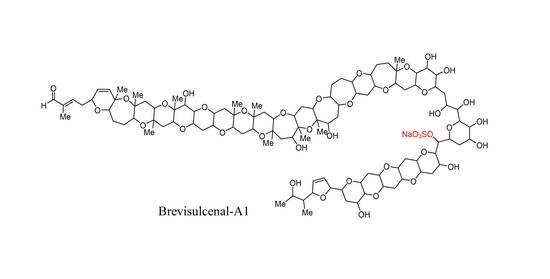
Brevisulcenals-A1 and A2, Sulfate Esters of Brevisulcenals, Isolated from the Red Tide Dinoflagellate Karenia brevisulcata
Abstract
:1. Introduction
2. Results
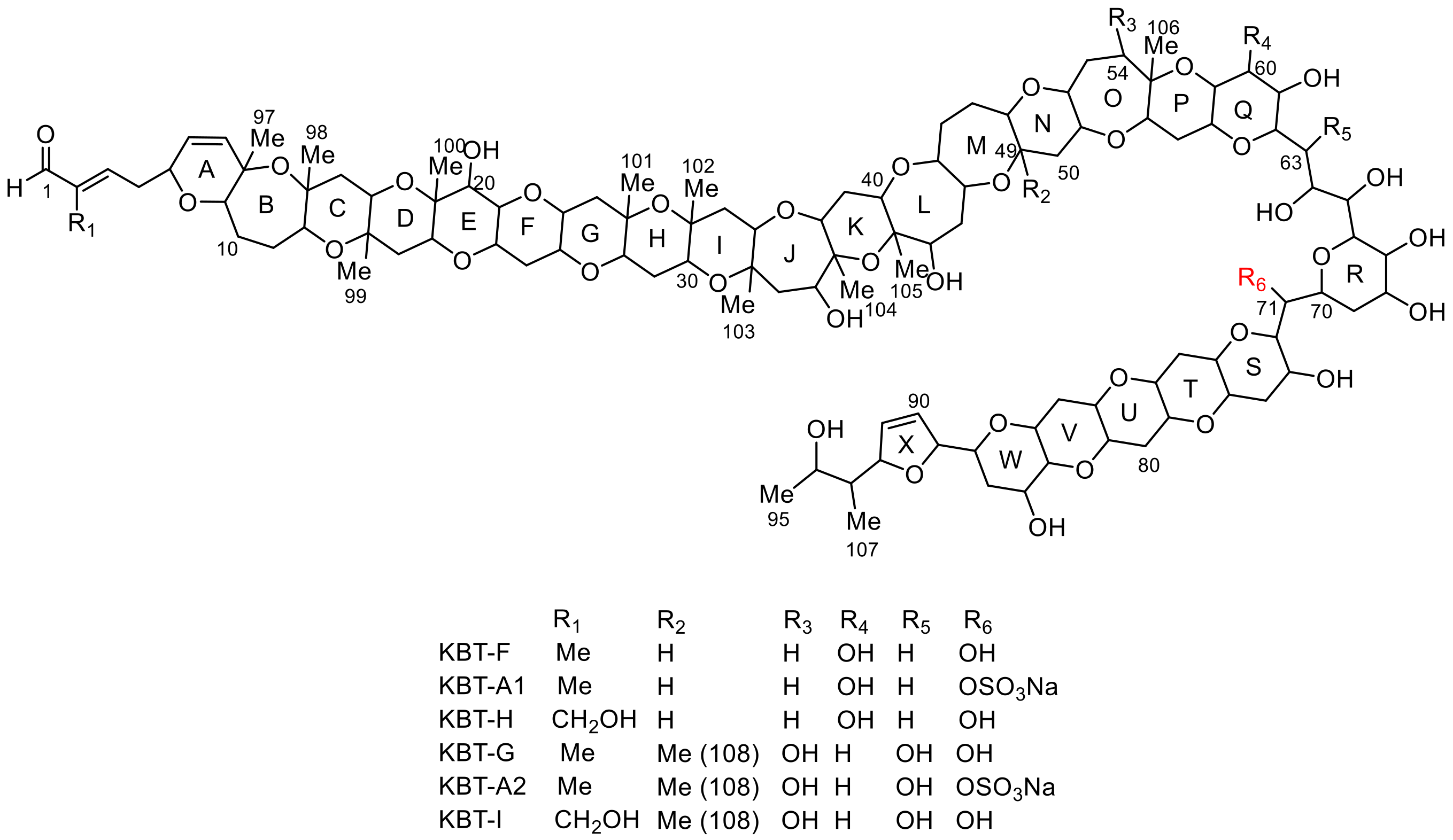
2.1. Extraction and Isolation of Brevisulcenals
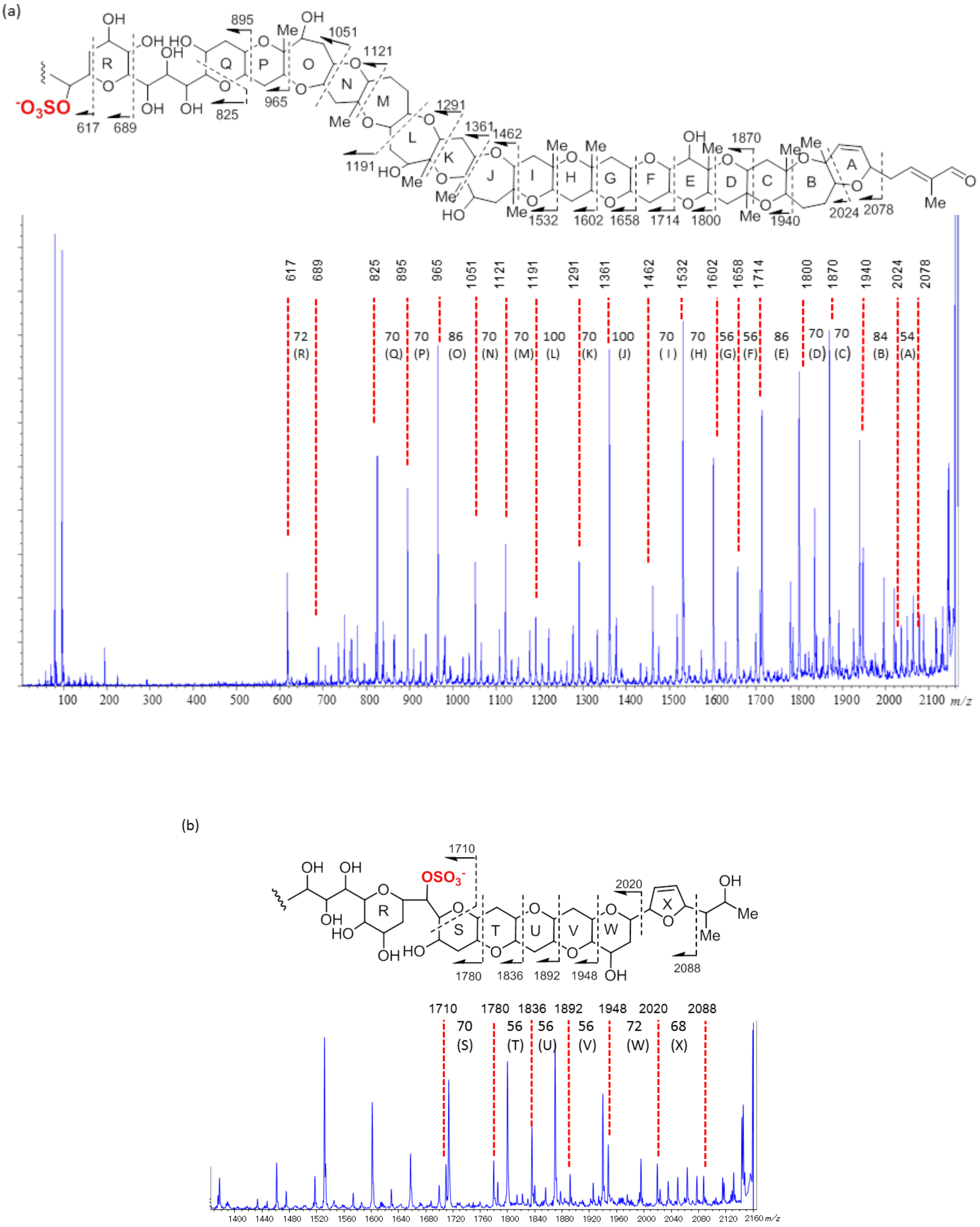
2.2. Structural Elucidation of Brevisulcenal-A2
2.3. Structural Elucidation of Brevisulcenal-A1
3. Discussion
4. Materials and Methods
4.1. General Methods
4.2. Culture Growth and Harvesting
4.3. Isolation of KBTs
4.4. MALDI MS and MALDI Tandem MS Measurements
4.5. Chemical Properties of Brevisulcenal-A2
4.6. Cytotoxicity
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoe Chang, F.H. Gymnodinium brevisulcatum sp. nov. (Gymnodiniales, Dinophyceae), a new species isolated from the 1998 summer toxic bloom in Wellington Harbour, New Zealand. Phycologia 1999, 38, 377–384. [Google Scholar] [CrossRef]
- Chang, F.H.; Chiswell, S.M.; Uddstrom, M.J. Occurrence and distribution of Karenia brevisulcata (Dinophyceae) during the 1998 summer toxic outbreaks on the central east coast of New Zealand. Phycologia 2001, 40, 215–221. [Google Scholar] [CrossRef]
- Holland, P.T.; Shi, F.; Satake, M.; Hamamoto, Y.; Ito, E.; Beuzenberg, V.; McNabb, P.; Munday, R.; Briggs, L.; Truman, P.; et al. Novel toxins produced by the dinoflagellate Karenia brevisulcata. Harmful Algae 2012, 13, 47–57. [Google Scholar] [CrossRef]
- Backer, L.C.; Fleming, L.E.; Rowan, A.; Cheng, Y.-S.; Benson, J.; Pierce, R.H.; Zaias, J.; Bean, J.; Bossart, G.D.; Jonson, D.; et al. Recreational exposure to aerosolized brevetoxins during Florida red tide events. Harmful Algae 2003, 2, 19–28. [Google Scholar] [CrossRef]
- Fleming, L.E.; Kirkpatrick, B.; Backer, L.C.; Bean, J.A.; Wanner, A.; Reich, A.; Cheng, Y.S.; Pierce, R.; Naar, J.; Abraham, W.M.; et al. Aerosolide red-tide toxins (brevetoxins) and asthma. CHEST 2007, 131, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Bean, J.A.; Fleming, L.E.; Kirkpatrick, B.; Backer, L.C.; Nierenberg, K.; Reich, A.; Cheng, Y.S.; Wanner, A.; Benson, J.; Naar, J.; et al. Florida red tide toxins (brevetoxins) and longitudinal respiratory effects in asthmatics. Harmful Algae 2011, 10, 744–748. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-Y.; Risk, M.; Ray, S.M.; Van Engen, D.; Clardy, J.; Golik, J.; James, J.C.; Nakanishi, K. Isolation and structure of brevetoxin B from the red tide dinoflagellate Ptychodiscus brevis (Gymnodinium breve). J. Am. Chem. Soc. 1981, 103, 6773–6775. [Google Scholar] [CrossRef]
- Shimizu, Y.; Chou, H.N.; Bando, H.; Van Duyne, G.; Clardy, J. Structure of brevetoxin A (GB-1 toxin), the most potent toxin in the Florida red tide organism Gymnodinium breve (Ptychodiscus brevis). J. Am. Chem. Soc. 1986, 108, 514–515. [Google Scholar] [CrossRef]
- Satake, M.; Shoji, M.; Oshima, Y.; Naoki, H.; Fujita, T.; Yasumoto, T. Gymnocin-A, a cytotoxic polyether from the notorious red tide dinoflagellate, Gymnodinium mikimotoi. Tetrahedron Lett. 2002, 43, 5829–5832. [Google Scholar] [CrossRef]
- Satake, M.; Tanaka, Y.; Ishikura, Y.; Oshima, Y.; Naoki, H.; Yasumoto, T.; Gymnocin, B. with the largest contiguous polyether rings from the red tide dinoflagellate Karenia (formerly Gymnodinium) mikimotoi. Tetrahedron Lett. 2005, 46, 3537–3540. [Google Scholar] [CrossRef]
- Suzuki, R.; Irie, R.; Harntaweesup, Y.; Tachibana, K.; Holland, P.T.; Harwood, D.T.; Shi, F.; Beuzenberg, V.; Itoh, Y.; Pascal, S.; et al. Brevisulcatic acids, marine ladder-frame polyethers frm the red tide dinoflagellate Karenia brevisulcata in New Zealand. Org. Lett. 2014, 16, 5850–5853. [Google Scholar] [CrossRef]
- Irie, R.; Suzuki, R.; Tachibana, K.; Holland, P.T.; Harwood, D.T.; Shi, F.; McNabb, P.; Beuzenberg, V.; Hayashi, F.; Zhang, H.; et al. Brevisulcatic acids from a marine microalgal species implicated in a toxic event in New Zealand. Heterocycles 2016, 92, 45–54. [Google Scholar]
- Hamamoto, Y.; Tachibana, K.; Holland, P.T.; Shi, F.; Beuzenberg, V.; Itoh, Y.; Satake, M. Brevisulcenal-F: A polycyclic ether toxin associated with massive Fish-kills in New Zealand. J. Am. Chem. Soc. 2012, 134, 4963–4968. [Google Scholar] [CrossRef]
- Satake, M.; Irie, R.; Hamamoto, Y.; Tachibana, K.; Holland, P.T.; Harwood, D.T.; Shi, F.; Beuzenberg, V.; Itoh, Y.; Hayashi, F.; et al. Polycyclic ether marine toxins from the dinoflagellate Karenia brevisulcata. Heterocycles 2018, 96, 2096–2105. [Google Scholar] [CrossRef]
- Harwood, D.T.; Shi, F.; Satake, M.; Holland, P.T. A sensitive LC-MS/MS assay for brevisulcenal and brevisulcatic acid toxins produced by the dinoflagellate Karenia brevisulcata. Toxicon 2014, 84, 19–27. [Google Scholar] [CrossRef]
- Satoh, T.; Sato, T.; Tamura, J. Development of a high-performance MALDI-TOF mass spectrometer utilizing a spiral ion trajectory. J. Am. Soc. Mass Spectrom. 2007, 18, 1318–1323. [Google Scholar] [CrossRef] [Green Version]
- Satoh, T.; Sato, T.; Kubo, A.; Tamura, J. Tandem time-of-flight mass spectrometer with high precursor ion selectivity employing spiral ion trajectory and improvement offset parabolic reflectron. J. Am. Soc. Mass Spectrom. 2011, 22, 797–803. [Google Scholar] [CrossRef] [Green Version]
- Murata, M.; Naoki, H.; Matsunaga, S.; Satake, M.; Yasumoto, T. Structure and partial stereochemical assignments for maitotoxin, the most toxic and largest natural non-biopolymer. J. Am. Chem. Soc. 1994, 116, 7098–7107. [Google Scholar] [CrossRef]
- Murata, M.; Kumagai, M.; Lee, J.S.; Yasumoto, T. Isolation and structure of yessotoxin, a novel polyether compound implicated in diarrhetic shellfish poisoning. Tetrahedron Lett. 1987, 28, 5869–5872. [Google Scholar] [CrossRef]
- Murata, M.; Gusovsky, F.; Sasaki, M.; Yokoyama, A.; Yasumoto, T.; Daly, J.W. Effect of maitotoxin analogues on calcium influx and phosphoinositide breakdown in cultured cells. Toxicon 1991, 29, 1085–1096. [Google Scholar] [CrossRef]
- Terao, K.; Ito, E.; Oarada, M.; Murata, M.; Yasumoto, T. Histopathological studies on experimental marine toxins poisoning-5. The effects in mice of yessotoxin isolated from Patinopecten yessoensis and of a desulfated derivative. Toxicon 1990, 28, 1095–1104. [Google Scholar] [CrossRef]
- Daiguji, M.; Satake, M.; Ramstad, H.; Aune, T.; Naoki, H.; Yasumoto, T. Structure and fluorometric HPLC determination of 1-desulfoyessotoxin, a new yessotoxin analog isolated from mussels from Norway. Nat. Toxins 1998, 6, 235–239. [Google Scholar] [CrossRef]
| Pos. | KBT-A1a | KBT-A2a | Pos. | KBT-A1a | KBT-A2a | Pos. | KBT-A1a | KBT-A2a | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | δH | δC | δH | δC | δH | δC | |||
| 1 | 9.51 | 197.1 | 9.54 | 197.1 | 36 | 4.11 | 78.7 | 4.11 | 78.3 | 70 | 4.92 | 70.7 | 4.92 | 72.6 |
| 2 | 143.3 | 143.3 | 37 | 82.7 | 82.7 | 71 | 5.72 | 71.1 | 5.70 | 73.3 | ||||
| 3 | 6.61 | 151.9 | 6.61 | 151.9 | 38 | 4.31 | 80.0 | 4.30 | 80.0 | 72 | 4.20 | 81.7 | 4.06 | 83.4 |
| 4a | 2.45 | 37.0 | 2.46 | 36.6 | 39a | 2.11 | 33.3 | 2.12 | 32.8 | 73 | 4.77 | 64.7 | 4.67 | 67.1 |
| 4b | 2.55 | 2.56 | 39b | 2.22 | 2.24 | 74a | 2.39 | 38.9 | 2.44 | 39.9 | ||||
| 5 | 4.37 | 76.8 | 4.35 | 76.2 | 40 | 4.13 | 78.5 | 4.10 | 77.8 | 74b | 2.69 | 2.83 | ||
| 6 | 5.45 | 127.3 | 5.45 | 127.0 | 41 | 82.7 | 82.7 | 75 | 3.35 | 79.8 | 3.19 | 79.7 | ||
| 7 | 5.97 | 140.6 | 6.01 | 139.9 | 42 | 4.00 | 75.7 | 4.02 | 75.9 | 76 | 3.71 | 76.4 | 3.48 | 79.1 |
| 8 | 78.3 | 78.3 | 43a | 2.22 | 42.5 | 2.20 | 42.5 | 77a | 2.16 | 37.7 | 1.82 | 37.9 | ||
| 9 | 3.85 | 80.8 | 3.85 | 80.0 | 43b | 2.64 | 2.43 | 77b | 2.51 | 2.49 | ||||
| 10a | 1.96 | 26.9 | 1.97 | 26.4 | 44 | 3.82 | 84.5 | 4.14 | 73.3 | 78 | 3.38 | 79.4 | 3.26 | 79.5 |
| 10b | 2.03 | 2.03 | 45 | 4.13 | 85.6 | 4.24 | 85.0 | 79 | 3.45 | 80.1 | 3.45 | 80.1 | ||
| 11a | 1.88 | 27.0 | 1.90 | 26.9 | 46a | 2.11 | 33.7 | 2.14 | 35.2 | 80a | 2.29 | 38.7 | 2.20 | 37.5 |
| 11b | 2.04 | 2.05 | 46b | 2.25 | 2.20 | 80b | 2.56 | 2.55 | ||||||
| 12 | 4.04 | 76.7 | 4.07 | 75.4 | 47a | 1.98 | 30.7 | 1.72 | 27.2 | 81 | 3.22 | 80.7 | 3.28 | 79.7 |
| 13 | 81.4 | 81.4 | 47b | 2.04 | 2.02 | 82 | 3.38 | 80.6 | 3.38 | 79.2 | ||||
| 14a | 2.03 | 44.7 | 2.05 | 43.7 | 48 | 3.09 | 85.6 | 3.17 | 88.5 | 83a | 1.72 | 39.1 | 1.74 | 38.4 |
| 14b | 2.08 | 2.08 | 49 | 3.21 | 85.3 | 78.3 | 83b | 2.50 | 2.52 | |||||
| 15 | 3.91 | 73.0 | 3.90 | 72.7 | 50a | 1.86 | 42.5 | 1.89 | 49.1 | 84 | 4.19 | 66.9 | 4.26 | 66.4 |
| 16 | 76.4 | 76.4 | 50b | 2.50 | 2.44 | 85 | 3.22 | 83.7 | 3.27 | 83.3 | ||||
| 17a | 1.99 | 41.9 | 1.98 | 41.6 | 51 | 3.41 | 82.6 | 4.39 | 79.7 | 86 | 4.37 | 67.8 | 4.40 | 67.4 |
| 17b | 2.24 | 2.22 | 52 | 3.21 | 83.1 | 3.52 | 83.0 | 87a | 1.95 | 34.6 | 2.01 | 34.0 | ||
| 18 | 4.52 | 74.7 | 4.53 | 74.2 | 53a | 1.67 | 32.0 | 2.14 | 39.0 | 87b | 2.55 | 2.58 | ||
| 19 | 79.6 | 79.6 | 53b | 1.97 | 2.48 | 88 | 3.67 | 79.1 | 3.69 | 78.4 | ||||
| 20 | 4.39 | 75.3 | 4.39 | 75.1 | 54 | 1.76 | 40.5 | 4.06 | 76.8 | 89 | 5.87 | 87.5 | 5.86 | 86.8 |
| 21 | 3.67 | 82.8 | 3.67 | 82.4 | 1.97 | 90 | 6.09 | 131.8 | 6.10 | 132.0 | ||||
| 22 | 4.20 | 75.3 | 4.20 | 75.4 | 55 | 80.1 | 82.0 | 91 | 6.31 | 132.8 | 6.31 | 132.3 | ||
| 23a | 1.89 | 38.5 | 1.90 | 38.0 | 56 | 3.31 | 83.7 | 4.26 | 77.1 | 92 | 5.13 | 92.3 | 5.01 | 90.7 |
| 23b | 2.72 | 2.72 | 57a | 2.08 | 32.9 | 2.08 | 34.5 | 93 | 1.58 | 48.9 | 1.49 | 48.4 | ||
| 24 | 3.42 | 81.8 | 3.41 | 81.8 | 57b | 2.31 | 2.33 | 94 | 4.38 | 68.8 | 4.4 | 67.4 | ||
| 25 | 3.59 | 80.6 | 3.59 | 80.6 | 58 | 4.04 | 71.6 | 3.25 | 80.9 | 95 | 1.36 | 23.8 | 1.37 | 23.1 |
| 26a | 1.73 | 45.9 | 1.74 | 46.13 | 59 | 3.63 | 72.8 | 3.76 | 71.0 | 96 | 1.74 | 11.8 | 1.74 | 11.4 |
| 26b | 2.32 | 2.39 | 60a | 4.43 | 70.8 | 1.67 | 41.9 | 97 | 1.40 | 24.1 | 1.47 | 24.3 | ||
| 27 | 76.3 | 76.3 | 60b | 2.41 | 98 | 1.56 | 23.3 | 1.61 | 22.9 | |||||
| 28 | 3.44 | 86.5 | 3.46 | 85.9 | 61 | 4.07 | 73.1 | 4.46 | 73.6 | 99 | 1.48 | 17.6 | 1.48 | 17.3 |
| 29a | 2.02 | 30.2 | 2.02 | 30.2 | 62 | 4.78 | 74.0 | 4.07 | 82.4 | 100 | 1.49 | 19.8 | 1.50 | 17.6 |
| 29b | 2.07 | 2.07 | 63a | 2.59 | 35.7 | 4.72 | 77.2 | 101 | 1.45 | 24.9 | 1.47 | 24.3 | ||
| 30 | 3.67 | 76.6 | 3.67 | 76.2 | 63b | 2.71 | 102 | 1.63 | 23.3 | 1.69 | 23.2 | |||
| 31 | 77.2 | 77.2 | 64 | 5.18 | 70.3 | 5.26 | 72.7 | 103 | 1.57 | 24.2 | 1.58 | 22.8 | ||
| 32a | 1.89 | 45.6 | 1.91 | 44.8 | 65 | 5.17 | 72.8 | 4.89 | 73.6 | 104 | 1.39 | 21.9 | 1.40 | 21.4 |
| 32b | 2.33 | 2.41 | 66 | 4.11 | 75.6 | 4.39 | 76.5 | 105 | 1.39 | 24.9 | 1.40 | 23.2 | ||
| 33 | 4.85 | 81.5 | 4.89 | 80.9 | 67 | 4.47 | 72.6 | 4.50 | 75.4 | 106 | 1.39 | 19.5 | 1.46 | 18.2 |
| 34 | 81.7 | 81.7 | 68 | 4.87 | 68.2 | 5.11 | 72.4 | 107 | 1.02 | 12.6 | 1.04 | 11.5 | ||
| 35a | 2.39 | 48.9 | 2.29 | 48.4 | 69a | 2.11 | 33.2 | 2.09 | 35.1 | 108 | 1.38 | 17.0 | ||
| 35b | 2.59 | 2.60 | 69b | 3.52 | 3.46 | |||||||||
| Pos. | ΔδH | ΔδC | Pos. | ΔδH | ΔδC | Pos. | ΔδH | ΔδC |
|---|---|---|---|---|---|---|---|---|
| 65 | +0.08 | 0.0 | 69a | +0.10 | +0.4 | 72 | +0.15 | −0.8 |
| 66 | +0.05 | 0.0 | 69b | −0.30 | 73 | −0.10 | −0.3 | |
| 67 | 0.00 | 0.0 | 70 | −0.19 | +1.6 | 74a | 0.00 | +1.7 |
| 68 | −0.61 | −0.3 | 71 | −0.65 | −6.2 | 74b | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satake, M.; Irie, R.; Holland, P.T.; Harwood, D.T.; Shi, F.; Itoh, Y.; Hayashi, F.; Zhang, H. Brevisulcenals-A1 and A2, Sulfate Esters of Brevisulcenals, Isolated from the Red Tide Dinoflagellate Karenia brevisulcata. Toxins 2021, 13, 82. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13020082
Satake M, Irie R, Holland PT, Harwood DT, Shi F, Itoh Y, Hayashi F, Zhang H. Brevisulcenals-A1 and A2, Sulfate Esters of Brevisulcenals, Isolated from the Red Tide Dinoflagellate Karenia brevisulcata. Toxins. 2021; 13(2):82. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13020082
Chicago/Turabian StyleSatake, Masayuki, Raku Irie, Patrick T. Holland, D Tim Harwood, Feng Shi, Yoshiyuki Itoh, Fumiaki Hayashi, and Huiping Zhang. 2021. "Brevisulcenals-A1 and A2, Sulfate Esters of Brevisulcenals, Isolated from the Red Tide Dinoflagellate Karenia brevisulcata" Toxins 13, no. 2: 82. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13020082