Zearalenone Induces Apoptosis and Cytoprotective Autophagy in Chicken Granulosa Cells by PI3K-AKT-mTOR and MAPK Signaling Pathways
Abstract
:1. Introduction
2. Results
2.1. Analysis of the Cell Viability
2.2. ZEA Induces Apoptosis in Chicken Granulosa Cells
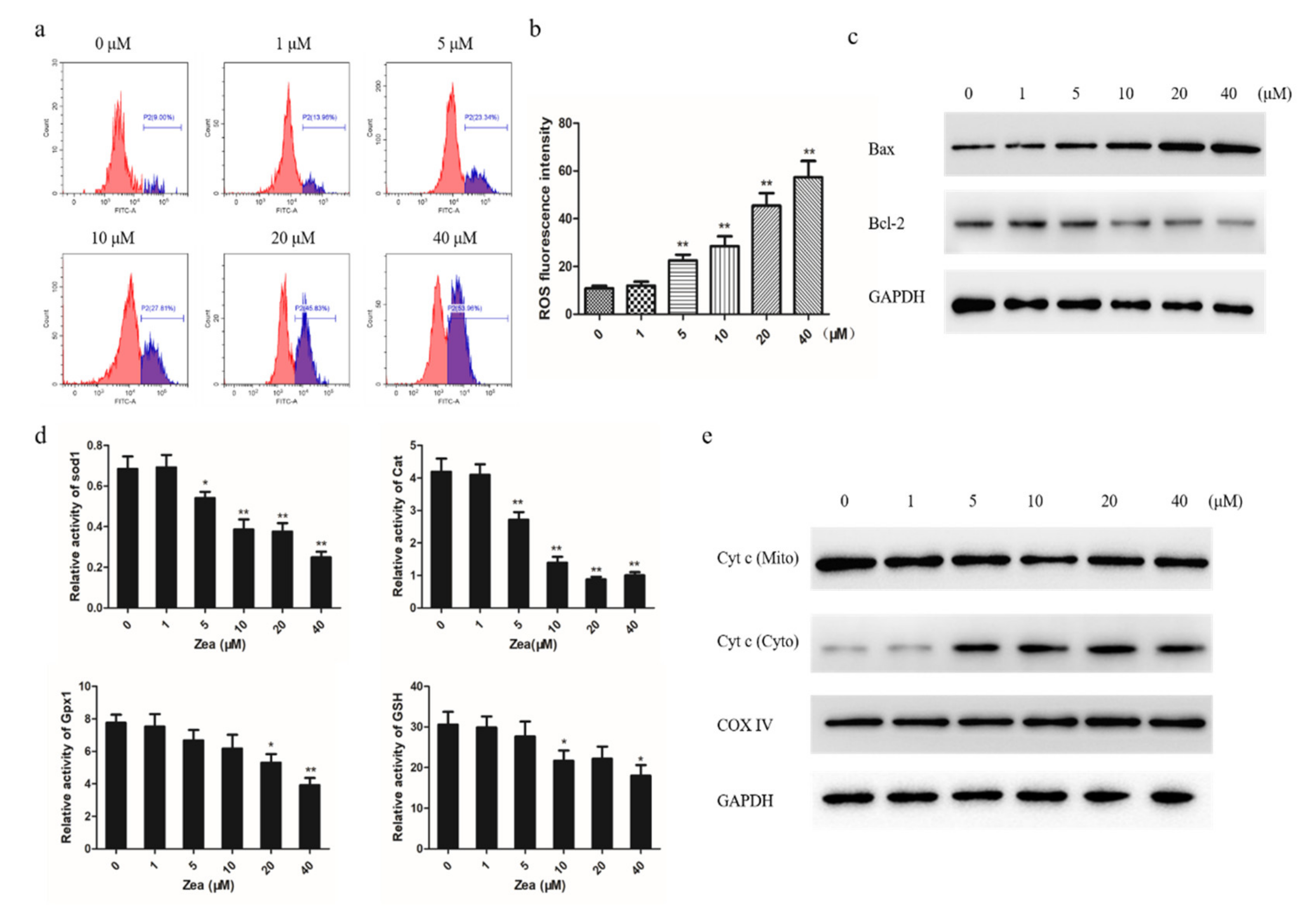
2.3. The Mitochondrial Apoptotic Pathway was Activated by ZEA
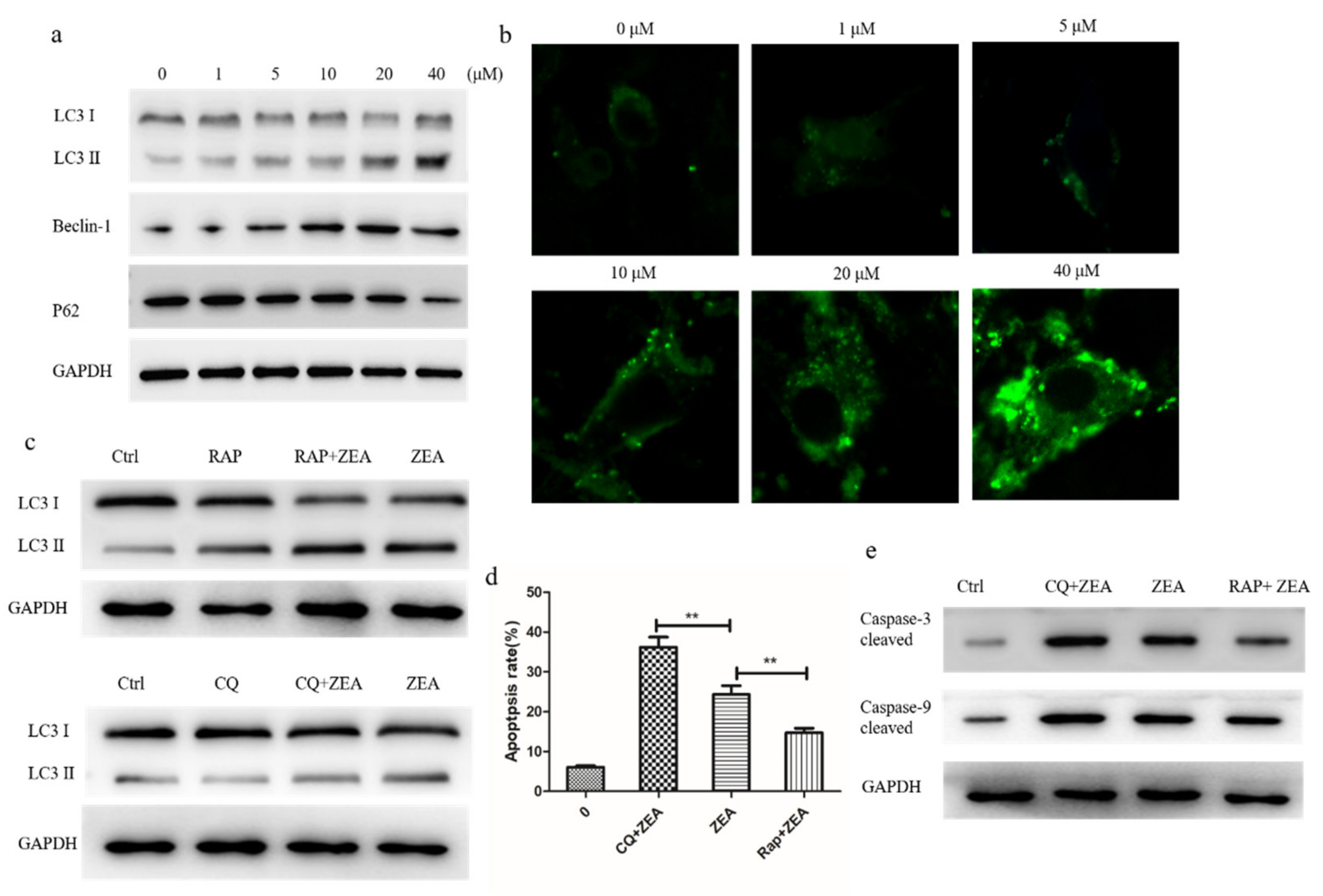
2.4. ZEA Induces Autophagy and Delays Apoptosis in Chicken Granulosa Cells
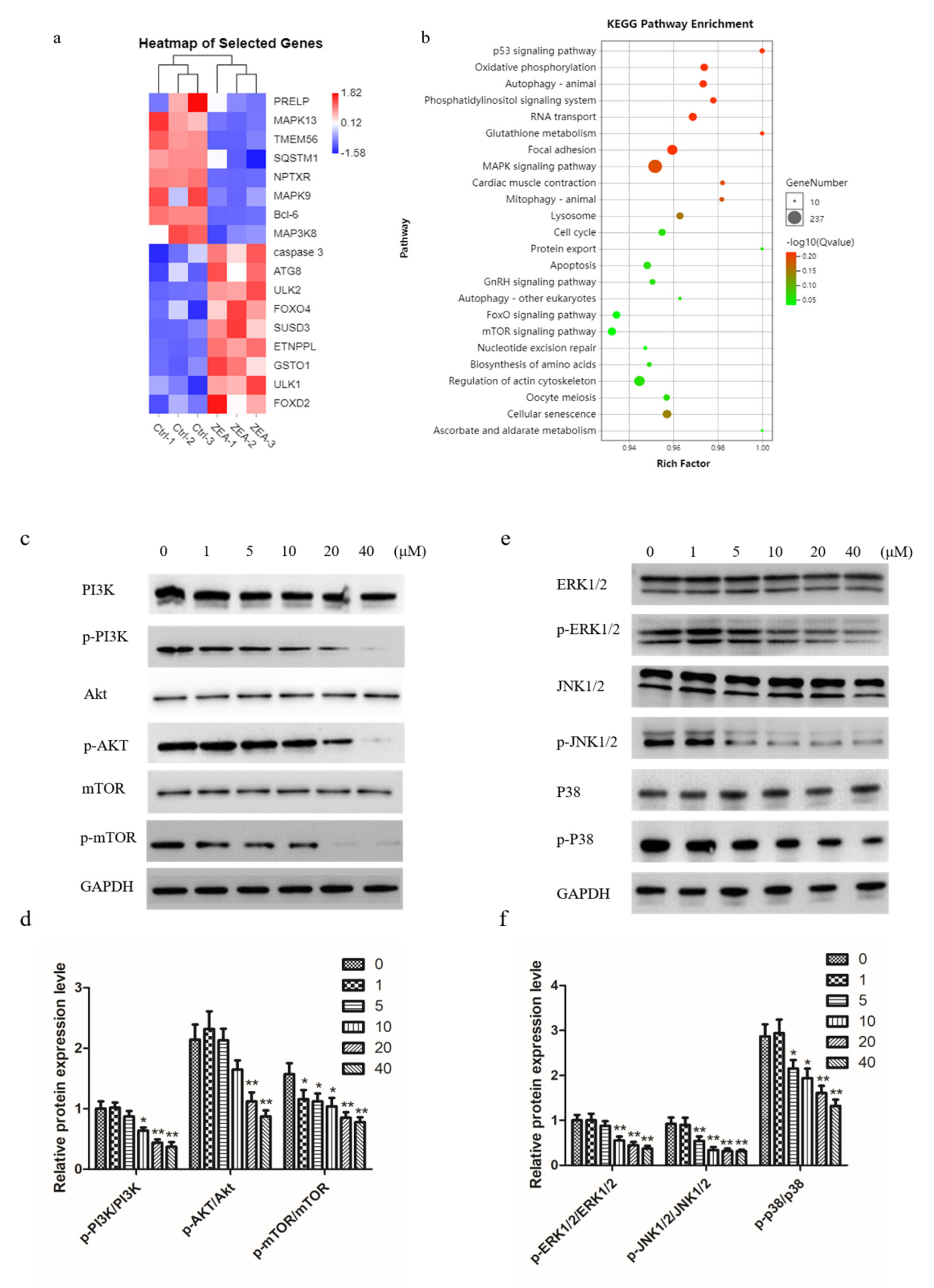
2.5. ZEA Inhibits PI3K/AKT/mTOR and MAPK Signaling Pathway in Chicken Granulosa Cells
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals
5.2. Granulosa Cells Culture
5.3. Intracellular Reactive Oxygen Species (ROS)
5.4. Cell Viability Assay
5.5. Flow Cytometry Apoptosis
5.6. Western Blotting Analysis.
5.7. Immunofluorescence Assay
5.8. TUNEL Assay
5.9. RNA Isolation and qRT-PCR
5.10. Autophagy Analysis
5.11. Antioxidative Enzymes Detection
5.12. RNA-seq
5.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, W.; Wang, B.; Si, M.; Zou, H.; Song, R.; Gu, J.; Yuan, Y.; Liu, X.; Zhu, G.; Bai, J. Zearalenone altered the cytoskeletal structure via ER stress- autophagy- oxidative stress pathway in mouse TM4 Sertoli cells. Sci. Rep. 2018, 8, 3320. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, W.; Bian, X.; Yuan, Y.; Gu, J.; Liu, X.; Liu, Z.; Bian, J. Zearalenone induces apoptosis and cytoprotective autophagy in primary Leydig cells. Toxicol. Lett. 2014, 226, 182–191. [Google Scholar] [CrossRef]
- Han, J.; Wang, T.; Fu, L.; Shi, L.Y.; Zhu, C.C.; Liu, J.; Zhang, Y.; Cui, X.S.; Kim, N.H.; Sun, S.C. Altered oxidative stress, apoptosis/autophagy, and epigenetic modifications in Zearalenone-treated porcine oocytes. Toxicol. Res. 2015, 4, 1184–1194. [Google Scholar] [CrossRef]
- Eppig, J.J. Intercommunication between mammalian oocytes and companion somatic cells. Bioessays New Rev. Mol. Cell. Dev. Biol. 1991, 13, 569–574. [Google Scholar] [CrossRef]
- Eppig, J.J. Maintenance of meiotic arrest and the induction of oocyte maturation in mouse oocyte-granulosa cell complexes developed in vitro from preantral follicles. Biol. Reprod. 1991, 45, 824–830. [Google Scholar] [CrossRef] [Green Version]
- Yuan, S.Y.; Hong, S.S.; Zheng, B.H.; Wei, L.I.; Ming, J.L.; Jing, H.T. Apoptosis in Granulosa cells during follicular atresia: relationship with steroids and insulin-like growth factors. Cell Res. 2004, 14, 341–346. [Google Scholar]
- Kolesarova, A.; Capcarova, M.; Medvedova, M.; Sirotkin, A.V.; Kovacik, J. In vitro assessment of iron effect on porcine ovarian granulosa cells: Secretory activity, markers of proliferation and apoptosis. Physiol. Res. 2011, 60, 503–510. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on the risks for animal and public health related to the presence of phomopsins in feed and food. Efsa J. 2019, 17, e05860. [Google Scholar]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O. Current Situation of Mycotoxin Contamination and Co-occurrence in Animal Feed—Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef] [Green Version]
- Reddy, K.; Salleh, B.; Saad, B.; Abbas, H.K.; Abel, C.A.; Shier, W.T. An overview of mycotoxin contamination in foods and its implications for human health. Toxin Rev. 2010, 29, 3–26. [Google Scholar] [CrossRef]
- Ben, S.I.; Boussabbeh, M.; Pires, D.S.J.; Guilbert, A.; Bacha, H.; Abidessefi, S.; Lemaire, C. SIRT1 protects cardiac cells against apoptosis induced by zearalenone or its metabolites α- and β-zearalenol through an autophagy-dependent pathway. Toxicol. Appl. Pharmacol. 2016, 314. [Google Scholar]
- M’Baye, M.; Hua, G.; Khan, H.A.; Yang, L. RNAi-mediated knockdown of INHBB increases apoptosis and inhibits steroidogenesis in mouse granulosacells. J. Reprod. Dev. 2015, 61, 391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Yuan, H.; Guo, C.; Lu, Y.; Deng, S.; Yang, Y.; Wei, Q.; Wen, L.; He, Z. Zearalenone induces apoptosis and necrosis in porcine granulosa cells via a caspase-3- and caspase-9-dependent mitochondrial signaling pathway. J. Cell. Physiol. 2012, 227, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Cao, M.; Lai, F.; Yang, F.; Ge, W.; Zhang, X.; Cheng, S.; Sun, X.; Qin, G.; Shen, W. Oxidative Stress Induced by Zearalenone in Porcine Granulosa Cells and Its Rescue by Curcumin In Vitro. PLoS ONE 2015, 10, e0127551. [Google Scholar] [CrossRef] [Green Version]
- Cortiella, J.; Niles, J.; Cantu, A.; Brettler, A.; Pham, A.; Vargas, G.; Winston, S.; Wang, J.; Walls, S.; Nichols, J.E. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng. Part A 2010, 16, 2565–2580. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.H.; Son, H.Y.; Cho, S.W.; Ha, C.S.; Kang, B.H. Zearalenone induces male germ cell apoptosis in rats. Toxicol. Lett. 2003, 138, 185–192. [Google Scholar] [CrossRef]
- Banjerdpongchai, R.; Kongtawelert, P.; Khantamat, O.; Srisomsap, C.; Chokchaichamnankit, D.; Subhasitanont, P.; Svasti, J. Mitochondrial and endoplasmic reticulum stress pathways cooperate in zearalenone-induced apoptosis of human leukemic cells. J. Hematol. Oncol. 2010, 3, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaji, T.; Nakamura, S.; Takematsu, H.; Kawasaki, T.; Kozutsumi, Y. Apoptosis of CTLL-2 cells induced by an immunosuppressant, ISP-I, is caspase-3-like protease-independent. J. Biochem. 2001, 129, 521–527. [Google Scholar]
- Zheng, W.L.; Wang, B.J.; Wang, L.; Shan, Y.P.; Zou, H.; Song, R.L.; Wang, T.; Gu, J.H.; Yuan, Y.; Liu, X.Z. ROS-Mediated Cell Cycle Arrest and Apoptosis Induced by Zearalenone in Mouse Sertoli Cells via ER Stress and the ATP/AMPK Pathway. Toxins 2018, 10, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sambuu, R.; Takagi, M.; Namula, Z.; Otoi, T.; Shiga, S.; Rodrigues, D.S.R.; Finkgremmels, J. Effects of exposure to zearalenone on porcine oocytes and sperm during maturation and fertilization in vitro. J. Reprod. Dev. 2011, 57, 547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomaszewski, J.; Miturski, R.; Semczuk, A.; Kotarski, J.; Jakowicki, J. Tissue zearalenone concentration in normal, hyperplastic and neoplastic human endometrium. Ginekologia Polska 1998, 69, 363. [Google Scholar] [PubMed]
- Ayed-Boussema, I.; Bouaziz, C.; Rjiba, K.; Valenti, K.; Laporte, F.; Bacha, H.; Hassen, W. The mycotoxin Zearalenone induces apoptosis in human hepatocytes (HepG2) via p53-dependent mitochondrial signaling pathway. Toxicol. In Vitro 2008, 22, 1671–1680. [Google Scholar] [CrossRef]
- Skommer, J. Brittain, T. Raychaudhuri, S. Bcl-2 inhibits apoptosis by increasing the time-to-death and intrinsic cell-to-cell variations in the mitochondrial pathway of cell death. Apoptosis 2010, 15, 1223–1233. [Google Scholar] [CrossRef] [Green Version]
- Bras, M.; Queenan, B.; Susin, S.A. Programmed cell death via mitochondria: Different modes of dying. Biochem. Biokhimiia 2005, 70, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Lee, K.T.; Chi, S.G.; Park, J.H. Costunolide induces apoptosis by ROS-mediated mitochondrial permeability transition and cytochrome C release. Biol. Pharm. Bull. 2002, 24, 303–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estaquier, J.; Vallette, F.; Vayssiere, J.L.; Mignotte, B. The mitochondrial pathways of apoptosis. J. Beijing Med Univ. 2002, 942, 157. [Google Scholar]
- Li, G.Y.; Xie, P.; Li, H.Y.; Hao, L.; Xiong, Q.; Qiu, T. Involment of p53, Bax, and Bcl-2 pathway in microcystins-induced apoptosis in rat testis. Environ. Toxicol. 2011, 26, 111–117. [Google Scholar] [CrossRef]
- Yu, J.Y.; Zheng, Z.H.; Son, Y.O.; Shi, X.; Jang, Y.O.; Lee, J.C. Mycotoxin zearalenone induces AIF- and ROS-mediated cell death through p53- and MAPK-dependent signaling pathways in RAW264.7 macrophages. Toxicol. In Vitro 2011, 25, 1654–1663. [Google Scholar] [CrossRef]
- Fan, W.; Shen, T.; Ding, Q.; Lv, Y.; Li, L.; Huang, K.; Yan, L.; Song, S. Zearalenone induces ROS-mediated mitochondrial damage in porcine IPEC-J2 cells. J. Biochem. Mol. Toxicol. 2017, 31, e21944. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Emr, S.D. Autophagy as a regulated pathway of cellular degradation. Science 2000, 290, 1717–1721. [Google Scholar] [CrossRef]
- Takagi, H.; Matsui, Y.; Sadoshima, J. The role of autophagy in mediating cell survival and death during ischemia and reperfusion in the heart. Antioxid. Redox Signal. 2007, 9, 1373–1381. [Google Scholar] [CrossRef]
- Han, R.; Liang, H.; Qin, Z.H.; Liu, C.Y. Crotoxin induces apoptosis and autophagy in human lung carcinoma cells in vitro via activation of the p38MAPK signaling pathway. Acta Pharmacol Sin. 2014, 35, 1323–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moscat, J.; Diaz-Meco, M.T. p62 at the Crossroads of Autophagy, Apoptosis, and Cancer. Cell 2009, 137, 1001–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiuri, M.C.; Zalckvar, E.; Kimchi, A.; Kroemer, G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Chen, M.; Jiang, Z.; Zhao, F.; Xi, B.; Xu, Z.; Fu, H.; Zhou, K. Platycodin-D Induced Autophagy in Non-Small Cell Lung Cancer Cells via PI3K/Akt/mTOR and MAPK Signaling Pathways. J. Cancer 2015, 6, 623–631. [Google Scholar] [CrossRef] [Green Version]
- Hirota, Y.; Yamashita, S.; Kurihara, Y.; Jin, X.; Aihara, M.; Saigusa, T.; Kang, D.; Kanki, T. Mitophagy is primarily due to alternative autophagy and requires the MAPK1 and MAPK14 signaling pathways. Autophagy 2015, 11, 332–343. [Google Scholar] [CrossRef] [Green Version]
- Herassandoval, D.; Pérezrojas, J.M.; Hernándezdamián, J.; Pedrazachaverri, J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell. Signal. 2014, 26, 2694–2701. [Google Scholar] [CrossRef]
- So, M.; Sha, S.; Antoniou, M.; Wu, S.; Tanun, K. Mycotoxin Zearalenone induced apoptosis in BEAS-2B cells through generation of ROS and activation of JNK and p38 MAPKs signalling pathways. BMC Genet. 2012, 6, 231–240. [Google Scholar]
- Gilbert, A.B.; Evans, A.J.; Perry, M.M.; Davidson, M.H. A method for separating the granulosa cells, the basal lamina and the theca of the preovulatory ovarian follicle of the domestic fowl (Gallus domesticus). J. Reprod. Fertil. 1977, 50, 179–181. [Google Scholar] [CrossRef] [Green Version]
- Ahumada-Solórzano, S.M.; Carranza, M.E.; Pedernera, E.; Rodríguez-Méndez, A.J.; Luna, M.; Arámburo, C. Local expression and distribution of growth hormone and growth hormone receptor in the chicken ovary: Effects of GH on steroidogenesis in cultured follicular granulosa cells. Gen. Comp. Endocrinol. 2012, 175, 297–310. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Wang, H.; Wang, J.; Han, S.; Zhang, Y.; Ma, M.; Zhu, Q.; Zhang, K.; Yin, H. Zearalenone Induces Apoptosis and Cytoprotective Autophagy in Chicken Granulosa Cells by PI3K-AKT-mTOR and MAPK Signaling Pathways. Toxins 2021, 13, 199. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13030199
Zhu Y, Wang H, Wang J, Han S, Zhang Y, Ma M, Zhu Q, Zhang K, Yin H. Zearalenone Induces Apoptosis and Cytoprotective Autophagy in Chicken Granulosa Cells by PI3K-AKT-mTOR and MAPK Signaling Pathways. Toxins. 2021; 13(3):199. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13030199
Chicago/Turabian StyleZhu, Yifeng, Heng Wang, Jianping Wang, Shunshun Han, Yao Zhang, Menggen Ma, Qing Zhu, Keying Zhang, and Huadong Yin. 2021. "Zearalenone Induces Apoptosis and Cytoprotective Autophagy in Chicken Granulosa Cells by PI3K-AKT-mTOR and MAPK Signaling Pathways" Toxins 13, no. 3: 199. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13030199




