Combining Patulin with Cadmium Induces Enhanced Hepatotoxicity and Nephrotoxicity In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
2.1. Combining PAT and CdCl2 Causes a Synergistic Apoptosis Induction In Vitro
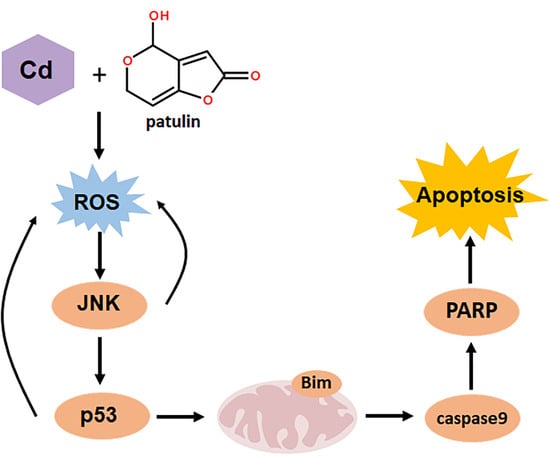
2.2. Synergistic Cell Death Caused by PAT and CdCl2 Is Mitochondria-Mediated Caspase-Dependent Apoptosis
2.3. Enhanced ROS Generation Plays a Critical Role in the Cell Death Caused by Co-Treatment with PAT and CdCl2
2.4. p53 Contributes to the Synergistic Effect Induced by the Combination of PAT and CdCl2
2.5. JNK1 Activation Contributes to the Synergistic Effect Caused by the Combination of PAT and CdCl2
2.6. Combination of PAT and CdCl2 Caused Hepatic and Renal Injury In Vivo
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Reagents
5.2. Cell Culture and Treatments
5.3. Apoptosis Evaluation
5.4. Crystal Violet Staining
5.5. Western Blotting
5.6. Measurement of ROS
5.7. Measurement of MMP
5.8. Combination Index Calculation
5.9. Animals and Treatments
5.10. Biochemical Assay
5.11. Histopathology
5.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Puel, O.; Galtier, P.; Oswald, I.P. Biosynthesis and Toxicological Effects of Patulin. Toxins 2010, 2, 613–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, S.; Singh, N.; Ansari, K.M. Toxicological effects of patulin mycotoxin on the mammalian system: An overview. Toxicol. Res. 2017, 6, 764–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.H.; Wu, T.S.; Yu, F.Y.; Wang, C.H. Mycotoxin patulin activates the p38 kinase and JNK signaling pathways in human embryonic kidney cells. Toxicol. Sci. 2006, 89, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Yin, S.; Song, X.; Zhang, E.; Fan, L.; Hu, H. 53 activation contributes to patulin-induced nephrotoxicity via modulation of reactive oxygen species generation. Sci. Rep. 2016, 13, 24455. [Google Scholar] [CrossRef] [PubMed]
- Manel, B.; Intidhar, B.S.; Alexandre, P.; Arnaud, G.; Hassen, B.; Salwa, A.E.; Christophe, L. Patulin induces apoptosis through ROS-mediated endoplasmic reticulum stress pathway. Toxicol. Sci. 2015, 144, 328–337. [Google Scholar] [CrossRef] [Green Version]
- Thévenod, F.; Lee, W.K. Toxicology of Cadmium and Its Damage to Mammalian Organs. Met. Ions Life Sci. 2013, 11, 415–490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zheng, S.; Wang, S.; Liu, Q.; Xu, S. Cadmium-induced oxidative stress promotes apoptosis and necrosis through the regulation of the miR-216a-PI3K/AKT axis in common carp lymphocytes and antagonized by selenium. Chemosphere 2020, 258, 127341. [Google Scholar] [CrossRef]
- Chuang, S.M.; Wang, I.C.; Yang, J.L. Roles of JNK, p38 and ERK mitogen-activated protein kinases in the growth inhibition and apoptosis induced by cadmium. Carcinogenesis 2000, 21, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.S.; Liao, Y.C.; Yu, F.Y.; Chang, C.H.; Liu, B.H. Mechanism of patulin-induced apoptosis in human leukemia cells (HL-60). Toxicol. Lett. 2009, 183, 105–111. [Google Scholar] [CrossRef]
- Cao, X.; Fu, M.; Bi, R.; Zheng, X.; Fu, B.; Tian, S.; Liu, C.; Li, Q.; Liu, J. Cadmium induced BEAS-2B cells apoptosis and mitochondria damage via MAPK signaling pathway. Chemosphere 2021, 263, 128346. [Google Scholar] [CrossRef]
- Lee, J.Y.; Tokumoto, M.; Fujiwara, Y.; Hasegawa, T.; Seko, Y.; Shimada, A.; Satoh, M. Accumulation of p53 via down-regulation of UBE2D family genes is a critical pathway for cadmium-induced renal toxicity. Sci. Rep. 2016, 25, 21968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, Y.O.; Lee, J.C.; Hitron, J.A.; Pan, J.; Zhang, Z.; Shi, X. Cadmium induces intracellular Ca2+- and H2O2-dependent apoptosis through JNK- and p53-mediated pathways in skin epidermal cell line. Toxicol. Sci. 2010, 113, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Assunção, R.; Pinhão, M.; Loureiro, S.; Alvito, P.; Silva, M.J. A multi-endpoint approach to the combined toxic effects of patulin and ochratoxin a in human intestinal cells. Toxicol. Lett. 2019, 313, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.J.; Macáková, P.; Juan-García, A.; Font, G. Cytotoxic effects of mycotoxin combinations in mammalian kidney cells. Food Chem. Toxicol. 2011, 49, 2718–2724. [Google Scholar] [CrossRef] [PubMed]
- Matović, V.; Buha, A.; Ðukić-Ćosić, D.; Bulat, Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. 2015, 78, 130–140. [Google Scholar] [CrossRef]
- Dai, X.; Xing, C.; Cao, H.; Luo, J.; Wang, T.; Liu, P.; Guo, X.; Hu, G.; Zhang, C. Alterations of mitochondrial antioxidant indexes and apoptosis in duck livers caused by Molybdenum or/and cadmium. Chemosphere 2018, 193, 574–580. [Google Scholar] [CrossRef]
- Heussner, A.H.; Dietrich, D.R.; O’ Brien, E. In vitro investigation of individual and combined cytotoxic effects of ochratoxin A and other selected mycotoxins on renal cells. Toxicol. In Vitro 2006, 20, 332–333. [Google Scholar] [CrossRef] [Green Version]
- Rai, A.; Maurya, S.K.; Khare, P.; Srivastava, A.; Bandyopadhyay, S. Characterization of developmental neurotoxicity of As, Cd, and Pb mixture: Synergistic action of metal mixture in glial and neuronal functions. Toxicol. Sci. 2010, 118, 586–601. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Nie, G.; Yang, F.; Chen, J.; Zhuang, Y.; Dai, X.; Liao, Z.; Yang, Z.; Cao, H.; Xing, C.; et al. Molybdenum and cadmium co-induce oxidative stress and apoptosis through mitochondria-mediated pathway in duck renal tubular epithelial cells. J. Hazard. Mater. 2020, 383, 121157. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, M.; Micali, A.; Marini, H.; Adamo, E.B.; Puzzolo, D.; Pisani, A.; Trichilo, V.; Altavilla, D.; Squadrito, F.; Minutoli, L. Cadmium, Organ Toxicity and Therapeutic Approaches: A Review on Brain, Kidney and Testis Damage. Curr. Med. Chem. 2017, 24, 3879–3893. [Google Scholar] [CrossRef] [PubMed]
- Fliege, R.; Metzler, M. Electrophilic properties of patulin. N-acetylcysteine and glutathione adducts. Chem. Res. Toxicol. 2000, 13, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Fliege, R.; Metzler, M. The mycotoxin patulin induces intra- and intermolecular protein crosslinks in vitro involving cysteine, lysine, and histidine side chains, and alpha-amino groups. Chem. Biol. Interact. 1999, 123, 85–103. [Google Scholar] [CrossRef]
- Mahfoud, R.; Maresca, M.; Garmy, N.; Fantini, J. The mycotoxin patulin alters the barrier function of the intestinal epithelium: Mechanism of action of the toxin and protective effects of glutathione. Toxicol. Appl. Pharmacol. 2002, 181, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qian, S.Y.; Guo, Q.; Jiang, J.; Waalkes, M.P.; Mason, R.P.; Kadiiska, M.B. Cadmium generates reactive oxygen- and carbon-centered radical species in rats: Insights from in vivo spin-trapping studies. Free Radic. Biol. Med. 2008, 45, 475–481. [Google Scholar] [CrossRef] [Green Version]
- O’ Connor, L.; Strasser, A.; O’ Reilly, L.A.; Hausmann, G.; Adams, J.M.; Cory, S.; Huang, D.C. Bim: A novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998, 17, 384–395. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, D.; Mnich, K.; Oommen, D.; Chakravarthy, R.; Almeida-Souza, L.; Krols, M.; Saveljeva, S.; Doyle, K.; Gupta, S.; Timmerman, V.; et al. HSPB1 facilitates ERK-mediated phosphorylation and degradation of BIM to attenuate endoplasmic reticulum stress-induced apoptosis. Cell Death Dis. 2017, 8, e3026. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Yin, S.; Zhao, C.; Fan, L.; Hu, H. Combining Patulin with Cadmium Induces Enhanced Hepatotoxicity and Nephrotoxicity In Vitro and In Vivo. Toxins 2021, 13, 221. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13030221
Cui J, Yin S, Zhao C, Fan L, Hu H. Combining Patulin with Cadmium Induces Enhanced Hepatotoxicity and Nephrotoxicity In Vitro and In Vivo. Toxins. 2021; 13(3):221. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13030221
Chicago/Turabian StyleCui, Jinling, Shutao Yin, Chong Zhao, Lihong Fan, and Hongbo Hu. 2021. "Combining Patulin with Cadmium Induces Enhanced Hepatotoxicity and Nephrotoxicity In Vitro and In Vivo" Toxins 13, no. 3: 221. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13030221