The Use of Sodium Hypochlorite at Point-of-Use to Remove Microcystins from Water Containers
Abstract
:1. Introduction
2. Results
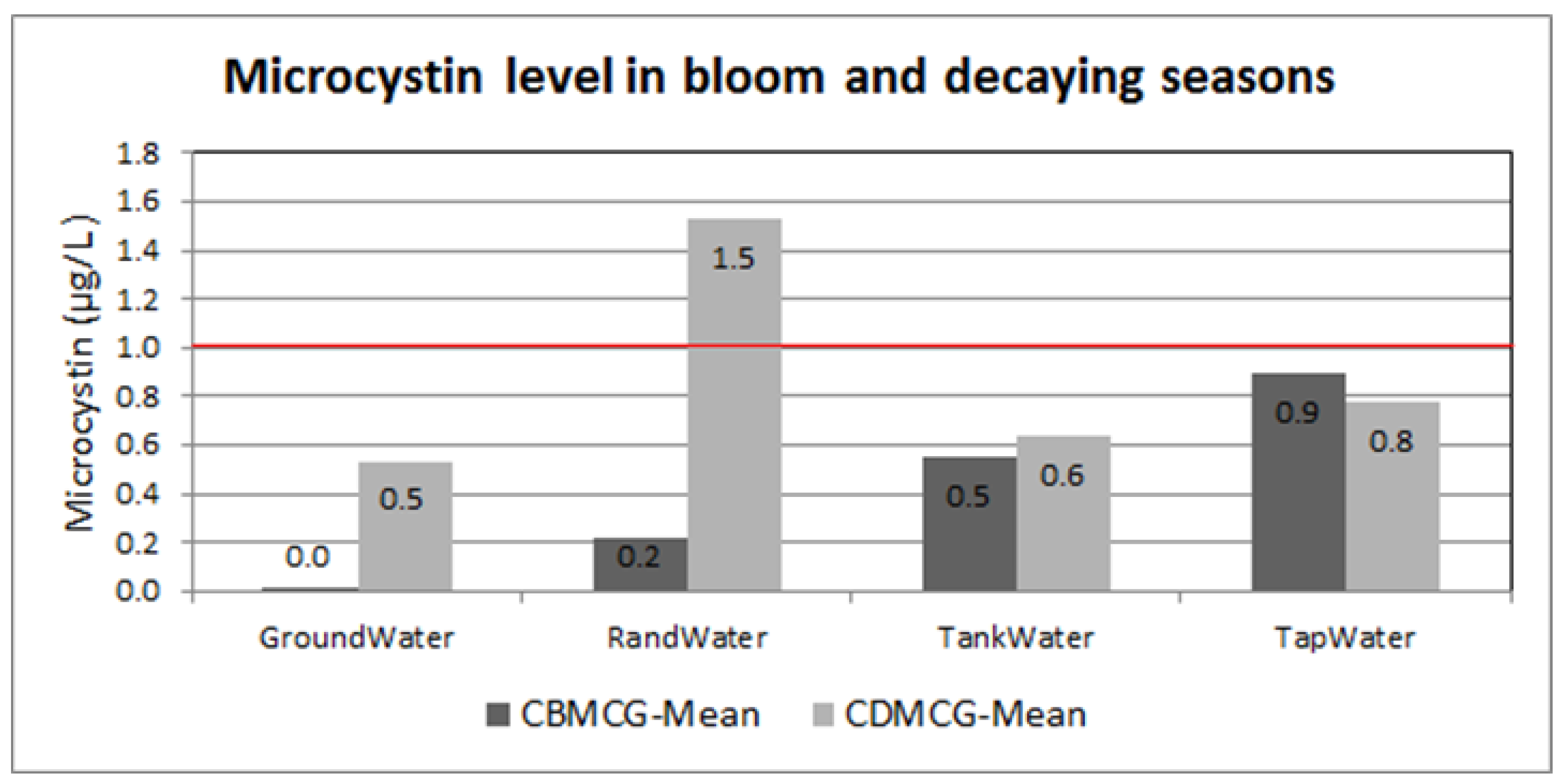
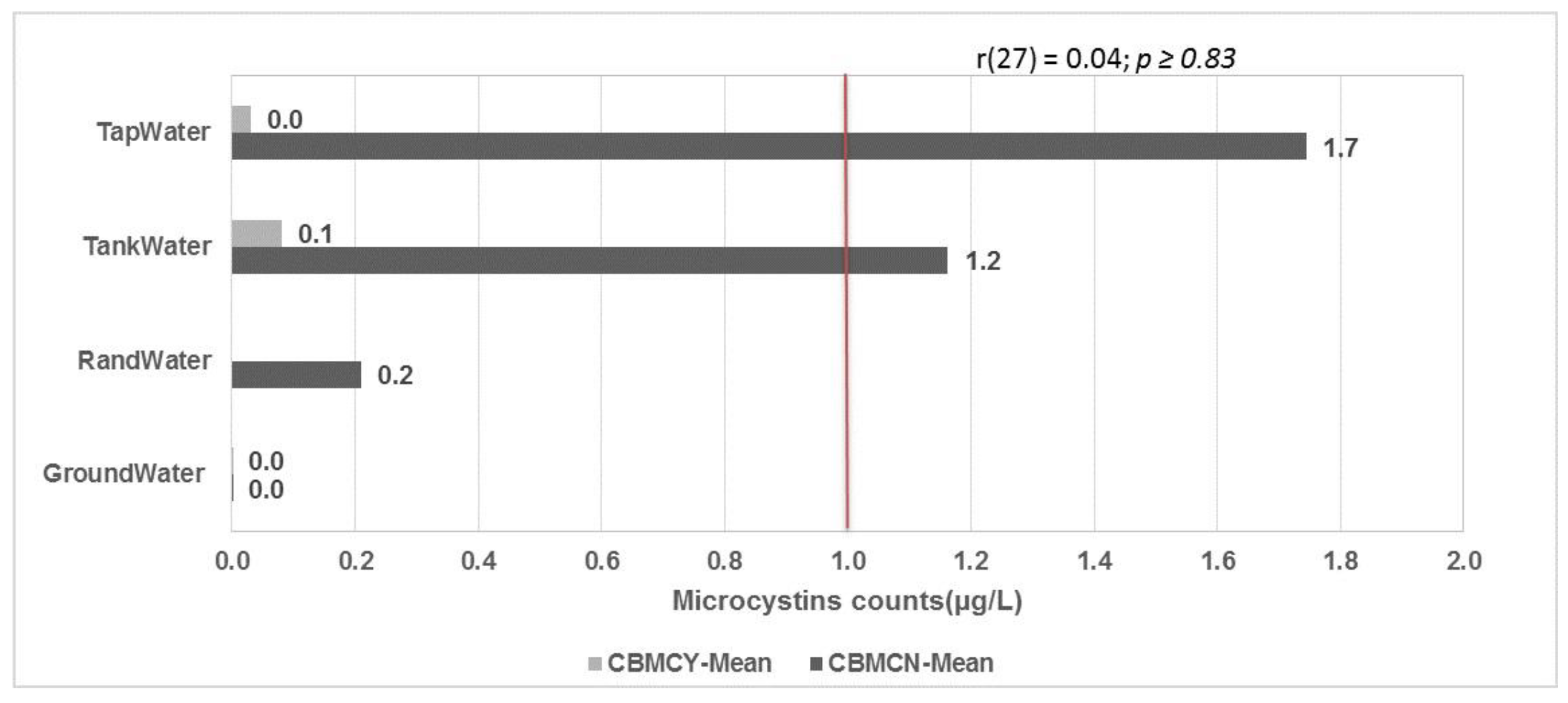
2.1. Microcystin Levels in Water Sources
2.2. Microcystin Levels in Water Containers
2.3. Microcystin Levels in Water Containers in the Blooming Season
3. Conclusions
4. Materials and Methods
4.1. Study Area
4.2. Study Population
4.3. Point-of-Use Water Treatment Using Sodium Hypochlorite (NaOCl)
4.4. Sample Collection
4.5. Microcystin Analysis and Interpretation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rutala, W.A.; Weber, D.J. Use of Inorganic Hypochlorite (Bleach) in health-care facilities. Clin. Microbiol. Rev. 1997, 10, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Tulchinsky, T.H. John Snow, Cholera, the Broad Street Pump; Waterborne Diseases Then and Now. Case Stud. Public Health 2018, 77–99. [Google Scholar] [CrossRef]
- WHO. Preventing disease through healthy environments: Exposure to mercury: A major public health concern. In WHO Document Production Services; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Bowman, G. The Fundamentals of Chlorine Chemistry and Disinfection; The Wisconsin State Lab of Hygiene and Rick Mealy, The Wisconsin Department of Natural Resources: Madison, WI, USA, 2007. Available online: https://dnr.wi.gov/regulations/labcert/documents/training/cl2chemistry.pdf (accessed on 17 February 2021).
- Van der Merwe, D. Cyanobacterial (blue-Green Algae) toxins. Handbook Toxicol. Chem. Warfare Agents 2015, 31, 421–429. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Ground Water and Drinking Water, Summary of Cyanotoxins Treatment in Drinking Water. 2018. Available online: https://www.epa.gov/ground-water-and-drinking-water/summary-cyanotoxins-treatment-drinking-water (accessed on 15 February 2021).
- Westrick, J.A. Chapter 13: Cyanobacterial toxin removal in drinking water treatment processes and recreational waters. In Proceedings of the Interagency, International Symposium on Cyanobacterial Harmful Algal Blooms Advances in Experimental Medicine & Biology; Hudnell, H.K., Ed.; WHOI—Woods Hole Oceanographic Institution: Falmouth, MA, USA, 2006. Available online: www.epa.gov/cyano_habs_symposium/monograph/Ch13.pdf (accessed on 22 April 2013).
- USEPA (the United States Environmental Protection Agency). Cyanobacteria and Cyanotoxins Information for Drinking Water Systems; EPA-810F11001; Office of Water: Washington, DC, USA, 2012. Available online: http://water.epa.gov/scitech/swguidance/standards/criteria/nutrients/upload/cyanobacteria_factsheet.pdf (accessed on 23 February 2015).
- Tsuji, K.; Watanuki, T.; Kondo, F.; Watanabe, M.F.; Nakazawa, H.; Suzuki, M.; Uchida, H.; Harada, K.I. Stability of Microcystins from cyanobacteria—iv. Effect of chlorination on decomposition. Toxicon 1997, 35, 1033–1041. [Google Scholar] [CrossRef]
- Nicholson, B.C.; Rositano, J.; Burch, M.D. Destruction of cyanobacterial peptide hepatotoxins by chlorine and chloramine. Water Res. 1994, 28, 1297–1303. [Google Scholar] [CrossRef]
- DWAF (Department of Water Affair and Forestry). South African Water Quality Guidelines, 2nd ed.; Agricultural Use: Livestock Watering; Department of Water Affairs and Forestry: Mokopane, South Africa; The Government Printer: Pretoria, South Africa, 1996; Volume 5.
- IFH (International Scientific Forum on Home Hygiene-Geneva). Guidelines for Prevention of Infection and Cross Infection in the Domestic Environment: Focus on Home Hygiene Issues in Developing Countries. Available online: https://www.ifh-homehygiene.org/care-guideline-best-practice/guidelines-prevention-infection-and-cross-infection-domestic-0 (accessed on 17 March 2017).
- Manage, P.M.; Edwards, C.; Lawton, L.A. Bacterial degradation of Microcystin-Biological Responses to Contaminations. In Interdisciplinary Studies on Environmental Chemistry-Biological Responses to Contaminants; Terrapub: Tokyo, Japan, 2010; pp. 97–104. [Google Scholar]
- Ali, M.E.; Das, R.; Maamor, A.; Hamid, S.B.A. Multifunctional carbon nanotubes (CNTs): A new dimension in environmental remediation. Adv. Mater. Res. 2014, 832, 328–332. [Google Scholar] [CrossRef]
- Daly, R.I.; Ho, L.; Brookes, J.D. Effect of Chlorination on Microcystis aeruginosa Cell integrity and subsequent Microcystin release and degradation. Environ. Sci. Technol. 2007, 41, 4447–4453. [Google Scholar] [CrossRef] [PubMed]
- Mishra, U.; Pabbi, S. Cyanobacteria: A potential Biofertilizer for Rice; Centre for Conservation and Utilisation of Blue-Green Algae, Indian Agricultural Research Institute: New Delhi, India; Indian Academy of Sciences: Bengaluru, India, 2004. [Google Scholar]
- Fosso-Kankeu, E.; Jagals, P.; Du Preez, H. Exposure of rural households to toxic cyanobacteria in container-stored water. Water SA 2008, 34, 631–636. [Google Scholar] [CrossRef] [Green Version]
- Chia, M.A.; Oniye, S.J.; Swanta, A.A. Domestic Water Quality Assessment: Microalgal and Cyanobacterial Contamination of Stored Water in Plastic Tanks in Zaria. Nigeria Eur. J. Sci. Res. 2013, 110, 501–510. [Google Scholar]
- Funari, E.; Testai, E. Human health risk assessment related to cyanotoxins exposure. Crit. Rev. Toxicol. 2008, 38, 97–125. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokoena, M.M.; Mudau, L.S.; Mokgobu, M.I.; Mukhola, M.S. The Use of Sodium Hypochlorite at Point-of-Use to Remove Microcystins from Water Containers. Toxins 2021, 13, 207. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13030207
Mokoena MM, Mudau LS, Mokgobu MI, Mukhola MS. The Use of Sodium Hypochlorite at Point-of-Use to Remove Microcystins from Water Containers. Toxins. 2021; 13(3):207. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13030207
Chicago/Turabian StyleMokoena, Matodzi Michael, Lutendo Sylvia Mudau, Matlou Ingrid Mokgobu, and Murembiwa Stanley Mukhola. 2021. "The Use of Sodium Hypochlorite at Point-of-Use to Remove Microcystins from Water Containers" Toxins 13, no. 3: 207. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13030207



