Saccharomyces cerevisiae Cell Wall-Based Adsorbent Reduces Aflatoxin B1 Absorption in Rats
Abstract
:1. Introduction
2. Results
2.1. In Vitro Preliminary Study of the Adsorption Capacity of the Tested Adsorbents toward AFB1
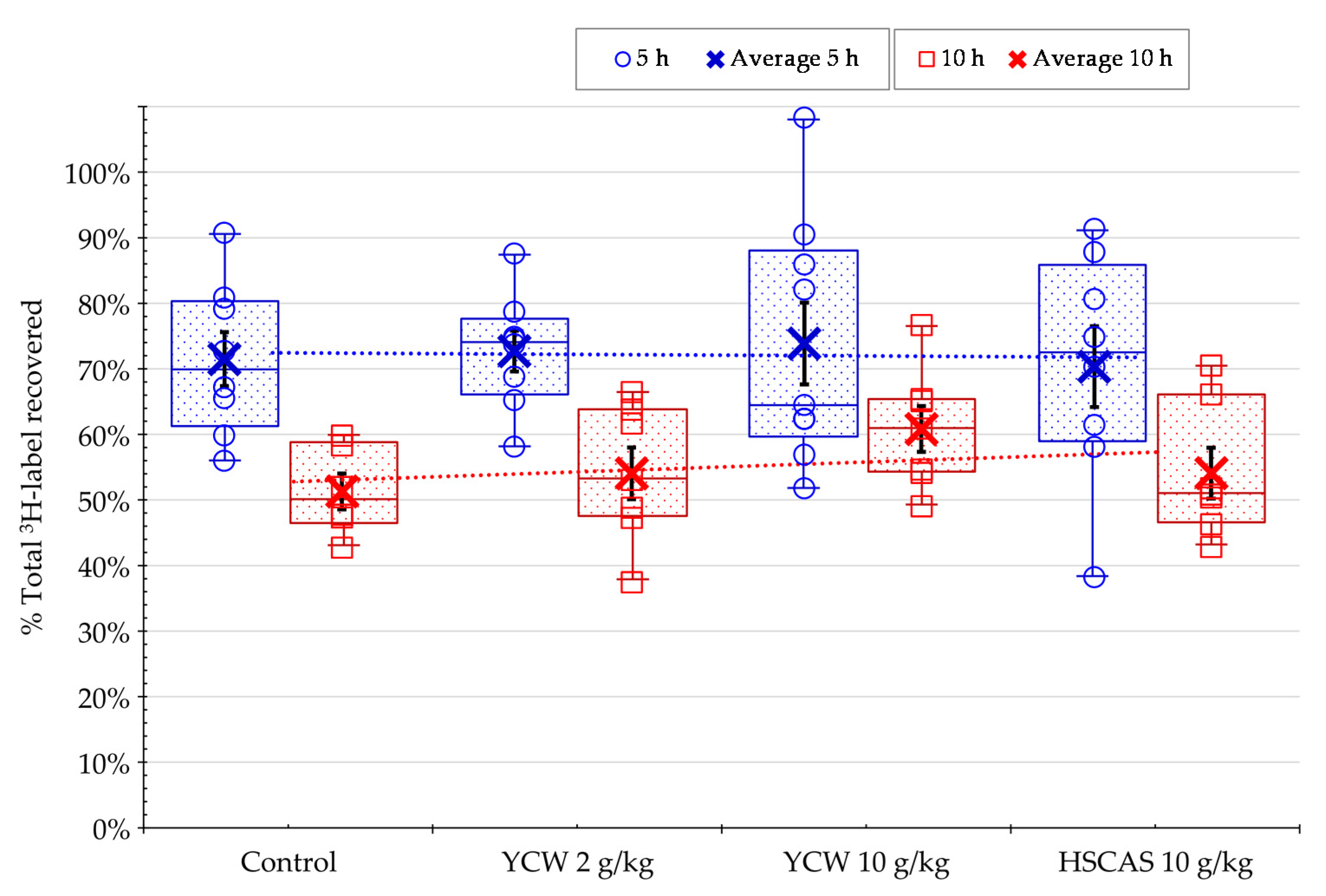
2.2. In Vivo Experiment on the Total Recovery of AFB1 in Rat Fed AFB1-Contaminated Diet
2.3. Evaluation of the Absorption Kinetics of AFB1 in Rat Fed AFB1-Contaminated Diet
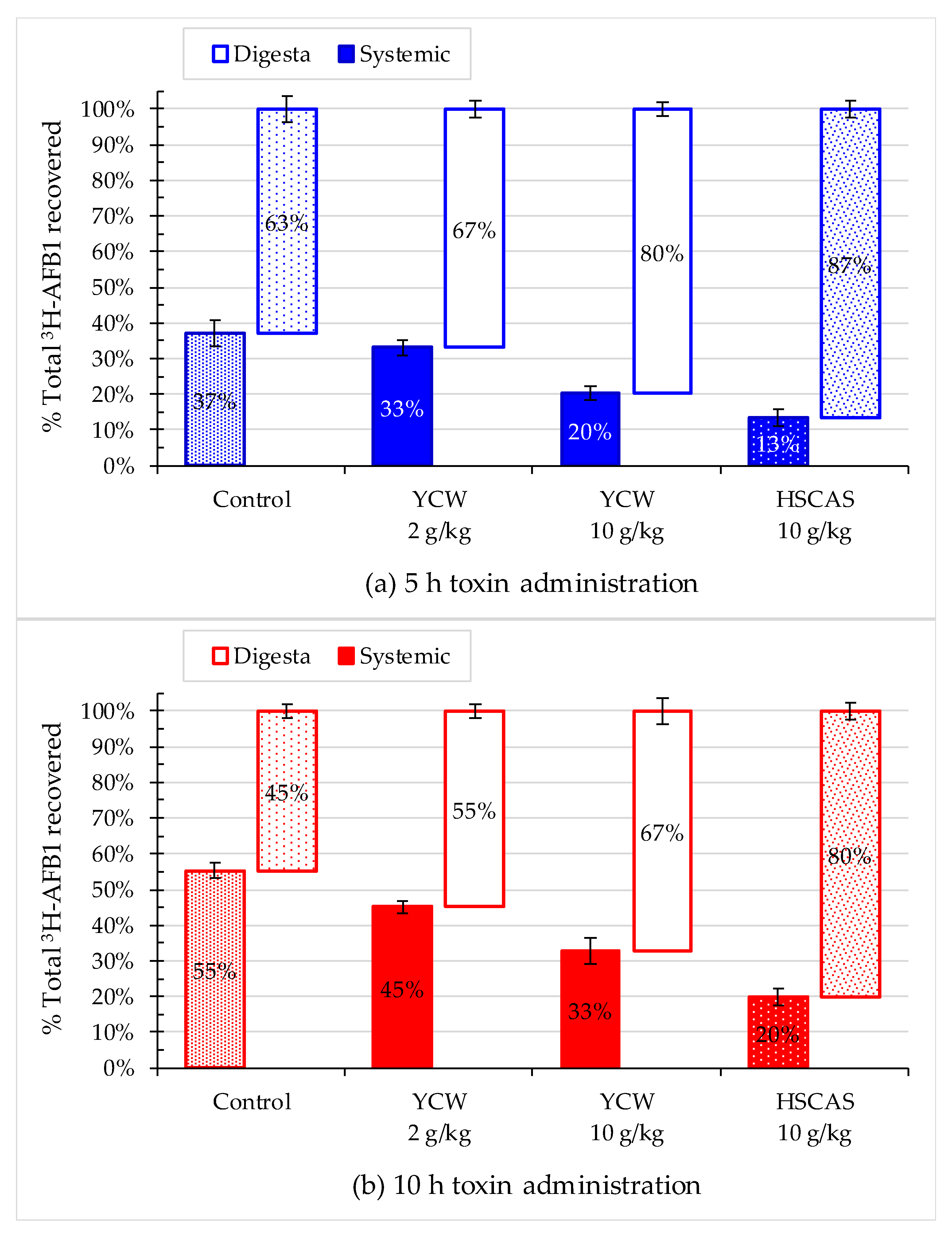
2.4. Effect of Mycotoxin Adsorbents on AFB1 Retention in the Gastrointestinal Tract
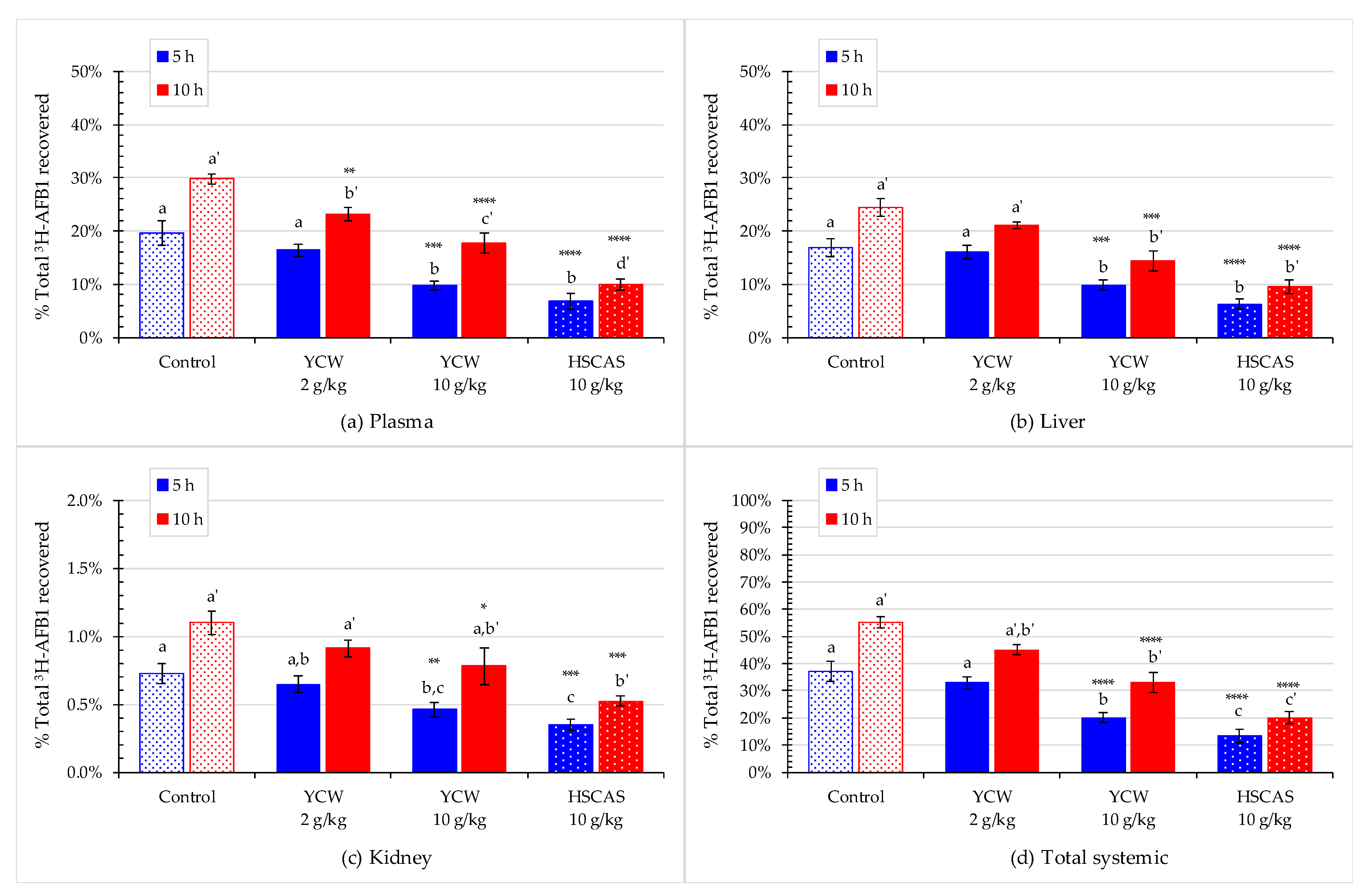
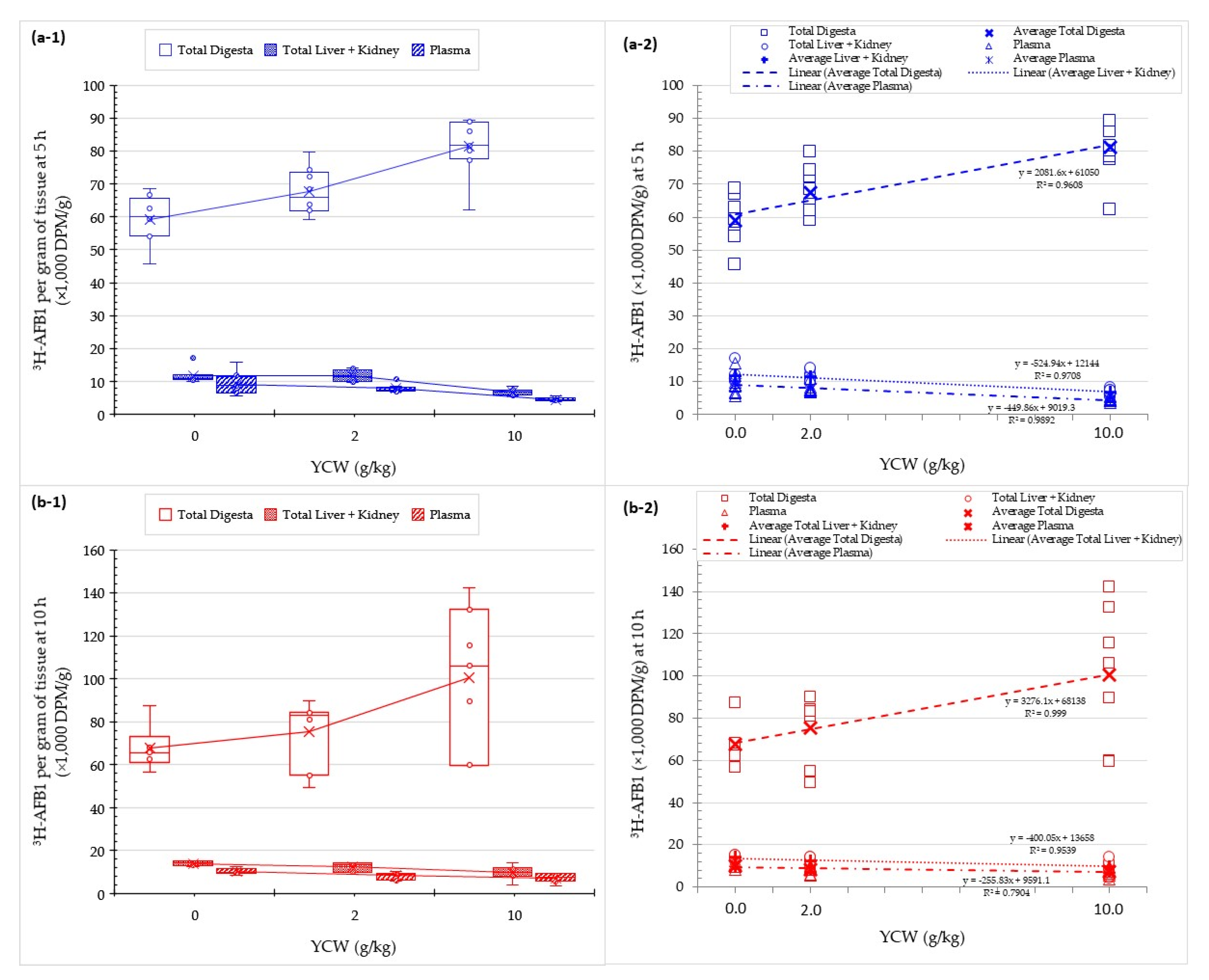
2.5. Effect of Mycotoxin Binders on AFB1 Absorption into Animal Tissues
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. In Vitro Main Study Assessing AFB1 Sequestration
5.2. In Vitro Kinetic of Adsorption
5.3. Animals and Diets
5.4. Animal Pre-Experimental Study
5.5. Animal Principal Experimental Study
5.6. Sample Collection
5.7. Radioactivity Determination
5.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Čolović, R.; Puvača, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Đuragić, O.; Kos, J.; Pinotti, L. Decontamination of Mycotox-in-contaminated feedstuffs and compound feed. Toxins 2019, 11, 617. [Google Scholar] [CrossRef] [Green Version]
- Yiannikouris, A.; Jouany, J.-P. Mycotoxins in feeds and their fate in animals: A review. Anim. Res. 2002, 51, 81–99. [Google Scholar] [CrossRef] [Green Version]
- Buckley, T.; Creighton, A.; Fogarty, U. Analysis of Canadian and Irish forage, oats and commercially available equine con-centrate feed for pathogenic fungi and mycotoxins. Ir. Vet. J. 2007, 60, 231–236. [Google Scholar] [CrossRef] [Green Version]
- CAST. Mycotoxins: Risks in Plant, Animal, and Human Systems; Council for Agricultural Science and Technology: Ames, IA, USA, 2003. [Google Scholar]
- Liu, Y.; Yamdeu, J.H.G.; Gong, Y.Y.; Orfila, C. A review of postharvest approaches to reduce fungal and mycotoxin contam-ination of foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1521–1560. [Google Scholar] [CrossRef] [PubMed]
- Robens, J.F.; Richard, J.L. Aflatoxins in Animal and Human Health. Rev. Environ. Contam. Toxicol. 1992, 127, 69–94. [Google Scholar] [CrossRef]
- Obuseh, A.F.; Jolly, E.P.; Kulczycki, A.; Ehiri, J.; Waterbor, J.; Desmond, R.A.; Preko, O.P.; Jiang, Y.; Piyathilake, C.J. Aflatoxin levels, plasma vitamins A and E concentrations, and their association with HIV and hepatitis B virus infections in Ghanaians: A cross-sectional study. J. Int. AIDS Soc. 2011, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Eaton, D.L.; Gallagher, E.P. Mechanisms of aflatoxin carcinogenesis. Annu. Rev. Pharm. Toxicol. 1994, 34, 135–172. [Google Scholar] [CrossRef] [PubMed]
- Susanto, A.; Laconi, E.B.; Astuti, D.A.; Bahri, S. In Vitro Testing to Aflatoxin Binding by Glucomannan Yeast Product and Glucomannan Extract from Amorphophallus oncophyllus. Media Peternak. 2014, 37, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Agag, B. Mycotoxins in foods and feeds: 1-aflatoxins. Ass. Univ. Bull. Environ. Res. 2004, 7, 173–205. [Google Scholar]
- WHO Task Group on Environmental Health Criteria for Mycotoxins & World Health Organization. Mycotoxins; World Health Organization: Geneva, Switzerland, 1979; Volume 11, pp. 1–127. [Google Scholar]
- Benkerroum, N. Chronic and Acute Toxicities of Aflatoxins: Mechanisms of Action. Int. J. Environ. Res. Public Health 2020, 17, 423. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Commission Regulation (EC) no 1881/2006 of 19 December 2006 setting maximum levels for certain contami-nants in foodstuffs L 364. Off. J. Eur. Union 2006, 49, 1–91. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2006:364:FULL&from=EN (accessed on 12 January 2020).
- US Food and Drug Administration. CPG Sec. Action Levels for Aflatoxins in Animal Feeds; US Food and Drug Administration: Silver Spring, MD, USA, 2020; Volume 683, p. 100. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cpg-sec-683100-action-levels-aflatoxins-animal-feeds (accessed on 18 December 2020).
- Jouany, J.P. Methods for preventing, decontaminating and minimizing the toxicity of mycotoxins in feeds. Anim. Feed Sci. Technol. 2007, 137, 342–362. [Google Scholar] [CrossRef]
- Diaz, D.E.; Hagler, W.M.; Hopkins, B.A.; Whitlow, L.W. Aflatoxin binders I: In vitro binding assay for aflatoxin B1 by several potential sequestering agents. Mycopathologia 2003, 156, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Grant, P.G.; Phillips, T.D. Isothermal Adsorption of Aflatoxin B1on HSCAS Clay. J. Agric. Food Chem. 1998, 46, 599–605. [Google Scholar] [CrossRef]
- Ramos, A.; Hernandez, E. In vitro aflatoxin adsorption by means of a montmorillonite silicate. A study of adsorption isotherms. Anim. Feed Sci. Technol. 1996, 62, 263–269. [Google Scholar] [CrossRef]
- Feddern, V.; Dors, G.C.; Tavernari, F.D.C.; Mazzuco, H.; Cunha, A.; Krabbe, E.L.; Scheuermann, G.N. Aflatoxins importance on animal nutrition. In Aflatoxins-Recent Advances and Future Prospects; InTech: London, UK, 2013; pp. 171–195. [Google Scholar]
- Vartiainen, S.; Yiannikouris, A.; Apajalahti, J.; Moran, A.C. Comprehensive Evaluation of the Efficiency of Yeast Cell Wall Extract to Adsorb Ochratoxin A and Mitigate Accumulation of the Toxin in Broiler Chickens. Toxins 2020, 12, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yiannikouris, A.; André, G.; Buléon, A.; Jeminet, G.; Canet, I.; François, J.; Bertin, G.; Jouany, J.P. Comprehensive conforma-tional study of key interactions involved in zearalenone complexation with β-D-glucans. Biomacromolecules 2004, 5, 2176–2185. [Google Scholar] [CrossRef] [PubMed]
- Yiannikouris, A.; André, G.; Poughon, L.; François, J.; Dussap, C.G.; Jeminet, G.; Bertin, G.; Jouany, J.P. Chemical and con-formational study of the interactions involved in mycotoxin complexation with β-D-glucans. Biomacromolecules 2006, 7, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Kolawole, O.; Meneely, J.; Greer, B.; Chevallier, O.; Jones, D.S.; Connolly, L.; Elliott, C. Comparative In Vitro Assessment of a Range of Commercial Feed Additives with Multiple Mycotoxin Binding Claims. Toxins 2019, 11, 659. [Google Scholar] [CrossRef] [Green Version]
- Yiannikouris, A.; Kettunen, H.; Apajalahti, J.; Pennala, E.; Moran, C.A. Comparison of the sequestering properties of yeast cell wall extract and hydrated sodium calcium aluminosilicate in three in vitro models accounting for the animal physiological bioavailability of zearalenone. Food Addit. Contam. Part A 2013, 30, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Yiannikouris, A.; Apajalahti, J.; Kettunen, H.; Ojanperä, S.; Bell, A.N.W.; Keegan, J.D.; Moran, C.A. Efficient Aflatoxin B1 Sequestration by Yeast Cell Wall Extract and Hydrated Sodium Calcium Aluminosilicate Evaluated Using a Multimodal In-Vitro and Ex-Vivo Methodology. Toxins 2021, 13, 24. [Google Scholar] [CrossRef]
- Díaz-Llano, G.; Smith, T.K.; Boermans, H.J.; Caballero-Cortes, C.; Friendship, R. Effects of feeding diets naturally contaminated with Fusarium mycotoxins on protein metabolism in late gestation and lactation of first-parity sows12. J. Anim. Sci. 2010, 88, 998–1008. [Google Scholar] [CrossRef]
- Merrill, M.L.; Bohnert, D.W.; Harmon, D.L.; Craig, A.M.; Schrick, F.N. The ability of a yeast-derived cell wall preparation to minimize the toxic effects of high-ergot alkaloid tall fescue straw in beef cattle. J. Anim. Sci. 2007, 85, 2596–2605. [Google Scholar] [CrossRef] [PubMed]
- Stanley, V.G.; Winsman, M.; Dunkley, C.; Ogunleye, T.; Daley, M.; Krueger, W.F.; Sefton, A.E.; Hinton, A. The Impact of Yeast Culture Residue on the Suppression of Dietary Aflatoxin on the Performance of Broiler Breeder Hens. J. Appl. Poult. Res. 2004, 13, 533–539. [Google Scholar] [CrossRef]
- Weaver, A.C.; King, W.D.; Verax, M.; Fox, U.; Kudupoje, M.B.; Mathis, G.; Lumpkins, B.; Yiannikouris, A. Impact of Chronic Levels of Naturally Multi-Contaminated Feed with Fusarium Mycotoxins on Broiler Chickens and Evaluation of the Mitigation Properties of Different Titers of Yeast Cell Wall Extract. Toxins 2020, 12, 636. [Google Scholar] [CrossRef]
- Aravind, K.L.; Patil, V.S.; Devegowda, G.; Umakantha, B.; Ganpule, S.P. Efficacy of esterified glucomannan to counteract mycotoxicosis in naturally contaminated feed on performance and serum biochemical and hematological parameters in broilers. Poult. Sci. 2003, 82, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Holanda, D.M.; Yiannikouris, A.; Kim, S.W. Investigation of the efficacy of a postbiotic yeast cell wall-based blend on new-ly-weaned pigs under a dietary challenge of multiple mycotoxins with emphasis on deoxynivalenol. Toxins 2020, 12, 504. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Holanda, D.M.; Gao, X.; Park, I.; Yiannikouris, A. Efficacy of a yeast cell wall extract to mitigate the effect of nat-urally co-occurring mycotoxins contaminating feed ingredients fed to young pigs: Impact on gut health, microbiome, and growth. Toxins 2019, 11, 633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swamy, H.V.; Smith, T.K.; Macdonald, E.J.; Boermans, H.J.; Squires, E.J. Effects of feeding a blend of grains naturally con-taminated with Fusarium mycotoxins on swine performance, brain regional neurochemistry, and serum chemistry and the efficacy of a polymeric glucomannan mycotoxin adsorbent. J. Anim. Sci. 2002, 80, 3257–3267. [Google Scholar] [CrossRef] [Green Version]
- Girgis, G.N.; Barta, J.R.; Girish, C.K.; Karrow, N.A.; Boermans, H.J.; Smith, T.K. Effects of feed-borne Fusarium mycotoxins and an organic mycotoxin adsorbent on immune cell dynamics in the jejunum of chickens infected with Eimeria maxima. Veter Immunol. Immunopathol. 2010, 138, 218–223. [Google Scholar] [CrossRef]
- Girish, C.; Smith, T.; Boermans, H.; Karrow, N. Effects of Feeding Blends of Grains Naturally Contaminated With Fusarium Mycotoxins on Performance, Hematology, Metabolism, and Immunocompetence of Turkeys. Poult. Sci. 2008, 87, 421–432. [Google Scholar] [CrossRef]
- Girish, C.K.; Devegowda, G. Efficacy of glucomannan-containing Yeast Product (Mycosorb®) and Hydrated sodium calcium aluminosilicate in Preventing the Individual and Combined Toxicity of aflatoxin and T-2 Toxin in Commercial Broilers. Asian Australas. J. Anim. Sci. 2006, 19, 877–883. [Google Scholar]
- Meissonnier, G.; Raymond, I.; Laffitte, J.; Cossalter, A.; Pinton, P.; Benoit, E.; Bertin, G.; Galtier, P.; Oswald, I. Dietary gluco-mannan improves the vaccinal response in pigs exposed to aflatoxin b1 or T-2 toxin. World Mycotoxin J. 2009, 2, 161–172. [Google Scholar] [CrossRef]
- Swamy, H.V.L.N.; Smith, T.K.; Macdonald, E.J.; Karrow, N.A.; Woodward, B.; Boermans, H.J. Effects of feeding a blend of grains naturally contaminated with Fusarium mycotoxins on growth and immunological measurements of starter pigs, and the efficacy of a polymeric glucomannan mycotoxin adsorbent. J. Anim. Sci. 2003, 81, 2792–2803. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, T.; Lin, G.; Li, M.; Zhu, R.; Yiannikouris, A.; Zhang, Y.; Mai, K. The Assessment of Diet Contaminated with Aflatoxin B1 in Juvenile Turbot (Scophthalmus maximus) and the Evaluation of the Efficacy of Mitigation of a Yeast Cell Wall Extract. Toxins 2020, 12, 597. [Google Scholar] [CrossRef]
- Dvorska, J.E.; Surai, P.F.; Speake, B.K.; Sparks, N.H. Protective effect of modified glucomannans against aurofusarin-induced changes in quail egg and embryo. Comp. Biochem. Physiol. C Toxicol. Pharm. 2003, 135C, 337–343. [Google Scholar] [CrossRef]
- Dvorska, J.E.; Pappas, A.C.; Karadas, F.; Speake, B.K.; Surai, P.F. Protective effect of modified glucomannans and organic selenium against antioxidant depletion in the chicken liver due to T-2 toxin-contaminated feed consumption. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 145, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Dvorska, J.E.; Surai, P.F. Effects of T-2 Toxin, Zeolite and Mycosorb on Antioxidant Systems of Growing Quail. Asian Australas. J. Anim. Sci. 2001, 14, 1752–1757. [Google Scholar] [CrossRef]
- Santos, R.; Fink-Gremmels, J. Mycotoxin syndrome in dairy cattle: Characterisation and intervention results. World Mycotoxin J. 2014, 7, 357–366. [Google Scholar] [CrossRef]
- Raju, M.; Devegowda, G. Influence of esterified-glucomannan on performance and organ morphology, serum biochemistry and haematology in broilers exposed to individual and combined mycotoxicosis (aflatoxin, ochratoxin and T-2 toxin). Br. Poult. Sci. 2000, 41, 640–650. [Google Scholar] [CrossRef]
- Raju, M.V.L.N.; Devegowda, G. Esterified-Glucomannan in Broiler Chicken Diets-Contaminated with Aflatoxin, Ochratoxin and T-2 Toxin: Evaluation of its Binding Ability (in vitro) and Efficacy as Immunomodulator. Asian Australas. J. Anim. Sci. 2002, 15, 1051–1056. [Google Scholar] [CrossRef]
- Firmin, S.; Gandia, P.; Morgavi, D.P.; Houin, G.; Jouany, J.; Bertin, G.; Boudra, H. Modification of aflatoxin B1and ochratoxin A toxicokinetics in rats administered a yeast cell wall preparation. Food Addit. Contam. Part A 2010, 27, 1153–1160. [Google Scholar] [CrossRef] [Green Version]
- Firmin, S.; Morgavi, D.; Yiannikouris, A.; Boudra, H. Effectiveness of modified yeast cell wall extracts to reduce aflatoxin B1 absorption in dairy ewes. J. Dairy Sci. 2011, 94, 5611–5619. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.A.; Apajalahti, J.; Yiannikouris, A.; Ojanperä, S.; Kettunen, H. Effects of low dietary aflatoxin b1 on broiler liver concentration without and with Mycosorb® toxin binder. J. Appl. Anim. Nutr. 2013, 2, e4. [Google Scholar] [CrossRef]
- Phillips, T.D.; Kubena, L.F.; Harvey, R.B.; Taylor, D.R.; Heidelbaugh, N.D. Hydrated Sodium Calcium Aluminosilicate: A High Affinity Sorbent for Aflatoxin. Poult. Sci. 1988, 67, 243–247. [Google Scholar] [CrossRef]
- FEFANA. Animal feeding-stuffs—determination of in vitro efficacy of Mycotoxin inactivators based on adsorption assay of aflatoxin B1. In DRAFT Mycotoxin Inactivators Adsorption Method; FEFANA: Brussels, Belgium, 2009. [Google Scholar]
- Lee, H.B.; Blaufox, M.D. Blood volume in the rat. J. Nucl. Med. 1985, 26, 72–76. [Google Scholar]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Yiannikouris, A.; Poughon, L.; Cameleyre, X.; Dussap, C.-G.; François, J.; Bertin, G.; Jouany, J.-P. A novel technique to evaluate interactions between Saccharomyces cerevisiae cell wall and mycotoxins: Application to zearalenone. Biotechnol. Lett. 2003, 25, 783–789. [Google Scholar] [CrossRef]
- Fasullo, M.; Smith, A.; Egner, P.; Cera, C. Activation of aflatoxin B1 by expression of human CYP1A2 polymorphisms in Saccharomyces cerevisiae. Mutat. Res. Toxicol. Environ. Mutagen. 2014, 761, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, E.P.; Kunze, K.L.; Stapleton, P.L.; Eaton, D.L. The kinetics of aflatoxin B1Oxidation by human cDNA-Expressed and human liver microsomal cytochromes P450 1A2 and 3A4. Toxicol. Appl. Pharm. 1996, 141, 595–606. [Google Scholar] [CrossRef]
- Kumagai, S. Intestinal absorption and excretion of aflatoxin in rats. Toxicol. Appl. Pharmacol. 1989, 97, 88–97. [Google Scholar] [CrossRef]
- Bastaki, S.A.; Osman, N.; Kochiyil, J.; Shafiullah, M.; Padmanabhan, R.; Abdulrazzaq, Y.M. Toxicokinetics of Aflatoxin in Pregnant Mice. Int. J. Toxicol. 2010, 29, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Wang, W.; Degroote, J.; Van Noten, N.; Yan, H.; Majdeddin, M.; Van Poucke, M.; Peelman, L.; Goderis, A.; Van De Mierop, K.; et al. Mycotoxin binder improves growth rate in piglets associated with reduction of toll-like re-ceptor-4 and increase of tight junction protein gene expression in gut mucosa. J. Anim. Sci. Biotechnol. 2017, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Grenier, B.; Applegate, T.J. Modulation of intestinal functions following mycotoxin ingestion: Meta-analysis of published ex-periments in animals. Toxins 2013, 5, 396–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parke, D.V.; Williams, R.T. Metabolism of toxic substances. Br. Med. Bull. 1969, 25, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xing, L.; Zhang, M.; Wang, J.; Zheng, N. The toxic effects of aflatoxin b1 and aflatoxin M1 on kidney through regulating L-proline and downstream apoptosis. Biomed. Res. Int. 2018, 2018, 9074861. [Google Scholar] [CrossRef] [Green Version]
- Grosman, M.E.; Elías, M.M.; Comin, E.J.; Garay, E.A.R. Alterations in renal function induced by aflatoxin B1 in the rat. Toxicol. Appl. Pharmacol. 1983, 69, 319–325. [Google Scholar] [CrossRef]
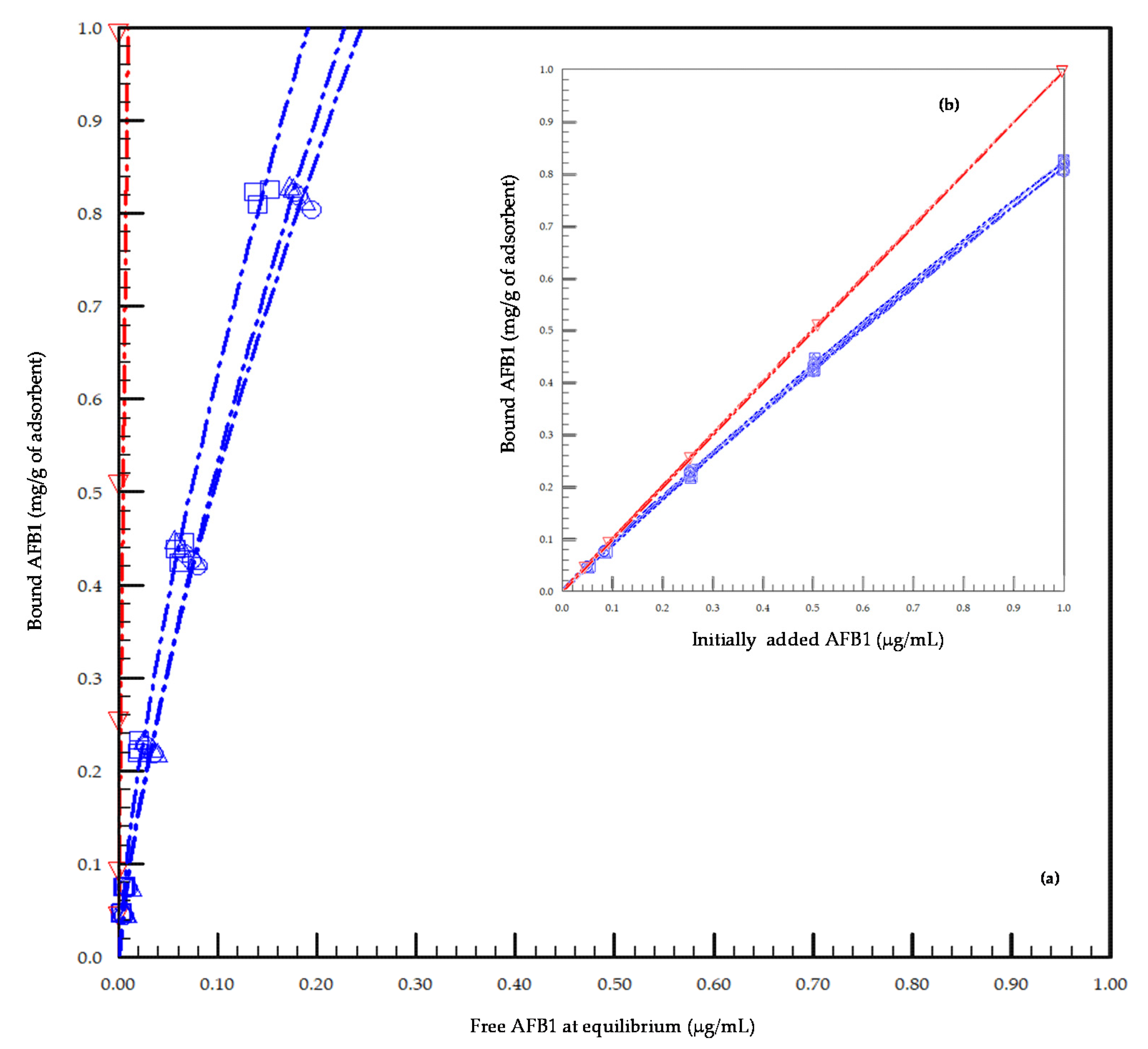


| Tested Adsorbent: | YCW | HSCAS | ||
|---|---|---|---|---|
| AFB1 Concentration (μg/mL) | #496992 | #496993 | #496994 | #50103379 |
| 1.00 | 85.58 ± 0.85 | 82.41 ± 0.85 | 81.53 ± 0.96 | 99.98 ± 0.03 |
| 0.50 | 87.75 ± 0.78 | 87.49 ± 2.24 | 85.27 ± 1.30 | 100.00 |
| 0.25 | 91.93 ± 0.38 | 88.61 ± 2.72 | 88.13 ± 1.94 | 100.00 |
| 0.10 | 93.88 ± 1.21 | 89.10 ± 0.26 | 88.93 ± 0.91 | 100.00 |
| 0.05 | 93.72 ± 0.45 | 92.72 ± 0.21 | 90.09 ± 1.68 | 100.00 |
| Average (%) | 90.57 ± 3.52 a | 88.07 ± 3.72 b,c | 86.79 ± 3.40 c | 100.00 d |
| CV (%) | 3.88 | 4.22 | 3.92 | 0.01 |
| Average Total (%) | 87.48 ± 3.73 a | 100.00 b | ||
| ANOVA | Dunnett | MLR | |||
|---|---|---|---|---|---|
| Tissue | YCW 2 g/kg | YCW 10 g/kg | HSCAS 10 g/kg | YCW | |
| Stomach (% DPM) | −16% | −25% | −8% | −21% | |
| Stomach (% recovery) | −31% | −31% | −9% | −27% | |
| Small intestine (% DPM) | +23% | +21% | +70% | +67% * | |
| Small intestine (% recovery) | +24% | +62% | +63% | +58% ** | |
| Cecum (% DPM) | ** | +26% | +70% ** | +49% * | +66% **** |
| Cecum (% recovery) | ** | +20% | +66% ** | +49% * | +64% **** |
| Colon (% DPM) | * | +29% | +83% | +96% * | +78% * |
| Colon (% recovery) | +27% | +78% | +123% | +74% * | |
| Total digesta (% DPM) | +8% | +32% | +35% | +31% * | |
| Total digesta (% recovery) | **** | +6% | +27% *** | +38% **** | +27% **** |
| Plasma (% DPM) | **** | −15% | −50% *** | −67% **** | −49% **** |
| Plasma (% recovery) | **** | −16% | −50% *** | −65% **** | −48% *** |
| Liver(% DPM) | **** | −2% | −42% **** | −64% **** | −44% **** |
| Liver (% recovery) | **** | −5% | −42% *** | −63% **** | −43% *** |
| Kidney (% DPM) | **** | −8% | −37% *** | −54% **** | −37% *** |
| Kidney (% recovery) | *** | −11% | −36% ** | −52% *** | −35% *** |
| Total systemic (% DPM) | **** | −9% | −46% **** | −66% **** | −46% **** |
| Total systemic (% recovery) | **** | −11% | −46% *** | −64% **** | −45% **** |
| ANOVA | Dunnett | MLR | |||
|---|---|---|---|---|---|
| Tissue | YCW 2 g/kg | YCW 10 g/kg | HSCAS 10 g/kg | YCW | |
| Stomach (% DPM) | +363% | +844% | +506% | +788% | |
| Stomach (% recovery) | +347% | +824% | +413 | +759% | |
| Small intestine (% DPM) | +13% | +160% | +49% | +167% ** | |
| Small intestine (% recovery) | +6% | +129% | +45% | +136% * | |
| Cecum (% DPM) | ** | +22% | +90% * | +86% ** | +89% ** |
| Cecum (% recovery) | *** | +15% | +62% ** | +80% *** | +61% ** |
| Colon (% DPM) | * | +38% | +43% | +92% * | +32% |
| Colon (% recovery) | * | +34% | +21% | +83% ** | +11% |
| Total digesta (% DPM) | **** | +29% | +76% *** | +86% *** | +71% *** |
| Total digesta (% recovery) | **** | +23% * | +50% **** | +79% **** | +46% **** |
| Plasma (% DPM) | **** | −15% | −30% * | −64% **** | −25% * |
| Plasma (% recovery) | **** | −22% ** | −40% **** | −67% **** | −35% *** |
| Liver(% DPM) | **** | −8% | −29% * | −58% **** | −28% ** |
| Liver (% recovery) | **** | −14% | −41% *** | −61% **** | −39% *** |
| Kidney (% DPM) | ** | −12% | −15% | −49% ** | −12% |
| Kidney (% recovery) | ** | −17% | −29% * | −52% *** | −25% |
| Total systemic (% DPM) | **** | −14% | −29% * | −61% **** | −26% ** |
| Total systemic (% recovery) | **** | −19% * | −41% **** | −64% **** | −37% **** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yiannikouris, A.; Apajalahti, J.; Siikanen, O.; Dillon, G.P.; Moran, C.A. Saccharomyces cerevisiae Cell Wall-Based Adsorbent Reduces Aflatoxin B1 Absorption in Rats. Toxins 2021, 13, 209. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13030209
Yiannikouris A, Apajalahti J, Siikanen O, Dillon GP, Moran CA. Saccharomyces cerevisiae Cell Wall-Based Adsorbent Reduces Aflatoxin B1 Absorption in Rats. Toxins. 2021; 13(3):209. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13030209
Chicago/Turabian StyleYiannikouris, Alexandros, Juha Apajalahti, Osmo Siikanen, Gerald Patrick Dillon, and Colm Anthony Moran. 2021. "Saccharomyces cerevisiae Cell Wall-Based Adsorbent Reduces Aflatoxin B1 Absorption in Rats" Toxins 13, no. 3: 209. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13030209





