Curcumin Alleviates LPS-Induced Oxidative Stress, Inflammation and Apoptosis in Bovine Mammary Epithelial Cells via the NFE2L2 Signaling Pathway
Abstract
:1. Introduction
2. Results
2.1. Curcumin (CUR) Rescued the Decrease of MAC-T Cell Viability and Cell Damage Induced by Lipopolysaccharide (LPS)
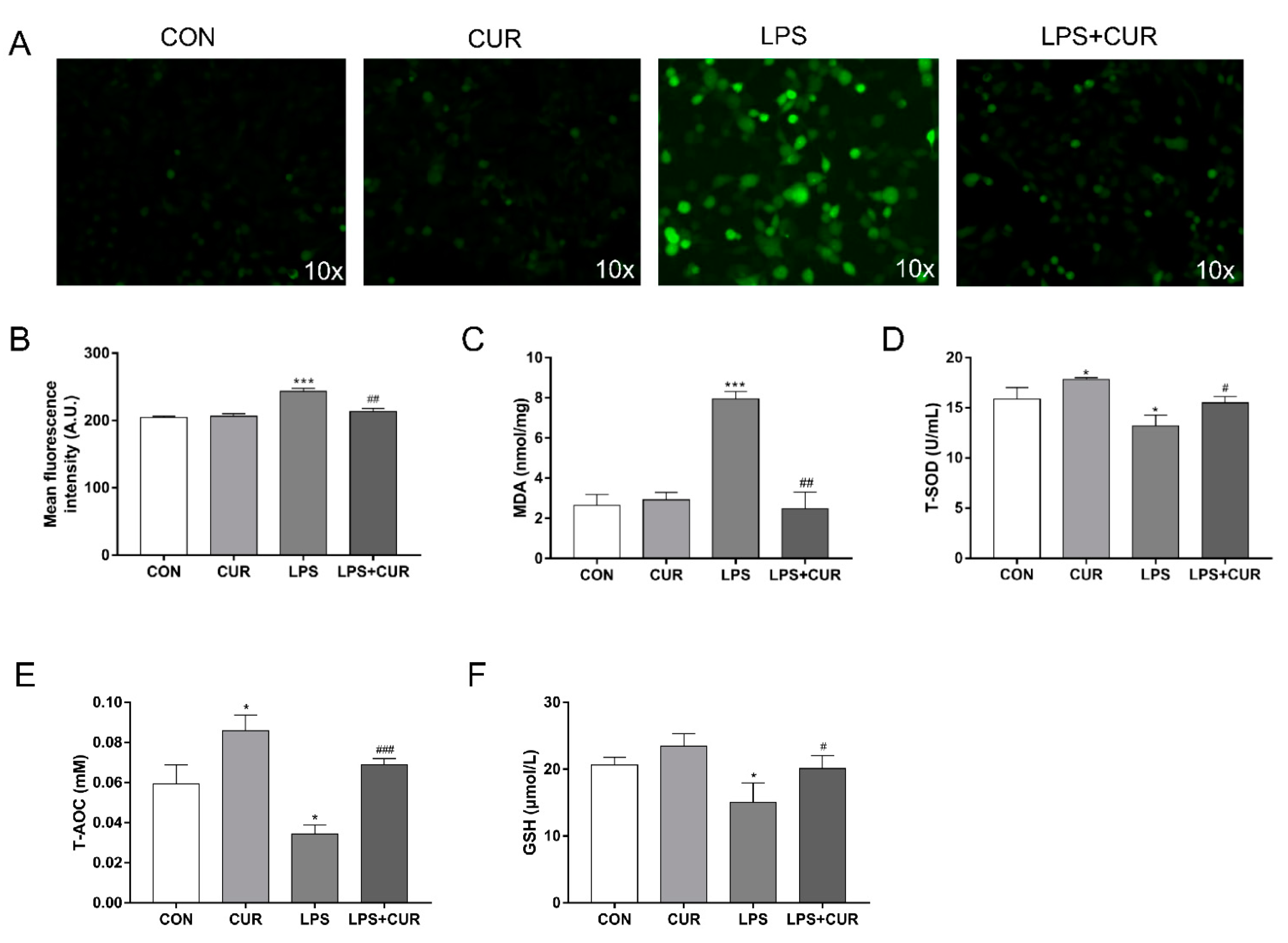
2.2. CUR Prevents LPS-Induced Oxidative Stress in MAC-T Cells
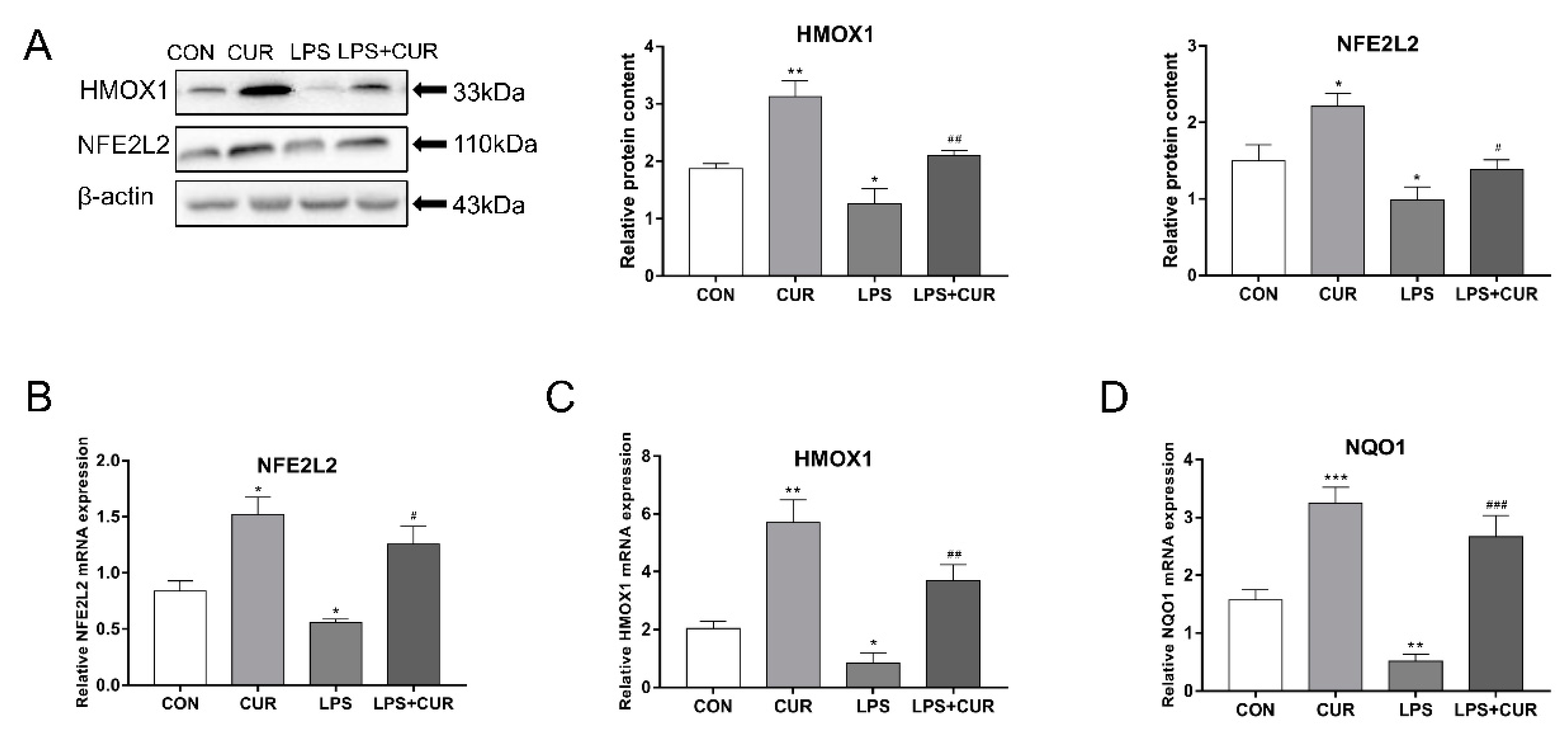
2.3. CUR Activated the Nuclear Factor E2-Related Factor 2 (NFE2L2)-Antioxidant Response Element (ARE) Pathway
2.4. CUR Prevents LPS-Induced Decrease in Mitochondrial Membrane Potential (ΔΨm, MMP)
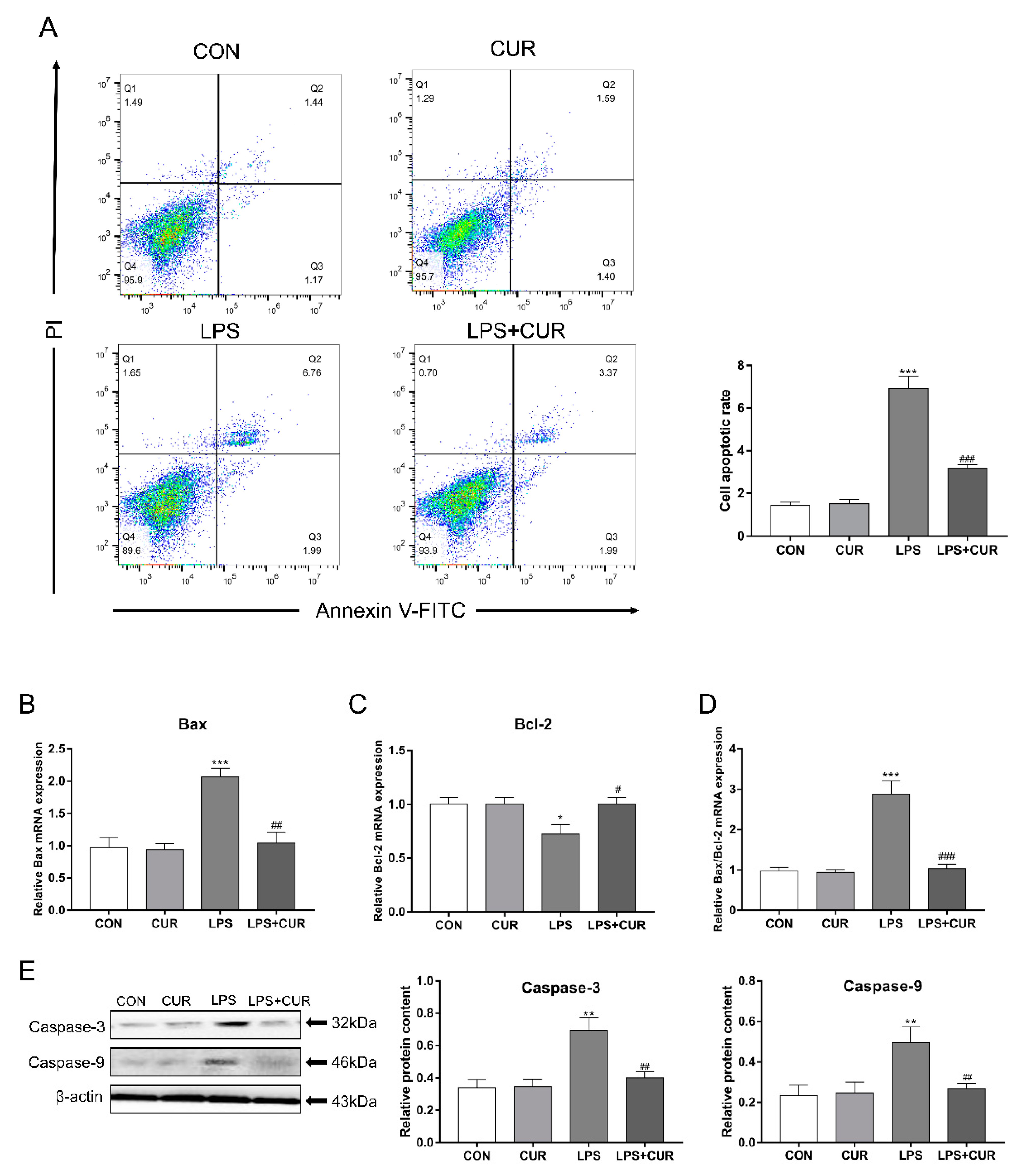
2.5. CUR Rescues the Apoptosis Caused by LPS Treatment of MAC-T Cells
2.6. CUR Rescued LPS-Elicited Nuclear Factor Kappa-B (NF-κB) Signaling Pathway Activity
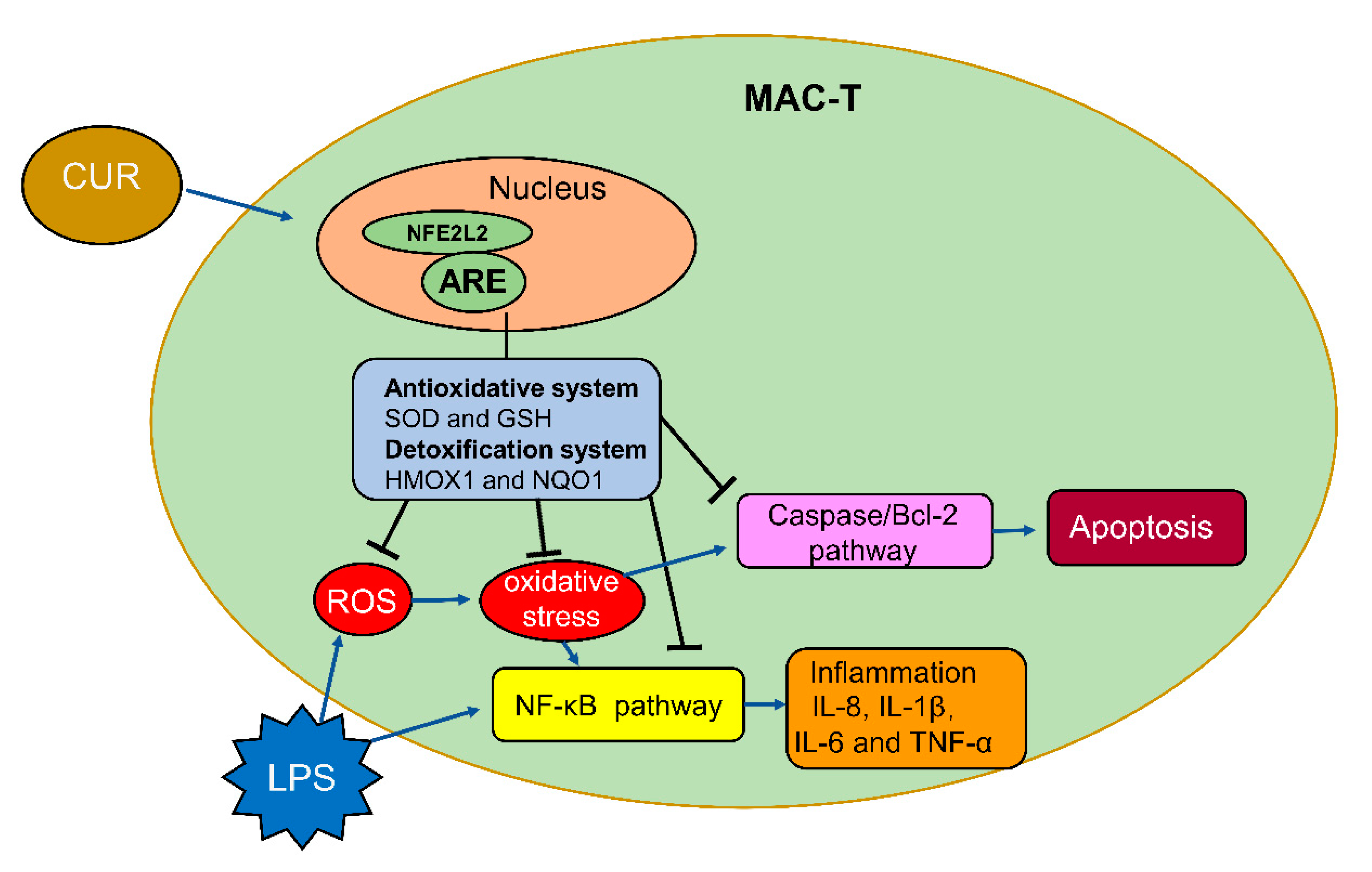
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Reagents
5.2. Cell Culture
5.3. Treatment Methods for Cells
5.4. Cell Viability Assay
5.5. Staining with Annexin V and Propidium Iodide (PI)
5.6. Fluorescence Measurements of Intracellular Oxidants
5.7. Measurement of Malondialdehyde (MDA), Total Superoxide Dismutase (T-SOD), Total Antioxidant Capacity (T-AOC) and Glutathione (GSH) Content
5.8. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (PCR)
5.9. Western Blot Analysis
5.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malcata, F.B.; Pepler, P.T.; O’Reilly, E.L.; Brady, N.; Eckersall, P.D.; Zadoks, R.N.; Viora, L. Point-of-care tests for bovine clinical mastitis: What do we have and what do we need? J. Dairy Res. 2020, 87, 60–66. [Google Scholar] [CrossRef]
- Yao, J.; Peng, D.; Cheng, Z. Research Progress on Diagnosis and Treatment of Bovine Mastitis. Asian Agric. Res. 2020, 12, 69–71. [Google Scholar]
- Swanson, K.; Stelwagen, K.; Dobson, J.; Henderson, H.; Davis, S.; Farr, V.; Singh, K. Transcriptome profiling of Streptococcus uberis-induced mastitis reveals fundamental differences between immune gene expression in the mammary gland and in a primary cell culture model. J. Dairy Sci. 2009, 92, 117–129. [Google Scholar] [CrossRef]
- Hillerton, J.E.; Kliem, K.E. Effective treatment of Streptococcus uberis clinical mastitis to minimize the use of antibiotics. J. Dairy Sci. 2002, 85, 1009–1014. [Google Scholar] [CrossRef]
- Algharib, S.A.; Dawood, A.; Xie, S. Nanoparticles for treatment of bovine Staphylococcus aureus mastitis. Drug Deliv. 2020, 27, 292–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bard, A.M.; Main, D.; Roe, E.; Haase, A.; Whay, H.R.; Reyher, K.K. To change or not to change? Veterinarian and farmer perceptions of relational factors influencing the enactment of veterinary advice on dairy farms in the United Kingdom. J. Dairy Sci. 2019, 102, 10379–10394. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, N.; Sahoo, P.; Behura, N.; Biswal, S. Prophylactic Effects of Turmeric against Bovine Mastitis. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 4080–4084. [Google Scholar] [CrossRef]
- Opal, S.M.; Scannon, P.J.; Vincent, J.; White, M.; Carroll, S.F.; Palardy, J.E.; Parejo, N.A.; Pribble, J.P.; Lemke, J.H. Relationship between Plasma Levels of Lipopolysaccharide (LPS) and LPS-Binding Protein in Patients with Severe Sepsis and Septic Shock. J. Infect. Dis. 1999, 180, 1584–1589. [Google Scholar] [CrossRef] [Green Version]
- Elin, R.J.; Wolff, S.M. Biology of Endotoxin. Annu. Rev. Med. 1976, 27, 127–141. [Google Scholar] [CrossRef]
- Hu, X.; Li, S.; Fu, Y.; Zhang, N. Targeting gut microbiota as a possible therapy for mastitis. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1409–1423. [Google Scholar] [CrossRef]
- Khafipour, E.; Krause, D.; Plaizier, J. A grain-based subacute ruminal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation. J. Dairy Sci. 2009, 92, 1060–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Xu, X.; Zhu, H.; Wang, Y.; Hou, Y.; Liu, Y. Dietary fish oil supplementation alters liver gene expressions to protect against LPS-induced liver injury in weanling piglets. Innate Immun. 2019, 25, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hou, X.; Sun, L.; He, T.; Wei, R.; Pang, M.; Wang, R. Staphylococcus aureus bacteriophage suppresses LPS-induced inflammation in MAC-T bovine mammary epithelial cells. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Wang, R.; Yu, S.; Lu, G.; Yu, Y.; Jiang, C. Anti-inflammatory activity of oligomeric proanthocyanidins via inhibition of NF-ΚB and MAPK in LPS-stimulated MAC-T Cells. J. Microbiol. Biotechnol. 2020, 30. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2016, 174, 1325–1348. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Zhang, J.; Zhou, Y.; Gao, Q.; Wang, R.; Fu, Y.; Zheng, L.; Yu, H. Transcriptome Investigation and In Vitro Verification of Curcumin-Induced HO-1 as a Feature of Ferroptosis in Breast Cancer Cells. Oxidative Med. Cell. Longev. 2020, 2020, 1–18. [Google Scholar] [CrossRef]
- Surh, Y.-J.; Kundu, J.K.; Na, H.-K. Nrf2 as a Master Redox Switch in Turning on the Cellular Signaling Involved in the Induction of Cytoprotective Genes by Some Chemopreventive Phytochemicals. Planta Medica 2008, 74, 1526–1539. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohamamdinejad, R.; Farkhondeh, T.; Samarghandian, S. Curcumin Activates the Nrf2 Pathway and Induces Cellular Protection Against Oxidative Injury. Curr. Mol. Med. 2020, 20, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Masuelli, L.; Benvenuto, M.; Di Stefano, E.; Mattera, R.; Fantini, M.; De Feudis, G.; De Smaele, E.; Tresoldi, I.; Giganti, M.G.; Modesti, A.; et al. Curcumin blocks autophagy and activates apoptosis of malignant mesothelioma cell lines and increases the survival of mice intraperitoneally transplanted with a malignant mesothelioma cell line. Oncotarget 2017, 8, 34405–34422. [Google Scholar] [CrossRef] [Green Version]
- Chan, M.M.-Y.; Ho, C.-T.; Huang, H.-I. Effects of three dietary phytochemicals from tea, rosemary and turmeric on inflammation-induced nitrite production. Cancer Lett. 1995, 96, 23–29. [Google Scholar] [CrossRef]
- Kaur, G.; Tirkey, N.; Bharrhan, S.; Chanana, V.; Rishi, P.; Chopra, K. Inhibition of oxidative stress and cytokine activity by curcumin in amelioration of endotoxin-induced experimental hepatoxicity in rodents. Clin. Exp. Immunol. 2006, 145. [Google Scholar] [CrossRef]
- Bradley, A. Bovine Mastitis: An Evolving Disease. Veter J. 2002, 164, 116–128. [Google Scholar] [CrossRef]
- Mehta, S.; Young, C.C.; Warren, M.R.; Akhtar, S.; Shefelbine, S.J.; Crane, J.D.; Bajpayee, A.G. Resveratrol and Curcumin Attenuate Ex Vivo Sugar-Induced Cartilage Glycation, Stiffening, Senescence, and Degeneration. Cartilage 2021. [Google Scholar] [CrossRef]
- Ahire, J.J.; Mokashe, N.U.; Patil, H.J.; Chaudhari, B.L. Antioxidative potential of folate producing probiotic Lactobacillus helveticus CD6. J. Food Sci. Technol. 2011, 50, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Castillo, C.; Hernandez, J.; Bravo, A.; Lopez-Alonso, M.; Pereira, V.; Benedito, J.L. Oxidative status during late pregnancy and early lactation in dairy cows. Vet. J. 2005, 169, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, T.D.N.; Son, Y.-O.; Lim, S.-S.; Shi, X.; Kim, J.-G.; Heo, J.S.; Choe, Y.; Jeon, Y.-M.; Lee, J.-C. Sodium fluoride induces apoptosis in mouse embryonic stem cells through ROS-dependent and caspase- and JNK-mediated pathways. Toxicol. Appl. Pharmacol. 2012, 259, 329–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [Green Version]
- Chan, W.-H.; Wu, H.-J.; Hsuuw, Y.-D. Curcumin Inhibits ROS Formation and Apoptosis in Methylglyoxal-Treated Human Hepatoma G2 Cells. Ann. N. Y. Acad. Sci. 2005, 1042, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.; Li, D.; Chen, Y.; Li, M.; Zhang, Y.; Sun, T.; Tang, S. Curcumin Ameliorates Copper-Induced Neurotoxicity Through Inhibiting Oxidative Stress and Mitochondrial Apoptosis in SH-SY5Y Cells. Cells 2020, 2020, 1–12. [Google Scholar]
- Li, J.; Zheng, X.; Ma, X.; Xu, X.; Du, Y.; Lv, Q.; Li, X.; Wu, Y.; Sun, H.; Yu, L.; et al. Melatonin protects against chromium(VI)-induced cardiac injury via activating the AMPK/Nrf2 pathway. J. Inorg. Biochem. 2019, 197, 110698. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, X.; Fan, H.; Liu, Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009, 1282, 133–141. [Google Scholar] [CrossRef]
- Oh, S.-H.; Lim, S.-C. A rapid and transient ROS generation by cadmium triggers apoptosis via caspase-dependent pathway in HepG2 cells and this is inhibited through N-acetylcysteine-mediated catalase upregulation. Toxicol. Appl. Pharmacol. 2006, 212, 212–223. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, Y.; Tang, L. Caffeine induces sustained apoptosis of human gastric cancer cells by activating the caspase-9/caspase-3 signalling pathway. Mol. Med. Rep. 2017, 16, 2445–2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perlman, H.; Zhang, X.; Chen, M.W.; Walsh, K.; Buttyan, R. An elevated bax/bcl-2 ratio corresponds with the onset of prostate epithelial cell apoptosis. Cell Death Differ. 1999, 6, 48–54. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Aitken, S.L. Impact of oxidative stress on the health and immune function of dairy cattle. Vet. Immunol. Immunopathol. 2009, 128, 104–109. [Google Scholar] [CrossRef]
- Notebaert, S.; Meyer, E. Mouse models to study the pathogenesis and control of bovine mastitis. A review. Vet. Q. 2006, 28, 2–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlahopoulos, S.; Boldogh, I.; Casola, A.; Brasier, A.R. Nuclear factor-κB-dependent induction of interleukin-8 gene expression by tumor necrosis factor α: Evidence for an antioxidant sensitive activating pathway distinct from nuclear translocation. Blood 1999, 94, 1878–1889. [Google Scholar] [CrossRef]
- Copray, J.C.; Mantingh, I.; Brouwer, N.; Biber, K.; Küst, B.M.; Liem, R.S.; Huitinga, I.; Tilders, F.J.; Van Dam, A.M.; Boddeke, H.W. Expression of interleukin-1 beta in rat dorsal root ganglia. J. Neuroimmunol. 2001, 118, 203–211. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Prgomet, C.; Schmitz, S.; Meyer, H.H.D. LPS effects on the MRNA expression of inflammatory factors in the mammary gland: Quantitative transcriptomics in various cell types using real-time RT-PCR. Tissue Antigens 2004, 64, 326–327. [Google Scholar]
- Whitfield, C.; Clarke, B.R. Lipopolysaccharides (Endotoxins)—ScienceDirect. In Encyclopedia of Microbiology, 4th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 791–802. [Google Scholar]
- Raetz, C.R.H.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikołajczyk, A.; Kozłowska, A.; Gonkowski, S. Distribution and Neurochemistry of the Porcine Ileocaecal Valve Projecting Sensory Neurons in the Dorsal Root Ganglia and the Influence of Lipopolysaccharide from Different Serotypes of Salmonella spp. on the Chemical Coding of DRG Neurons in the Cell Cultures. Int. J. Mol. Sci. 2018, 19, 2551. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Li, D.; Ouyang, J.; Tian, X.; Zhao, Y.; Peng, X.; Li, S.; Yu, G.; Yang, J. Leonurine alleviates LPS-induced myocarditis through suppressing the NF-кB signaling pathway. Toxicology 2019, 422. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Jurk, K.; Kopp, M.; Kröller-Schön, S.; Mikhed, Y.; Schwierczek, K.; Roohani, S.; Kashani, F.; Oelze, M.; Klein, T.; et al. Glucagon-like peptide-1 receptor signalling reduces microvascular thrombosis, nitro-oxidative stress and platelet activation in endotoxaemic mice. Br. J. Pharmacol. 2017, 174, 1620–1632. [Google Scholar] [CrossRef] [Green Version]
- Gavish, L.; Perez, L.S.; Reissman, P.; Gertz, S.D. Irradiation with 780 nm diode laser attenuates inflammatory cytokines but upregulates nitric oxide in lipopolysaccharide-stimulated macrophages: Implications for the prevention of aneurysm progression. Lasers Surg. Med. 2008, 40, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Soderquist, R.G.; Sloane, E.M.; Loram, L.C.; Harrison, J.A.; Dengler, E.C.; Johnson, S.M.; Amer, L.D.; Young, C.S.; Lewis, M.T.; Poole, S.; et al. Release of Plasmid DNA-Encoding IL-10 from PLGA Microparticles Facilitates Long-Term Reversal of Neuropathic Pain Following a Single Intrathecal Administration. Pharm. Res. 2010, 27, 841–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibeagha-Awemu, E.M.; Lee, J.-W.; Ibeagha, A.E.; Bannerman, D.D.; Paape, M.J.; Zhao, X. Bacterial lipopolysaccharide induces increased expression of toll-like receptor (TLR) 4 and downstream TLR signaling molecules in bovine mammary epithelial cells. Vet. Res. 2007, 39, 11–12. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-Y.; Sun, S.-P.; Zhu, H.-S.; Jiao, X.-Q.; Zhong, K.; Guo, Y.-J.; Zha, G.-M.; Han, L.-Q.; Yang, G.-Y.; Li, H.-P. GABA regulates the proliferation and apoptosis of MAC-T cells through the LPS-induced TLR4 signaling pathway. Res. Vet. Sci. 2018, 118, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.J.; Li, H.P.; Zhu, H.S.; Sui, S.P.; Chen, P.G.; Deng, Y.; Sui, T.M.; Wang, Y.Y. NF-κB is involved in the LPS-mediated proliferation and apoptosis of MAC-T epithelial cells as part of the subacute ruminal acidosis response in cows. Biotechnol. Lett. 2016, 38, 1–11. [Google Scholar] [CrossRef]
- Markiewicz, H.; Krumrych, W. Inmufort Bov—Możliwość stymulacji mechanizmów odporności nieswoistej gruczołu mlekowego krów. Prace Klin. Kazuistyczne 2020, 95, 2. [Google Scholar]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Steward, W.P.; Gescher, A.J. Pharmacokinetics and pharmacodynamics of curcumin. Adv. Exp. Med. Biol. 2007, 595, 453–470. [Google Scholar] [PubMed] [Green Version]
- Mücke, N.; da Silva, T.B.V.; de Oliveira, A.; Moreira, T.F.M.; Venancio, C.D.S.; Marques, L.L.M.; Valderrama, P.; Gonçalves, O.H.; da Silva-Buzanello, R.A.; Yamashita, F.; et al. Use of Water-Soluble Curcumin in TPS/PBAT Packaging Material: Interference on Reactive Extrusion and Oxidative Stability of Chia Oil. Food Bioprocess Technol. 2021, 14, 471–482. [Google Scholar] [CrossRef]
- Fereydouni, N.; Movaffagh, J.; Amiri, N.; Darroudi, S.; Gholoobi, A.; Goodarzi, A.; Hashemzadeh, A.; Darroudi, M. Synthesis of nano-fibers containing nano-curcumin in zein corn protein and its physicochemical and biological characteristics. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Narula, P.; Saini, K.; Saini, M.; Singla, D.; Chauhan, A.S.; Kakkar, V. Assay and Dermatokinetics of Tetrahydrocurcumin Lipidic Nanostructures Using Reverse Phase-High Performance Liquid Chromatography. Pharm. Nanotechnol. 2021, 9, 1–13. [Google Scholar] [CrossRef]



| Gene | Gene ID | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|---|
| β-actin | 280979 | CCCTGGAGAAGAGCTACGAG | GTAGTTTCGTGAATGCCGCAG |
| HMOX1 | 513221 | TTAAGCTGGTGATGGCGTCT | GGGAGTGTAGACGGGGTTCT |
| NFE2L2 | 497024 | CCCAGTCCAACCTTTGTCGT | TGGAGAGCTTTTGCCCGTAG |
| NQO1 | 519632 | CACTCTGCACTTCTGTGGCT | CAGGATCTGAACTCGGGCAT |
| Bax | 280730 | GCTCTGAGCAGATCATGAAGAC | CAATTCATCTCCGATGCGCT |
| Bcl-2 | 281020 | GATGACCGAGTACCTGAACC | AGAGACAGCCAGGAGAAATCA |
| NF-κB P65 | 508233 | ACCTGGGGATCCAGTGTGTA | ACGGCATTCAGGTCGTAGT |
| NF-κB P50 | 616115 | AAACACTGTGAGGATGGCGT | AGGCATCTGTCATTCGTGCT |
| IL-8 | 280828 | ATGACTTCCAAGCTGGCTGTT | GGTTTAGGCAGACCTCGTTT |
| IL-1β | 281251 | GTCCTCCGACGAGTTTCTGT | AGAGCCTTCAGCACACATGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Fang, H.; Shen, J.; Jin, Y.; Zhao, Y.; Wang, R.; Fu, Y.; Tian, Y.; Yu, H.; Zhang, J. Curcumin Alleviates LPS-Induced Oxidative Stress, Inflammation and Apoptosis in Bovine Mammary Epithelial Cells via the NFE2L2 Signaling Pathway. Toxins 2021, 13, 208. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13030208
Li R, Fang H, Shen J, Jin Y, Zhao Y, Wang R, Fu Y, Tian Y, Yu H, Zhang J. Curcumin Alleviates LPS-Induced Oxidative Stress, Inflammation and Apoptosis in Bovine Mammary Epithelial Cells via the NFE2L2 Signaling Pathway. Toxins. 2021; 13(3):208. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13030208
Chicago/Turabian StyleLi, Ruihua, Hengtong Fang, Jinglin Shen, Yongcheng Jin, Yun Zhao, Rui Wang, Yurong Fu, Yue Tian, Hao Yu, and Jing Zhang. 2021. "Curcumin Alleviates LPS-Induced Oxidative Stress, Inflammation and Apoptosis in Bovine Mammary Epithelial Cells via the NFE2L2 Signaling Pathway" Toxins 13, no. 3: 208. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13030208





