrial atrophy | 1) Improved (medium a/high a), NSD (low) 2) Less severe adhesion (medium/high), More severe adhesion (low) 3) Improved (medium b/high a), NSD (low) |
| 8 | Zhang (2015) [34] | In vivo | Gloydius brevicaudus venom (Snake venom) | Female SD rats (autotransplantation method) | 0.75 mg/kg (low dose), 1.5 mg/kg (high dose) | 3 weeks | 1) Volume of ectopic foci 2) Expression of MMP-9 | 1) Decreased a 2) Decreased a |
| In vitro | Rat ectopic endometrial cells | Disintegrin 1.5 µg/mL | - | 1) Expression of MMP-9 | 1) Decreased b |
| Study ID | Author (Year) | Study Design | Sample Size (TG/CG) | Mean Age ± SD (Min, Max) | Main Symptoms | Diagnostic Criteria | Intervention | Control Intervention | Treatment Period | Outcome Measures | Main Results | AE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Wu (1993) [35] | Case series | 60 | n.r. (20,48) | Dysmenorrhea, infertility, menstrual disorder | Symptom, gynecological exam, ultrasonography | Herbal medicine | - | n.r. | 1) TER | 91.67% | n.r. |
| 2 | Zhang (2007) [36] | Case series | 30 | 32 (23,43) | Dysmenorrhea, mittelschmerz, endometrioma | Symptom, gynecological exam, ultrasonography | Herbal medicine | - | 3 mo | VAS | Positivea | n.r. |
| 3 | Xu (2007) [37] | Case series | 52 | n.r. (23,46) | Dysmenorrhea, pelvic pain, anal pain, dyspareunia, menstrual disorder, infertility | Symptom, gynecological exam, ultrasonography | Herbal medicine | - | 3 mo | 1) TER | 88.46% | none |
| 4 | Zhang (2007) [38] | Case series | 78 | 34.5 (21,46) | Dysmenorrhea, pelvic mass, infertility | Gynecological exam, ultrasonography, laparoscopy | Herbal medicine | - | 3 mo | 1) TER for dysmenorrhea 2) TER for pelvic mass 3) Pregnancy rate (within 2 years) | 1) 94.11% 2) 87.5% 3) 19.05% | n.r. |
| 5 | Feng (2014) [39] | Case series | 35 | n.r. (23,42) | Infertility | Symptom, gynecological exam, ultrasonography | Herbal medicine | - | 1–6 mo | 1) TER for pregnancy 2) Spontaneous abortion rate in early pregnancy | 1) 82.86% 2) 17.14% | n.r. |
| 6 | Han (2009) [40] | RCT | 156 (78/78) | 28.5 (18,48) | n.r. | Symptom, laparoscopy | Herbal medicine(A) | Herbal medicine(B) | 9 mo | 1) TER 2) Pregnancy rate 3) Recurrence rate | 1) Positive b 2) 11.53%(TG) vs 0%(CG) 3) Positive b | n.r. |
| 7 | Zhang (2009) [41] | RCT | 128 (45/43/40) | n.r. (n.r.) | Dysmenorrhea, pelvic mass | Symptom, gynecological exam, ultrasonography, laparoscopy, antiendometrial antibody, CA125 | Herbal medicine(A) | CG1: Herbal medicine(B) CG2: Herbal medicine(C) | 3–6 mo | 1) TER 2) Dysmenorrhea score 3) Pelvic mass score | 1) Positive (vs CG1 a /CG2 a) 2) Positive (vs CG1 b /CG2 b) 3) Positive (vs CG1 b /CG2 b) | n.r. |
| 8 | Lin (2011) [42] | RCT | 70 (40/30) | n.r. (n.r.) | Dysmenorrhea | Symptom, ultrasonography, CA125 | Herbal medicine | Mifepristone | 6 mo | 1) TER 2) TER for dysmenorrhea 3) CA125 | 1) NSD 2) Positive a 3) Positive a | none |
| 9 | Meng (2012) [43] | RCT | 312 (156/156) | 28.5 (18,48) | n.r. | Symptom, gynecological exam, ultrasonography | Herbal medicine(A)+Control intervention | Herbal medicine(B) | 9 mo | 1) TER 2) Pregnancy rate 3) Recurrence rate | 1) Positive b 2) 11.54%(TG) vs 0%(CG) 3) Positive b | n.r. |
| 10 | Guo (2013) [44] | RCT | 94 (48/46) | TG: 32.0±9.6 (22,42) CG: 31.0±7.8 (23,40) | Infertility | Symptom, gynecological exam, ultrasonograph, CA125 | Herbal medicine +Control intervention | Desogestrel and Ethinyl Estradiol | 3 mo | Pregnancy rate | Positive b | n.r. |
| 11 | Liu (2017) [45] | RCT | 72 (36/36) | n.r. (24,45) | Dysmenorrhea | Symptom, gynecological exam, ultrasonography | Herbal medicine | Sanjie Zhentong Capsule | 3 mo | 1) TER 2) VAS | 1) Positive a 2) Positive a | none |
| 12 | Yi (2018) [46] | RCT | 60 (30/30) | TG: 29.41±4.7 (n.r.) CG: 30.0±4.1(n.r.) | Dysmenorrhea, dyspareunia | Symptom, ultrasonography, antiendometrial antibody, CA125 | Herbal medicine +Control intervention | Gestrinone | 3 mo | 1) TER 2) VAS | 1) Positive a 2) Positive a | n.r. |
| Study ID | Author (Year) | Herbal Medicine | Component | Toxic Animal-Based Medicinal Materials | Dosage, Frequency | Dosing Period | |
|---|---|---|---|---|---|---|---|
| 1 | Wu (1993) [35] | n.r. | Sparganii Rhizoma 9 g, Curcumae Rhizoma 8 g, Liquidambaris Fructus 9 g, Hirudo 9 g, Paeoniae Radix Rubra 9 g, Manitis Squama 12 g, Eupolyphaga 12 g, Moutan Radicis Cortex 12 g, Salviae Miltiorrhizae Radix 12 g, Cyperi Rhizoma 12 g, Prunellae Spica 12 g | Eupolyphaga, Hirudo | n.r. | Medication discontinued during menstruation | |
| 2 | Zhang (2007) [36] | n.r. | Salviae Miltiorrhizae Radix, Persicae Semen, Corydalis Tuber, Curcumae Rhizoma, Hirudo, Linderae Radix, Olibanum, Myrrha, Cinnamomi Cortex | Hirudo | One dose per day, bid | n.r. | |
| 3 | Xu (2007) [37] | Leech Tongluo Capsule (Shuizhi tongluo jiaonang) | Hirudo 0.5 g, Manitis Squama 0.5 g, Astragali Radix 0.5 g, Draconis Sanguis 0.5 g, Codonopsis Pilosulae Radix 0.5 g, Sparganii Rhizoma 0.5 g, Curcumae Rhizoma 0.5 g, Scolopendra 0.5 g, Cnidii Rhizoma 0.5 g, Glycyrrhizae Radix et Rhizoma 0.5 g | Hirudo, Scolopendra | Six granules/time, tid | From MCD 5 | |
| 4 | Zhang (2007) [38] | Quyu Jiedu Xiaozheng Decoction (Quyu Jiedu Xiaozheng tang) | Sargentodoxa cuneata 15–30 g, Hedyotidis Herba 15–30 g, Astragali Radix 30 g, Coicis Semen 30 g, Scapharcae seu Tegillarcae Concha 15 g, Litchi Semen 15 g, Cremastrae Tuber 9–12 g, Draconis Sanguis 6–9 g, Olibanum 6–9 g, Myrrha 6–9 g, Eupolyphaga 6 g, Scolopendra 1–2 pieces, Agastachis Herba 9 g, Glycyrrhizae Radix et Rhizoma 6 g | Eupolyphaga, Scolopendra | One dose per day, n.r. | n.r. | |
| 5 | Feng (2014) [39] | Xiaoyi Zhuyun tang | Angelicae Gigantis Radix 12 g, Paeoniae Radix Rubra 12 g, Paeoniae Radix Alba 12 g, Cyperi Rhizoma 12 g, Morindae Radix 12 g, Patriniae Radix 12 g, Sparganii Rhizoma 20 g, Curcumae Rhizoma 20 g, Astragali Radix 20 g, Galli Gigeriae Endothelium Corneum 20 g, Gleditsiae Spina 20 g, Lycopi Herba 20 g, Dipsaci Radix 20 g, Drynariae Rhizoma 20 g, Eucommiae Cortex 20 g, Coicis Semen 20 g, Bupleuri Radix 9 g, Hirudo 6 g, Cervi Cornu 10 g | Hirudo | One dose per day, bid | From MCD 6 to MCD 15 (10 days) | |
| 6 | Han (2009) [40] | A | n.r. | Hirudo 4–5 g | Hirudo | n.r., bid | From 3–4 days before menstruation to the end of menstruation |
| B | n.r. | Salviae Miltiorrhizae Radix 10–15 g, Angelicae Gigantis Radix 8–10 g, Salvia chinensis Benth 8–10 g, Myrrha 10 g, Sparganii Rhizoma 8 g, Leonuri Herba 8 g | |||||
| 7 | Zhang (2009) [41] | Quyu Jiedu Xiaozheng Decoction (Quyu Jiedu Xiaozheng tang) | Sargentodoxa cuneata 30 g, Hedyotidis Herba 30 g, Coicis Semen 30 g, Astragali Radix 30 g, Scapharcae seu Tegillarcae Concha 15 g, Litchi Semen 15 g, Cremastrae Tuber 10 g, Agastachis Herba 9 g, Scolopendra two pieces, Draconis Sanguis 6 g, Eupolyphaga 6 g, Olibanum 6 g, Myrrha 6 g, Glycyrrhizae Radix et Rhizoma 6 g | Eupolyphaga, Scolopendra | One dose per day, bid | n.r. | |
| 8 | Lin (2011) [42] | Whole scorpion bergamot powder (Quanxie Foshou san) | Scorpio 6 g, Angelicae Gigantis Radix 15 g, Cnidii Rhizoma 10 g, Leonuri Herba 15 g, Cyperi Rhizoma 15 g | Scorpio | One dose per day, n.r. | From 3–4 days before menstruation | |
| 9 | Meng (2012) [43] | A | n.r. | Hirudo 1–2 g | Hirudo | n.r., tid | From 3–4 days before menstruation to the end of menstruation |
| B | n.r. | Angelicae Gigantis Radix 10–15 g, Cnidii Rhizoma 6–10 g, Leonuri Herba 15 g, Paeoniae Radix Rubra 10 g, Paeoniae Radix Alba 10 g, Curcumae Longae Rhizoma 10 g, Sparganii Rhizoma 10 g, Curcumae Rhizoma 10 g, Sargentodoxa cuneata 30 g, Sepiae Endoconcha 12 g | |||||
| 10 | Guo (2013) [44] | Bushen Quyu Decoction (Bushen Quyu tang) | Lycii Fructus 15 g, Rehmanniae Radix Preparata 15 g, Ligustri Lucidi Fructus 15 g, Cuscutae Semen 15 g, Angelicae Gigantis Radix 10 g, Paeoniae Radix Rubra 15 g, Paeoniae Radix Alba 15 g, Achyranthis Radix 12 g, Bupleuri Radix 10 g, Sappan Lignum 10 g, Sparganii Rhizoma 10 g, Curcumae Rhizoma 10 g, Hirudo 10 g | Hirudo | One dose per day, bid | From MCD 1 | |
| 11 | Liu (2017) [45] | Tongjingxiao Granules (Tongjingxiao keli) | Salviae Miltiorrhizae Radix 20 g, Paeoniae Radix Rubra 20 g, Angelicae Gigantis Radix 10 g, Scolopendra 6 g, Corydalis Tuber 20 g, Meliae Fructus 10 g, Glycyrrhizae Radix et Rhizoma 6 g | Scolopendra | One dose per day, bid | From 7 days before menstruation to the end of menstruation | |
| 12 | Yi (2018) [46] | Xiaoyi Analgesic Soup (Xiaoyi Zhitong tang) | Cinnamomi Ramulus 20 g, Poria Sclerotium 30 g, Salviae Miltiorrhizae Radix 20 g, Persicae Semen 20 g, Curcumae Rhizoma 10 g, Scolopendra two pieces, Eupolyphaga 10 g, Hirudo 10 g, Pelodiscis Carapax 20 g | Eupolyphaga, Hirudo, Scolopendra | n.r., bid | From the end of menstruation | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.-I.; Yoon, Y.-J.; Sung, S.-H.; Ha, K.-T.; Park, J.-K. Toxic Animal-Based Medicinal Materials Can Be Effective in Treating Endometriosis: A Scoping Review. Toxins 2021, 13, 145. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13020145
Hwang S-I, Yoon Y-J, Sung S-H, Ha K-T, Park J-K. Toxic Animal-Based Medicinal Materials Can Be Effective in Treating Endometriosis: A Scoping Review. Toxins. 2021; 13(2):145. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13020145
Chicago/Turabian StyleHwang, Su-In, Young-Jin Yoon, Soo-Hyun Sung, Ki-Tae Ha, and Jang-Kyung Park. 2021. "Toxic Animal-Based Medicinal Materials Can Be Effective in Treating Endometriosis: A Scoping Review" Toxins 13, no. 2: 145. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13020145





