Use of AbobotulinumtoxinA for Cosmetic Treatments in the Neck, and Middle and Lower Areas of the Face: A Systematic Review
Abstract
:1. Introduction
2. Results
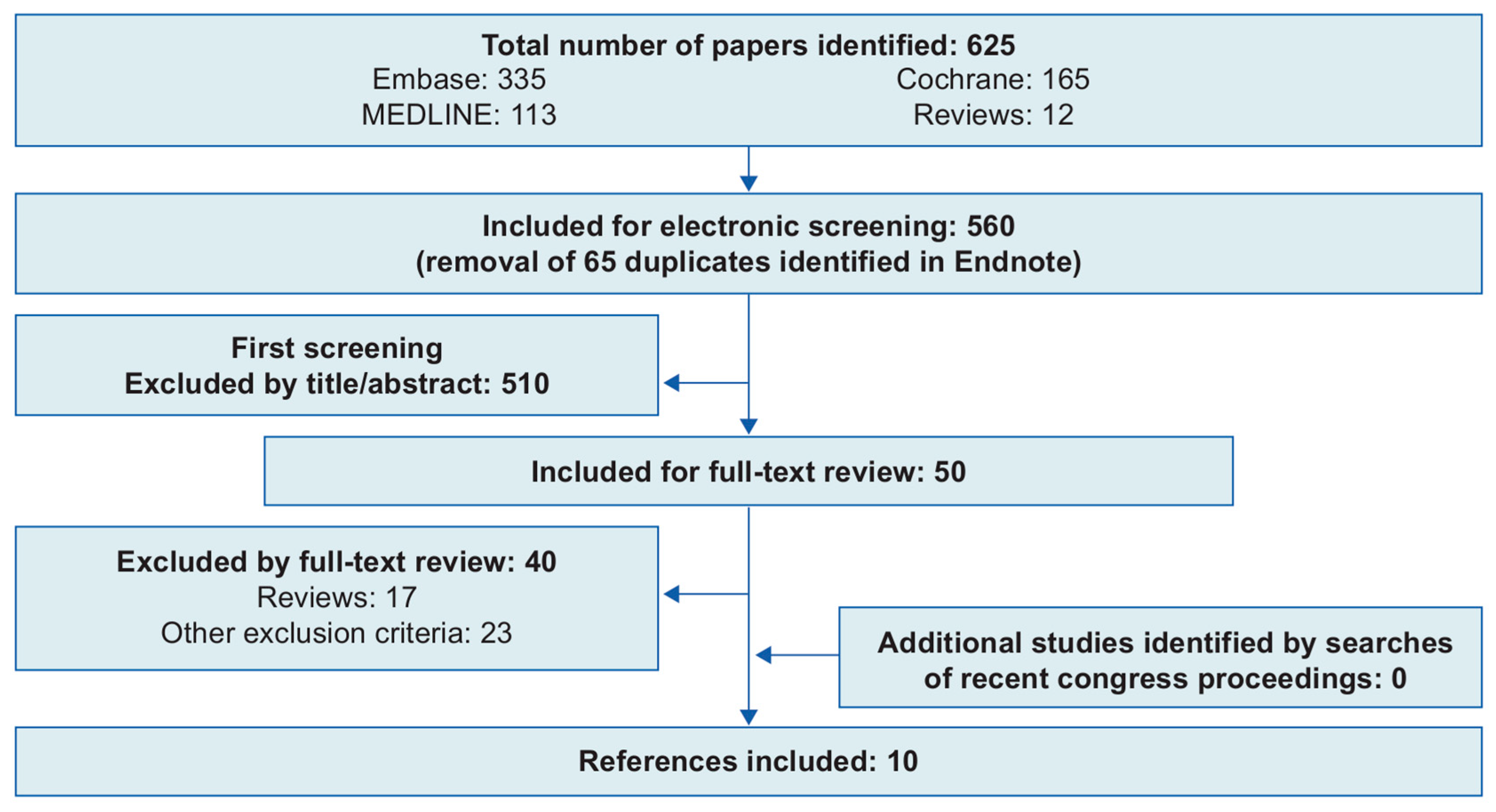
2.1. Systematic Review of the Literature
2.2. Treatment Approaches
2.3. Efficacy Outcomes
2.4. Safety Outcomes
2.5. Patient Satisfaction and Quality of Life
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Search Strategy
5.2. Eligibility Criteria
5.3. Data Extraction
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Society for Dermatologic Surgery. 2019 ASDS Consumer Survey on Cosmetic Dermatologic Procedures. Available online: https://www.asds.net/medical-professionals/practice-resources/asds-consumer-survey-on-cosmetic-dermatologic-procedures (accessed on 16 February 2021).
- American Society of Plastic Surgeons. 2016 National Plastic Surgery Statistics. Available online: https://d2wirczt3b6wjm.cloudfront.net/News/Statistics/2016/2016-plastic-surgery-statistics-report.pdf (accessed on 16 February 2021).
- International Society of Aesthetic Plastic Surgery. ISAPS International Survey on Aesthetic/Cosmetic Procedures Performed in 2019. Available online: https://www.isaps.org/wp-content/uploads/2020/12/Global-Survey-2019.pdf (accessed on 16 February 2021).
- Sundaram, H.; Signorini, M.; Liew, S.; de Almeida, A.R.T.; Wu, Y.; Vieira Braz, A.; Fagien, S.; Goodman, G.J.; Monheit, G.; Raspaldo, H.; et al. Global aesthetics consensus: Botulinum toxin type A—Evidence-based review, emerging concepts, and consensus recommendations for aesthetic use, including updates on complications. Plast. Reconstr. Surg. 2016, 137, 518e–529e. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Foster, J.A.; Rogachefsky, A.S. Pharmacology of botulinum toxin. J. Am. Acad. Dermatol. 2000, 43, 249–259. [Google Scholar] [CrossRef]
- Pirazzini, M.; Rossetto, O.; Eleopra, R.; Montecucco, C. Botulinum neurotoxins: Biology, pharmacology, and toxicology. Pharmacol. Rev. 2017, 69, 200–235. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A. Terminology for preparations of botulinum neurotoxins: What a difference a name makes. JAMA 2011, 305, 89–90. [Google Scholar] [CrossRef]
- Samizadeh, S.; De Boulle, K. Botulinum neurotoxin formulations: Overcoming the confusion. Clin. Cosmet. Investig. Dermatol. 2018, 11, 273–287. [Google Scholar] [CrossRef] [Green Version]
- Field, M.; Splevins, A.; Picaut, P.; van der Schans, M.; Langenberg, J.; Noort, D.; Snyder, D.; Foster, K. AbobotulinumtoxinA (Dysport®), onabotulinumtoxinA (Botox®), and incobotulinumtoxinA (Xeomin®) neurotoxin content and potential implications for duration of response in patients. Toxins 2018, 10, E535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United States Food and Drug Administration. DYSPORT® (abobotulinumtoxinA) for Injection, for Intramuscular Use—Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125274s107lbl.pdf (accessed on 16 February 2021).
- United States Food and Drug Administration. XEOMIN (incobotulinumtoxinA) for Injection, for Intramuscular or Intraglandular Use—Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125360s086s092lbl.pdf (accessed on 16 February 2021).
- Walker, T.J.; Dayan, S.H. Comparison and overview of currently available neurotoxins. J. Clin. Aesthetic. Dermatol. 2014, 7, 31–39. [Google Scholar]
- Electronic Medicines Compendium. Azzalure, 125 Speywood Units, Powder for Solution for Injection—Summary of Product Characteristics. Available online: https://www.medicines.org.uk/emc/product/6584/smpc (accessed on 16 February 2021).
- Ascher, B.; Talarico, S.; Cassuto, D.; Escobar, S.; Hexsel, D.; Jaen, P.; Monheit, G.D.; Rzany, B.; Viel, M. International consensus recommendations on the aesthetic usage of botulinum toxin type A (Speywood Unit)—Part II: Wrinkles on the middle and lower face, neck and chest. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Maas, C.; Kane, M.A.; Bucay, V.W.; Allen, S.; Applebaum, D.J.; Baumann, L.; Cox, S.E.; Few, J.W.; Joseph, J.H.; Lorenc, Z.P.; et al. Current aesthetic use of abobotulinumtoxinA in clinical practice: An evidence-based consensus review. Aesthetic Surg. J. 2012, 32, 8S–29S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redaelli, A.; Saromytskaya, A.; Rowland Payne, C.; Manturova, N.; Battistella, M.; Saban, Y.; Panova, O.; Wollina, U.; Landau, M.; Atamanov, V.; et al. International expert consensus on the use of AboBotulinum Toxin A (AboTA) for facial rejuvenation and primary hyperhidrosis. Cosmet. Med. 2017, 2.17, 70–80. [Google Scholar]
- Rzany, B.; Fratila, A.A.; Fischer, T.C.; Hilton, S.; Pavicic, T.; Rothhaar, A.; Sattler, G.; Sommer, B.; Pickett, A. Recommendations for the best possible use of botulinum neurotoxin type a (Speywood units) for aesthetic applications. J. Drugs Dermatol. JDD 2013, 12, 80–84. [Google Scholar]
- Bonaparte, J.P.; Ellis, D.; Quinn, J.G.; Ansari, M.T.; Rabski, J.; Kilty, S.J. A comparative assessment of three formulations of botulinum toxin A for facial rhytides: A systematic review and meta-analyses. Syst. Rev. 2013, 2, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahai, F.; Lorenc, Z.P.; Kenkel, J.M.; Fagien, S.; Hirmand, H.; Nestor, M.S.; Sclafani, A.P.; Sykes, J.M.; Waldorf, H.A. A review of abobotulinumtoxinA (Dysport). Aesthetic 2013, 33, 13S–17S. [Google Scholar] [CrossRef] [Green Version]
- Redaelli, A.; Battistella, M. Abobotulinum toxin A and fillers for facial rejuvenation: My experience and technique. Glob. Dermatol. 2017, 4, 1–4. [Google Scholar]
- Hevia, O. Retrospective review of 500 patients treated with abobotulinumtoxinA. J. Drugs Dermatol. JDD 2010, 9, 1081–1084. [Google Scholar]
- Mazzuco, R.; Hexsel, D. Gummy smile and botulinum toxin: A new approach based on the gingival exposure area. J. Am. Acad. Dermatol. 2010, 63, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Petchngaovilai, C. Midface lifting with botulinum toxin: Intradermal technique. J. Cosmet. Dermatol. 2009, 8, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, S.F.; Kechichian, E.G.; Awaida, C.J.; Tomb, R.R.; Nasr, M.W. Botulinum toxin for neck rejuvenation: Assessing efficacy and redefining patient selection. Plast. Reconstr. Surg. 2017, 140, 9e–17e. [Google Scholar] [CrossRef]
- Kim, N.H.; Chung, J.H.; Park, R.-H.; Park, J.B. The use of botulinum toxin type A in aesthetic mandibular contouring. Plast. Reconstr. Surg. 2005, 115, 919–930. [Google Scholar] [CrossRef]
- Awaida, C.J.; Jabbour, S.F.; Rayess, Y.A.; El Khoury, J.S.; Kechichian, E.G.; Nasr, M.W. Evaluation of the microbotox technique: An algorithmic approach for lower face and neck rejuvenation and a crossover clinical trial. Plast. Reconstr. Surg. 2018, 142, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.S.; Chang, B.L.; Lanni, M.; Wilson, A.J.; Beer, J.; Percec, I. Perioral rejuvenation: A prospective, quantitative dynamic three-dimensional analysis of a dual modality treatment. Aesthetic 2018, 38, 1225–1236. [Google Scholar] [CrossRef]
- Yu, C.C.; Chen, P.K.T.; Chen, Y.R. Botulinum toxin A for lower facial contouring: A prospective study. Aesthetic Plast. Surg. 2007, 31, 445–451. [Google Scholar] [CrossRef]
- Hexsel, D.; Brum, C.; Porto, M.D.; Soirefmann, M.; Siega, C.; Schilling-Souza, J.; Rodrigues, T.C. Quality of life and satisfaction of patients after full-face injections of abobotulinum toxin type A: A randomized, phase IV clinical trial. J. Drugs Dermatol. JDD 2013, 12, 1363–1367. [Google Scholar] [PubMed]
- Hexsel, D.; Brum, C.; Porto, M.D.; Soirefmann, M.; Siega, C.; Schilling-Souza, J.; Rodrigues, T.C. Full-face injections of variable total doses of abobotulinum toxin type A: Arandomized, phase IV clinical trial of safety and efficacy. J. Drugs Dermatol. JDD 2013, 12, 1356–1362. [Google Scholar] [PubMed]
- Geister, T.L.; Blessmann-Gurk, B.; Rzany, B.; Harrington, L.; Gortelmeyer, R.; Pooth, R. Validated assessment scale for platysmal bands. Dermatol. Surg. 2013, 39, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Biskup, N.; Mattison, G.; Leis, A. Development and validation of a clinical assessment tool for platysmal banding in cervicomental aesthetics of the female neck. Aesthetic Surg. J. 2015, 35, NP141–NP146. [Google Scholar] [CrossRef] [Green Version]
- Chang, B.L.; Wilson, A.J.; Taglienti, A.J.; Chang, C.S.; Folsom, N.; Percec, I. Patient perceived benefit in facial aesthetic procedures: FACE-Q as a tool to study botulinum toxin injection outcomes. Aesthetic Surg. J. 2016, 36, 810–820. [Google Scholar] [CrossRef] [Green Version]
- Levy, P.M. The ‘Nefertiti lift’: A new technique for specific re-contouring of the jawline. J. Cosmet. Laser Ther. 2007, 9, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Klassen, A.F.; Cano, S.J.; Scott, A.; Snell, L.; Pusic, A.L. Measuring patient-reported outcomes in facial aesthetic patients: Development of the FACE-Q. Facial Plast. Surg. 2010, 26, 303–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pusic, A.L.; Klassen, A.F.; Scott, A.M.; Cano, S.J. Development and psychometric evaluation of the FACE-Q satisfaction with appearance scale: A new patient-reported outcome instrument for facial aesthetics patients. Clin. Plast. Surg. 2013, 40, 249–260. [Google Scholar] [CrossRef]
- Nestor, M.; Cohen, J.L.; Landau, M.; Hilton, S.; Nikolis, A.; Haq, S.; Viel, M.; Andriopoulos, B.; Prygova, I.; Foster, K.; et al. Onset and duration of abobotulinumtoxinA for aesthetic use in the upper face: A systematic literature review. J. Clin. Aesthetic Dermatol. 2020, 13, E56–E83. [Google Scholar]
- Yiannakopoulou, E. Serious and long-term adverse events associated with the therapeutic and cosmetic use of botulinum toxin. Pharmacology 2015, 95, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Eleopra, R.; Tugnoli, V.; Caniatti, L.; De Grandis, D. Botulinum toxin treatment in the facial muscles of humans: Evidence of an action in untreated near muscles by peripheral local diffusion. Neurology 1996, 46, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- PRISMA. Transparent Reporting of Systematic Reviews and Meta-Analyses. Available online: http://www.prisma-statement.org/ (accessed on 16 February 2021).


| Study | Study Design | Country | Age, Years | Participants, N (n [%] Women, n [%] Men) | Area(s) of Injection. Further Details | Indication Assessed | Total Dose and Number of Injection Points | Injection Frequency | AbobotulinumtoxinA Dilution |
|---|---|---|---|---|---|---|---|---|---|
| Awaida et al., 2018 [26] | Non-randomized controlled trial | Lebanon | Mean (SD): 55.9 (5.8) | 25 (25 [100%] women) | Neck, lower face. Injections were administered over the entire anterior neck | Oral commissures, marionette lines, jowls, neck volume, platysmal bands at rest and at maximal contraction | Mean (SD) dose of 154 (28.6) U in 150 points of injection | NR | 500 U vial reconstituted in normal saline to a concentration of 70 U/mL |
| Chang et al., 2018 [27] | Non-randomized controlled trial | USA | Mean: 51.7 Range: 28.8–72.4 | 32 (32 [100%] women) | Lower face. Left upper, right upper, left lower, and right lower cutaneous lip | Perioral rhytids, marionette lines, chin, nasolabial fold, oral commissures, cheeks | 4–5 U per point in four points (left upper, right upper, left lower and right lower cutaneous lip; 18 U in total) | One session | NR |
| Hevia 2010 [21] | Observational study | USA | Mean: 50 1 Range: 21.0–78.0 1 | 43 2 (NR) | Neck. Platysma | Platysma | 4–12 injections (50–160 U in total) | NR | 300 U was reconstituted with 2.25 mL of 0.9% saline, resulting in concentration of 133 U/mL |
| Hexsel et al., 2013 [30] | RCT | Brazil | Mean (SD): 48.3 (7.2) Range: 30.0–60.0 | 85 (82 [96.5%] women; 3 [3.5%] men) | Mid, lower face. In each third of the face, at least two of the assessed indications (see next column) were injected | Lower eyelid wrinkles, nasal wrinkles, malar wrinkles, perioral wrinkles, marionette lines, gummy/asymmetric smile, cellulitic chin | Comparison of 120‒165 U, 166‒205 U and 206‒250 U Number of injection points NR | ≥2 sessions | 500 U reconstituted in 2 mL of 0.9% sterile saline, resulting in 250 U/mL |
| Hexsel et al., 2013 [29] | RCT | Brazil | As above (Hexsel et al., 2013 [30]) | ||||||
| Jabbour et al., 2017 [24] | Non-randomized controlled trial | Lebanon | Mean (SD): 54.8 (5.3) | 30 (30 [100%] women) | Lower face, neck. Injections administered 1–2 cm apart on a horizontal line under the mandibular border, followed by platysmal band injections 2 cm apart, vertically | Jowls, platysmal bands at rest and at maximal contraction, marionette lines, neck volume, oral commissures | 125 U maximum for global neck treatment per injection session (5 U per point in 2–4 points on each platysmal bands and for mandibular border) Mean (SD) dose of 114.3 (13.7) U | Two sessions | 500 U reconstituted in 2.5 mL of sterile saline |
| Kim et al., 2005 [25] | Non-randomized controlled trial | South Korea | Age ranges: 13‒19 years (n = 10) 20‒29 years (n = 293) 30‒39 years (n = 70) 40‒49 years (n = 9) | 383 (355 [92.7%] women; 28 [7.3%] men) | Lower face. Within 1.5 cm of the mandible angle border | Masseter muscle | 100–140 U on each side | 1–2 injections | 500 U reconstituted in 4 mL sterile saline to a final concentration of 125 U/mL |
| Mazzuco and Hexsel 2010 [22] | Observational study | Brazil | NR | 16 (NR) | Lower face. Each side of the nasolabial fold and/or the malar region, depending on type of indication | Gummy smile (anterior/posterior/mixed) | 5–15 U and 2–6 injection points depending on gummy smile type (see Mazzuco and Hexsel [22] for full details) | One session | 500 U diluted in 2 mL of 0.9% sodium chloride solution |
| Petchngaovilai 2009 [23] | Observational study | Thailand | Range: 27.0–72.0 | 261 (NR) | Mid-lower face. Mid-face lifting involving injection of the platysma and lateral part of the orbicularis oculi | Mid-face, including the platysma | 50–70 U per side Number of injection points NR | NR | 500 U diluted in 7 mL of normal saline |
| Yu et al., 2007 [28] | Non-randomized controlled trial | Taiwan | Mean: 32.8 Range: 25.0–46.0 | 10 (10 [100%] women) | Lower face. At 1 cm intervals on the masseter muscle | Masseter muscle | 120 U per masseteric muscle, 20 U per 0.1 mL over six injections | One session | 500 U per vial diluted in 2.5 mL of sterile distilled water to a concentration of 200 U/mL |
| Regions of the Neck and Middle and Lower Areas of the Face | Doses Used in Individual Studies (Actual Dose or Mean/Range Dose Across Patients) 1 | Number of Injection Points Used in Individual Studies 2 |
|---|---|---|
| Marionette lines | 18 U; 120‒250 U; 114.3 U; 154 U (Chang; Hexsel; Jabbour; Awaida) | 2–4; 4; 150 (Jabbour; Chang; Awaida) |
| Jowls | 114.3 U; 154 U (Jabbour; Awaida) | 2–4; 150 (Jabbour; Awaida) |
| Nasolabial folds/nasal wrinkles | 18 U; 120‒250 U (Chang; Hexsel) | 4 (Chang) |
| Chin | 18 U; 120‒250 U (Chang; Hexsel) | 4 (Chang) |
| Perioral rhytids | 18 U; 120‒250 U (Chang; Hexsel) | 4 (Chang) |
| Neck volume/platysma | 50–70 U; 114.3 U; 50–160 U; 154 U (Petchngaovilai; Jabbour; Hevia; Awaida) | 2–4; 4–12; 150 (Jabbour; Hevia; Awaida) |
| Masseter muscle reduction | 100–140 U; 120 U (Kim; Yu) | 6 (Yu) |
| Malar wrinkles | 120‒250 U (Hexsel) | NR (Hexsel) |
| Gummy/asymmetric smile | 5–15 U; 120‒250 U (Mazzuco; Hexsel) | 2–6 (Mazzuco) |
| Oral commissures | 18 U; 114.3 U; 154 U (Chang; Jabbour; Awaida) | 2–4; 4; 150 (Jabbour; Chang; Awaida) |
| Efficacy | Safety | ||||
|---|---|---|---|---|---|
| Study | Assessment Methods | Clinical Effect | Key Findings | AEs Reported | Pain and/or Other Safety Findings |
| Awaida et al. 2018 [26] | Validated photonumeric scales. Investigator Global Aesthetic Improvement Scale used to assess improvement in the overall appearance of the lower face and neck | NR | There was statistically significant improvement in jowls (p < 0.0001), platysmal bands with contraction (p < 0.0001) and neck volume (p < 0.0008) 15 days post-treatment compared with pre-treatment There was no improvement in platysmal bands at rest, marionette lines and oral commissures | Injection-point ecchymosis lasting 2 days (n = 3 with micro injections of aboBoNT-A, n = 6 with Nefertiti technique) Mild dysphagia lasting 2 weeks (n = 1 with Nefertiti technique) | Mean (SD) VAS scores for pain from injections were 4.6 (2.3) for the micro injection technique and 0.6 (2.3) for Nefertiti technique (on scale of 0–10) |
| Chang et al. 2018 [27] | Digital image correlation | Improvement was observed at first 14-day follow-up Duration: 90 days (final follow-up) | At day 14, there were significant reductions in the magnitude of strain in the cheek (12%; p = 0.001), chin (7.8%; p = 0.022), marionette lines (17%; p < 0.001), upper lip (6.3%; p = 0.001) and perioral region as a whole (9.3%; p = 0.001). There was a 5.9% reduction in nasolabial folds (not statistically significant, p = 0.057) At day 14, there were significant increases in perioral volume in the nasolabial folds (p = 0.004), marionette lines (p = 0.006), upper lip (p = 0.004) and oral commissures (p < 0.001) There were further reductions in strain at day 90 There was no significant change in facial strain symmetry from baseline to day 90 By day 90, only the increase in volume in the marionette lines remained significant (p = 0.039), with volumes in the other three regions returning close to baseline levels | NR | No patients had any complications as a result of injections |
| Hevia 2010 [21] | NR | NR | NR | No AEs were reported for patients who received treatment | NR |
| Hexsel et al. 2013 [30] | Dermatological evaluation, wrinkle severity assessment, review of standardized photographs | Improvement was observed at first 4-week follow-up Duration: up to 20 weeks (participants reporting improvement in nasal wrinkles and lower eyelid wrinkles at follow-up) | There was a reduction in the severity of marionette lines between baseline and week 4 (p value not reported) At week 4, most of the participants presented at least 50% improvement in lower eyelid wrinkles, nasal wrinkles, perioral wrinkles and chin At week 16, more than 15% of the participants maintained at least 50% improvement in lower eyelid wrinkles, and more than 50% of the participants maintained at least 25% improvement in nasal and lower eyelid wrinkles At week 20, 18% of participants maintained at least 25% improvement in nasal wrinkles and 28% of the subjects maintained at least 25% improvement in lower eyelid wrinkles | Excessive perioral weakness (n = 30/77, AEs linked to injection dose) Lip asymmetry (n = 3) No serious AEs | Pain after injection was reported in two participants (although the area of face treated in these participants was not reported; thus, these may be participants that received treatment in the upper face) |
| Hexsel et al. 2013 [29] | NR | NR | NR | NR | NR |
| Jabbour et al. 2017 [24] | Validated photonumeric scales | Improvement was observed at 15- and 30-day follow-up visits Duration: NR | There was significant improvement in wrinkles/lines in the platysmal bands with contraction (p < 0.001) and rest (p < 0.009) No significant improvement observed in the jowls, marionette lines and oral commissures No significant improvement in neck volume scores | Injection-point ecchymosis (n = 5) Mild dysphagia and minor neck muscle weakness for 2 weeks post-injection (n = 1) | Mean (SD) VAS score for pain from injections was 1.2 (1.1) (on scale of 0–10) |
| Kim et al. 2005 [25] | Ultrasonogram | Onset: 2–4 weeks Duration: maximum effect was at 10–12 weeks | At 3 months, the mean thickness of the masseter muscle was reduced by 31% | Crunching power is decreased (n = 192) In crunching, muscle is protruded (n = 38) Unnatural smiling appearance (n = 8) Disappearance of facial dimple (n = 4) | NR |
| Mazzuco and Hexsel 2010 [22] | Clinician assessment of photographs (with the aid of two computer programs, the area of gum exposed was measured before and after treatment, to evaluate the level of improvement) | Onset: NR Duration: 3–5 months | A decrease in the degree of gum display was measured in all patients 20–30 days following treatment The average improvement of gingival exposure was: 75.09% in the overall sample 96% in those with anterior gummy smile 61.06% in those with posterior gummy smile 90.1% in those with mixed gummy smile 71.93% in those with asymmetric gummy smile | Asymmetric smile (n = 1) Difficulty in smiling (n = 1) | NR |
| Petchngao-vilai 2009 [23] | Assessment of photographs | Onset: In some cases instantaneous, but in most cases changes seen within 5–10 days Duration: 10–14 weeks | 24.9% of participants (n = 65) attained high improvements with cheek lift, softening of nasolabial folds and re-defining of the facial contour 1 65.52% of participants (n = 171) attained moderate improvements with cheek lift and facial contouring1 9.58% of participants (n = 25) attained minimal improvements of facial contour 1 | Minor facial asymmetry (n = 8) | NR |
| Yu et al. 2007 [28] | CT scan to measure muscle volume, patient-reported scores on a VAS to record facial change | Onset: 2 weeks Duration: NR | At 3 months, the volume of the masseter muscle was: decreased to 69.36% of baseline volume on the right side; decreased to 70.44% of baseline volume on the left side; and reduced by 30% overall (p < 0.001) There was no significant reduction in the volume of the other masticating muscles (temporalis, medial pterygoid, lateral pterygoid) compared with baseline (p > 0.001) Mean score reported by patients on facial improvement reached its maximum of 7.1 at 6 months One patient reported no change to facial appearance at any point during the study At the end of the study, only one patient reported a meaningful score of 8 | Injection-point ecchymosis and swelling the day after injection that subsided 1 week later (n = 1) Soreness of bilateral masseters 1 day after the injection, which was aggravated when chewing food (n = 4) Easily fatigued while chewing food 2 days after the injection (n = 10) Bite weakness while eating vegetables or thick meat (n = 8) Less food intake because of more chewing effort required, but there was no interference to daily life (n = 1) Depression of the cheek on the right side (n = 2) | Mean VAS score 3 (on scale of 1–5) Nine participants reported a bearable discomfort during the injection |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galadari, H.; Galadari, I.; Smit, R.; Prygova, I.; Redaelli, A. Use of AbobotulinumtoxinA for Cosmetic Treatments in the Neck, and Middle and Lower Areas of the Face: A Systematic Review. Toxins 2021, 13, 169. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13020169
Galadari H, Galadari I, Smit R, Prygova I, Redaelli A. Use of AbobotulinumtoxinA for Cosmetic Treatments in the Neck, and Middle and Lower Areas of the Face: A Systematic Review. Toxins. 2021; 13(2):169. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13020169
Chicago/Turabian StyleGaladari, Hassan, Ibrahim Galadari, Riekie Smit, Inna Prygova, and Alessio Redaelli. 2021. "Use of AbobotulinumtoxinA for Cosmetic Treatments in the Neck, and Middle and Lower Areas of the Face: A Systematic Review" Toxins 13, no. 2: 169. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13020169





