An Innovative Synbiotic Formulation Decreases Free Serum Indoxyl Sulfate, Small Intestine Permeability and Ameliorates Gastrointestinal Symptoms in a Randomized Pilot Trial in Stage IIIb-IV CKD Patients
Abstract
:1. Introduction
2. Results
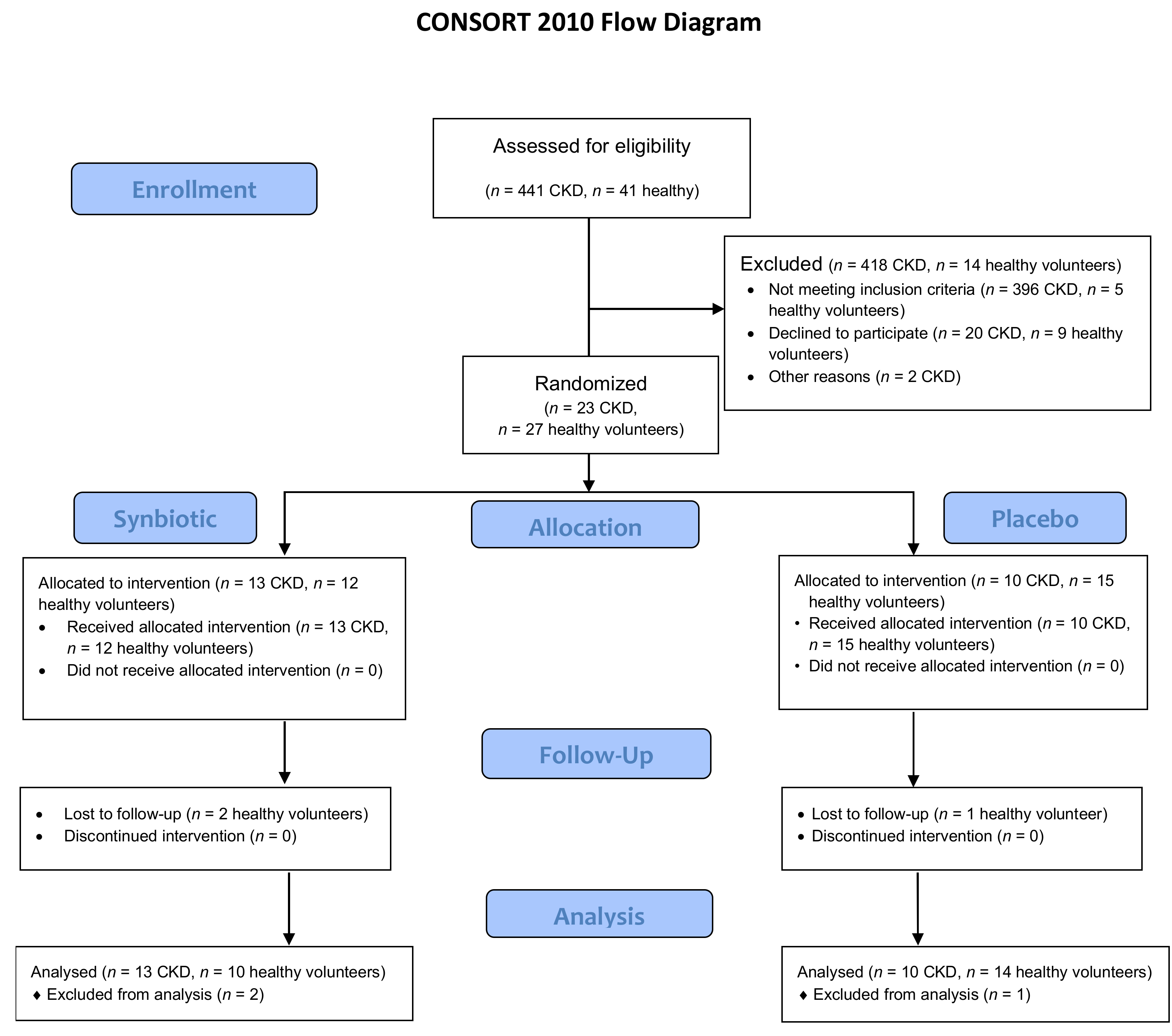
2.1. Clinical Trial, Baseline Characteristics and Clinical Results
2.2. NATUREN G® Reduces Serum Concentration of Free IS Only in CKD Patients
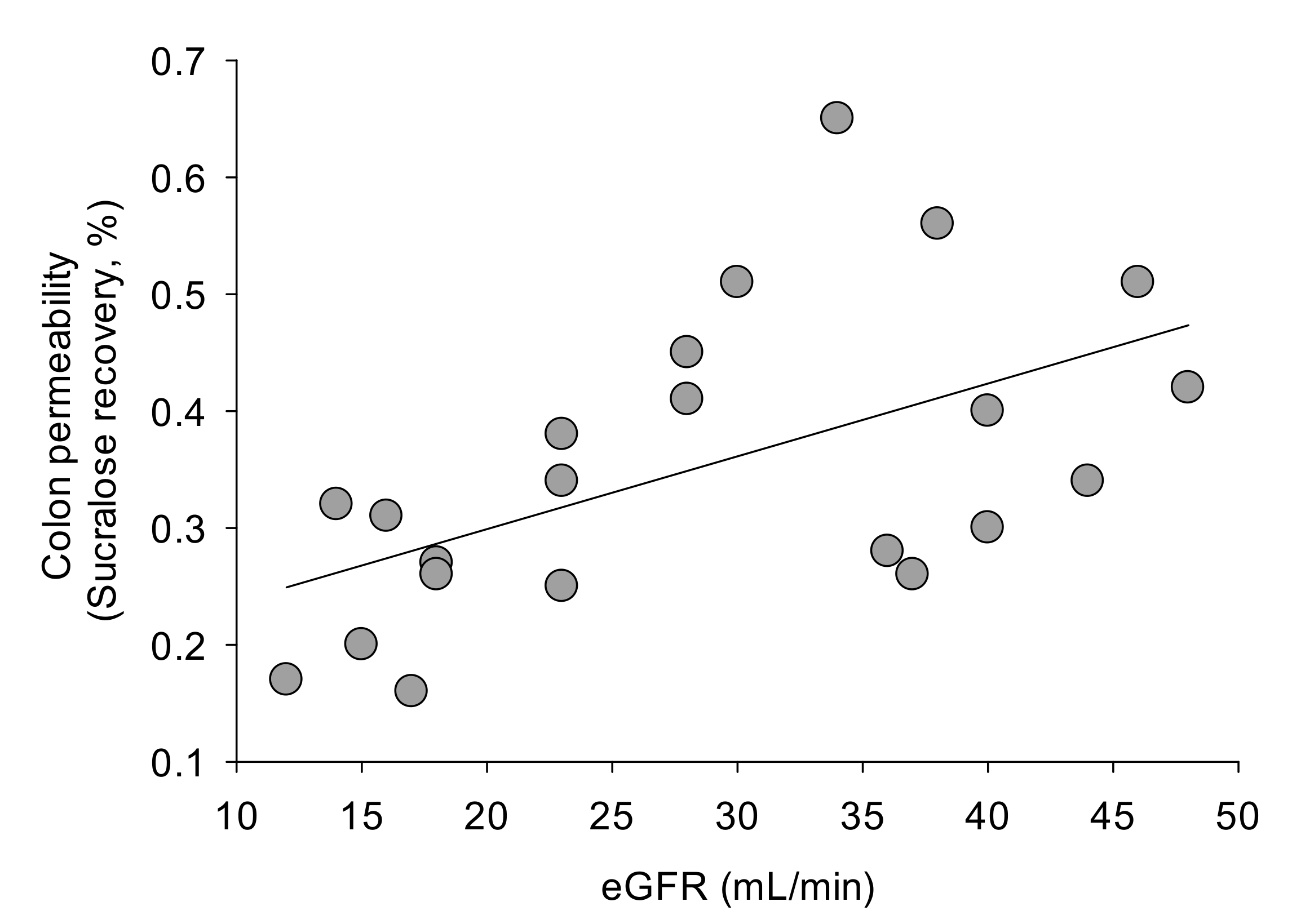
2.3. Whole Gut Permeability in Healthy Subjects and in CKD Patients
2.4. NATUREN G® Ameliorates Gastrointestinal Symptoms in the CKD Group
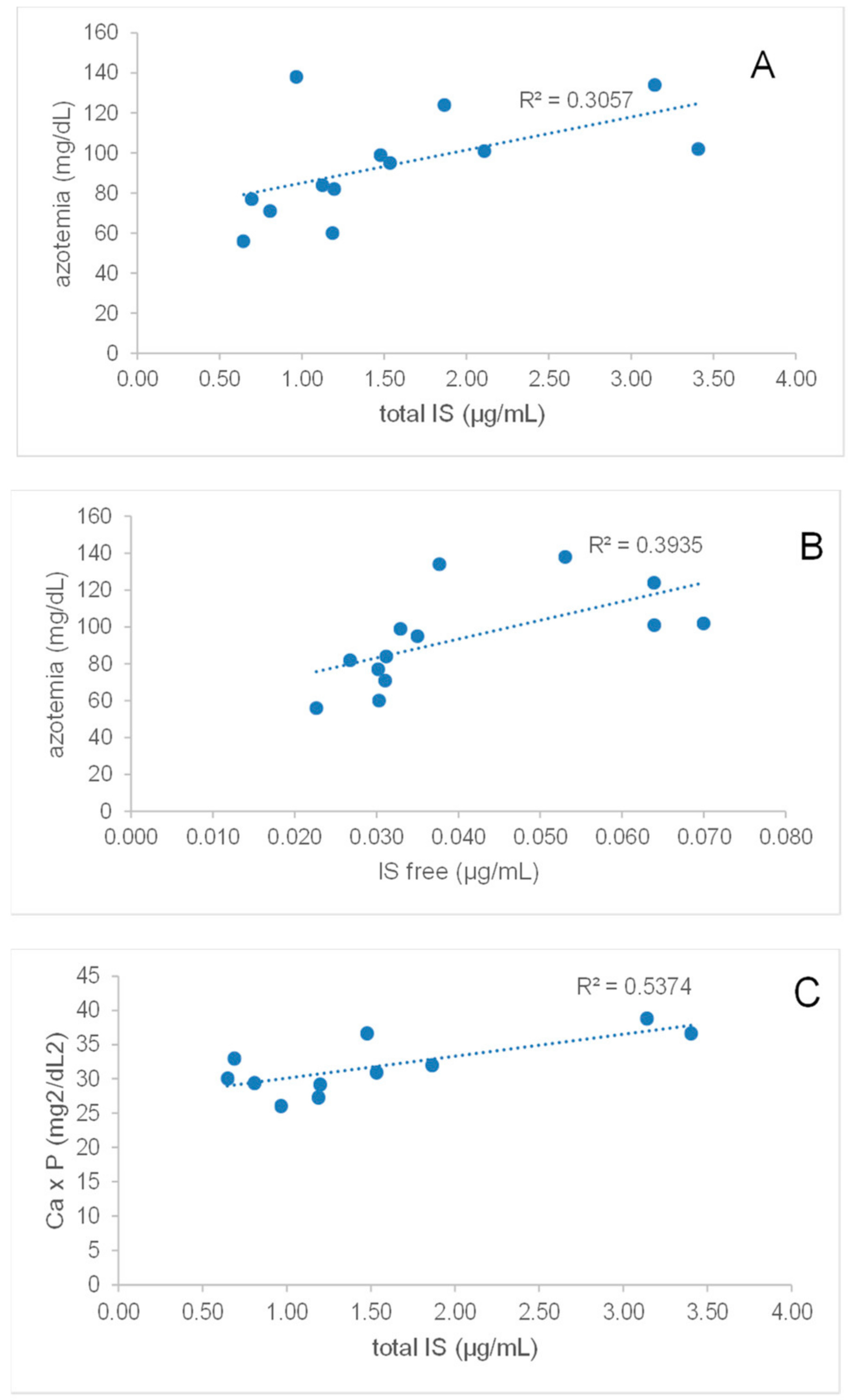
2.5. Total and Free IS Directly Correlate with Azotemia in Synbiotic-Treated CKD Patients
3. Discussion
4. Materials and Methods
4.1. Study Design, Clinical Parameters and GSRS/Bristol Scores
4.2. Liquid Chromatography/Electrospray Ionization–Mass Spectrometry/Mass Spectrometry (LC/ESI–MS/MS) for Quantification of pCS and IS
4.3. Measurement of Gut Permeability
4.4. IL6 ELISA
4.5. Statistical Analysis and Correlations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castillo-Rodriguez, E.; Fernandez-Prado, R.; Esteras, R.; Perez-Gomez, M.V.; Gracia-Iguacel, C.; Fernandez-Fernandez, B.; Kanbay, M.; Tejedor, A.; Lazaro, A.; Ruiz-Ortega, M.; et al. Impact of Altered Intestinal Microbiota on Chronic Kidney Disease Progression. Toxins 2018, 10, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holle, J.; Kirchner, M.; Okun, J.; Bayazit, A.K.; Obrycki, L.; Canpolat, N.; Bulut, I.K.; Azukaitis, K.; Duzova, A.; Ranchin, B.; et al. Serum indoxyl sulfate concentrations associate with progression of chronic kidney disease in children. PLoS ONE 2020, 15, e0240446. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.-W.; Hsu, K.-H.; Lee, C.-C.; Sun, C.-Y.; Hsu, H.-J.; Tsai, C.-J.; Tzen, C.-Y.; Wang, Y.-C.; Lin, C.-Y.; Wu, M.-S. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol. Dial. Transplant. 2010, 26, 938–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramezani, A.; Massy, Z.A.; Meijers, B.; Evenepoel, P.; Vanholder, R.; Raj, D.S. Role of the Gut Microbiome in Uremia: A Potential Therapeutic Target. Am. J. Kidney Dis. 2016, 67, 483–498. [Google Scholar] [CrossRef] [Green Version]
- Cosola, C.; Rocchetti, M.T.; Cupisti, A.; Gesualdo, L. Microbiota metabolites: Pivotal players of cardiovascular damage in chronic kidney disease. Pharmacol. Res. 2018, 130, 132–142. [Google Scholar] [CrossRef]
- Ikee, R.; Sasaki, N.; Yasuda, T.; Fukazawa, S. Chronic Kidney Disease, Gut Dysbiosis, and Constipation: A Burdensome Triplet. Microorganisms 2020, 8, 1862. [Google Scholar] [CrossRef]
- Sumida, K.; Molnar, M.Z.; Potukuchi, P.K.; Thomas, F.; Lu, J.L.; Matsushita, K.; Yamagata, K.; Kalantar-Zadeh, K.; Kovesdy, C.P. Constipation and Incident CKD. J. Am. Soc. Nephrol. 2016, 28, 1248–1258. [Google Scholar] [CrossRef] [Green Version]
- Vaziri, N.D.; Zhao, Y.-Y.; Pahl, M.V. Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: The nature, mechanisms, consequences and potential treatment. Nephrol. Dial. Transplant. 2015, 31, 737–746. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Lim, S.Y.; Ko, Y.S.; Lee, H.Y.; Oh, S.W.; Kim, M.G.; Cho, W.Y.; Jo, S.K. Intestinal barrier disruption and dysregulated mucosal immunity contribute to kidney fibrosis in chronic kidney disease. Nephrol. Dial. Transplant. 2019, 34, 419–428. [Google Scholar] [CrossRef]
- Shah, N.B.; Allegretti, A.S.; Nigwekar, S.U.; Kalim, S.; Zhao, S.; Lelouvier, B.; Servant, F.; Serena, G.; Thadhani, R.I.; Raj, D.S.; et al. Blood Microbiome Profile in CKD: A Pilot Study. Clin. J. Am. Soc. Nephrol. 2019, 14, 692–701. [Google Scholar] [CrossRef]
- Gonzalez, A.; Krieg, R.; Massey, H.D.; Carl, D.; Ghosh, S.; Gehr, T.W.B.; Ghosh, S.S. Sodium butyrate ameliorates insulin resistance and renal failure in CKD rats by modulating intestinal permeability and mucin expression. Nephrol. Dial. Transplant. 2019, 34, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Caggiano, G.; Cosola, C.; Di Leo, V.; Gesualdo, M.; Gesualdo, L. Microbiome modulation to correct uremic toxins and to preserve kidney functions. Curr. Opin. Nephrol. Hypertens. 2020, 29, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, M.T.; Cosola, C.; Di Bari, I.; Magnani, S.; Galleggiante, V.; Scandiffio, L.; Dalfino, G.; Netti, G.S.; Atti, M.; Corciulo, R.; et al. Efficacy of Divinylbenzenic Resin in Removing Indoxyl Sulfate and P-cresol Sulfate in Hemodialysis Patients: Results from an In Vitro Study and an In Vivo Pilot Trial (xuanro4-Nature 3.2). Toxins 2020, 12, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavan, M. Influence of prebiotic and probiotic supplementation on the progression of chronic kidney disease. Minerva Urol. Nefrol. 2014, 68, 222–226. [Google Scholar]
- Takkavatakarn, K.; Wuttiputinun, T.; Phannajit, J.; Praditpornsilpa, K.; Eiam-Ong, S.; Susantitaphong, P. Protein-bound uremic toxin lowering strategies in chronic kidney disease: A systematic review and meta-analysis. J. Nephrol. 2021, 1–13. [Google Scholar] [CrossRef]
- Sumida, K.; Yamagata, K.; Kovesdy, C.P. Constipation in CKD. Kidney Int. Rep. 2020, 5, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Ramos, C.I.; Armani, R.G.; Canziani, M.E.F.; Dalboni, M.A.; Dolenga, C.J.R.; Nakao, L.S.; Campbell, K.L.; Cuppari, L. Effect of prebiotic (fructooligosaccharide) on uremic toxins of chronic kidney disease patients: A randomized controlled trial. Nephrol. Dial. Transplant. 2019, 34, 1876–1884. [Google Scholar] [CrossRef]
- Armani, R.G.; Carvalho, A.B.; Ramos, C.I.; Hong, V.; Bortolotto, L.A.; Cassiolato, J.L.; Oliveira, N.F.; Cieslarova, Z.; do Lago, C.L.; Klassen, A.; et al. Effect of fructooligosaccharide on endothelial function in CKD patients: A randomized controlled trial. Nephrol. Dial. Transplant. 2021, 7, 335. [Google Scholar] [CrossRef]
- Poesen, R.; Evenepoel, P.; De Loor, H.; Delcour, J.A.; Courtin, C.M.; Kuypers, D.; Augustijns, P.; Verbeke, K.; Meijers, B. The Influence of Prebiotic Arabinoxylan Oligosaccharides on Microbiota Derived Uremic Retention Solutes in Patients with Chronic Kidney Disease: A Randomized Controlled Trial. PLoS ONE 2016, 11, e0153893. [Google Scholar] [CrossRef]
- Guida, B.; Germanò, R.; Trio, R.; Russo, D.; Memoli, B.; Grumetto, L.; Barbato, F.; Cataldi, M. Effect of short-term synbiotic treatment on plasma p-cresol levels in patients with chronic renal failure: A randomized clinical trial. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1043–1049. [Google Scholar] [CrossRef]
- Rossi, M.; Johnson, D.W.; Morrison, M.; Pascoe, E.M.; Coombes, J.S.; Forbes, J.M.; Szeto, C.-C.; McWhinney, B.C.; Ungerer, J.P.; Campbell, K.L. Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY): A Randomized Trial. Clin. J. Am. Soc. Nephrol. 2016, 11, 223–231. [Google Scholar] [CrossRef]
- Di Iorio, B.R.; Rocchetti, M.T.; De Angelis, M.; Cosola, C.; Marzocco, S.; Di Micco, L.; Di Bari, I.; Accetturo, M.; Vacca, M.; Gobbetti, M.; et al. Nutritional Therapy Modulates Intestinal Microbiota and Reduces Serum Levels of Total and Free Indoxyl Sulfate and P-Cresyl Sulfate in Chronic Kidney Disease (Medika Study). J. Clin. Med. 2019, 8, 1424. [Google Scholar] [CrossRef] [Green Version]
- Saldanha, J.F.; Leal, V.O.; Rizzetto, F.; Grimmer, G.H.; Ribeiro-Alves, M.; Daleprane, J.B.; Carraro-Eduardo, J.C.; Mafra, D. Effects of Resveratrol Supplementation in Nrf2 and NF-κB Expressions in Nondialyzed Chronic Kidney Disease Patients: A Randomized, Double-Blind, Placebo-Controlled, Crossover Clinical Trial. J. Ren. Nutr. 2016, 26, 401–406. [Google Scholar] [CrossRef]
- Migliori, M.; Panichi, V.; De La Torre, R.; Fitó, M.; Covas, M.; Bertelli, A.; Muñoz-Aguayo, D.; Scatena, A.; Paoletti, S.; Ronco, C. Anti-Inflammatory Effect of White Wine in CKD Patients and Healthy Volunteers. Blood Purif. 2015, 39, 218–223. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, H.; Shi, K.; Ren, Y.; Zhang, P.; Cheng, S. Gut bacterial translocation is associated with microinflammation in end-stage renal disease patients. Nephrology 2012, 17, 733–738. [Google Scholar] [CrossRef]
- Shi, K.; Wang, F.; Jiang, H.; Liu, H.; Wei, M.; Wang, Z.; Xie, L. Gut Bacterial Translocation May Aggravate Microinflammation in Hemodialysis Patients. Dig. Dis. Sci. 2014, 59, 2109–2117. [Google Scholar] [CrossRef]
- Fujii, R.; Yamada, H.; Munetsuna, E.; Yamazaki, M.; Ohashi, K.; Ishikawa, H.; Maeda, K.; Hagiwara, C.; Ando, Y.; Hashimoto, S.; et al. Associations of Circulating MicroRNAs (miR-17, miR-21, and miR-150) and Chronic Kidney Disease in a Japanese Population. J. Epidemiol. 2020, 30, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Shen, J.; Cheng, J.; Fan, X. MicroRNA-21 regulates intestinal epithelial tight junction permeability. Cell Biochem. Funct. 2015, 33, 235–240. [Google Scholar] [CrossRef]
- Lv, L.-L.; Cao, Y.-H.; Ni, H.-F.; Xu, M.; Liu, D.; Liu, H.; Chen, P.-S.; Liu, B.-C. MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am. J. Physiol. Physiol. 2013, 305, F1220–F1227. [Google Scholar] [CrossRef] [Green Version]
- Chao, G.; Wang, Y.; Zhang, S.; Yang, W.; Ni, Z.; Zheng, X. MicroRNA-29a increased the intestinal membrane permeability of colonic epithelial cells in irritable bowel syndrome rats. Oncotarget 2017, 8, 85828–85837. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Souba, W.W.; Croce, C.M.; Verne, G.N. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut 2009, 59, 775–784. [Google Scholar] [CrossRef] [Green Version]
- Simenhoff, M.L.; Saukkonen, J.J.; Burke, J.F.; Wesson, J.L.G.; Schaedler, R.W.; Gordon, S.J. Bacterial Populations of the Small Intestine in Uremia. Nephron 1978, 22, 63–68. [Google Scholar] [CrossRef]
- Strid, H.; Simrén, M.; Stotzer, P.-O.; Ringström, G.; Abrahamsson, H.; Björnsson, E.S. Patients with Chronic Renal Failure Have Abnormal Small Intestinal Motility and a High Prevalence of Small Intestinal Bacterial Overgrowth. Digestion 2003, 67, 129–137. [Google Scholar] [CrossRef]
- Riordan, S.M.; McIver, C.J.; Thomas, D.H.; Duncombe, V.M.; Bolin, T.D.; Thomas, M.C. Luminal Bacteria and Small-Intestinal Permeability. Scand. J. Gastroenterol. 1997, 32, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Di Palo, D.M.; Garruti, G.; Di Ciaula, A.; Molina-Molina, E.; Shanmugam, H.; De Angelis, M.; Portincasa, P. Increased Colonic Permeability and Lifestyles as Contributing Factors to Obesity and Liver Steatosis. Nutrients 2020, 12, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julious, S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. 2005, 4, 287–291. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E.; et al. A 14-Item Mediterranean Diet Assessment Tool and Obesity Indexes among High-Risk Subjects: The PREDIMED Trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svedlund, J.; Dotevall, G. GSRS? A clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig. Dis. Sci. 1988, 33, 129–134. [Google Scholar] [CrossRef]
- Cosola, C.; De Angelis, M.; Rocchetti, M.T.; Montemurno, E.; Maranzano, V.; Dalfino, G.; Manno, C.; Zito, A.; Gesualdo, M.; Ciccone, M.M.; et al. Beta-Glucans Supplementation Associates with Reduction in P-Cresyl Sulfate Levels and Improved Endothelial Vascular Reactivity in Healthy Individuals. PLoS ONE 2017, 12, e0169635. [Google Scholar] [CrossRef] [Green Version]
- Del Valle-Pinero, A.Y.; Van Deventer, H.E.; Fourie, N.H.; Martino, A.C.; Patel, N.S.; Remaley, A.T.; Henderson, W.A. Gastrointestinal permeability in patients with irritable bowel syndrome assessed using a four probe permeability solution. Clin. Chim. Acta 2013, 418, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Bonfrate, L.; Di Palo, D.M.; Celano, G.; Albert, A.; Vitellio, P.; De Angelis, M.; Gobbetti, M.; Portincasa, P. Effects of Bifidobacterium longum BB536 and Lactobacillus rhamnosus HN001 in IBS patients. Eur. J. Clin. Investig. 2020, 50, e13201. [Google Scholar] [CrossRef]



| P | S | Difference at Baseline (p Value) | |
|---|---|---|---|
| Gender | 7 M, 3 F | 7 M, 6 F | - |
| Age | 51.5 ± 2.8 | 51 ± 4.3 | 0.6 |
| BMI | 25.8 ± 0.9 | 27.2 ± 1 | 0.4 |
| eGFR mL/min | 23.9 ± 3.6 | 31.5 ± 2.8 | 0.1 |
| Serum creatinine mg/dl | 3.2 ± 0.4 | 2.3 ± 0.2 | 0.04 |
| sys BP (mmHg) | 126.5 ± 3.6 | 124.2 ± 3.6 | 0.7 |
| dia BP (mmHg) | 82.5 ± 2.5 | 82.7 ± 2.5 | 1.0 |
| Serum albumin (g/dL) | 3.9 ± 0.1 | 3.9 ± 0.1 | 0.8 |
| Azotemia (mg/dL) | 97 ± 16 | 94.9 ± 7.2 | 0.9 |
| BUN (mg/dL) | 45.3 ± 7.5 | 44.3 ± 3.3 | 0.9 |
| Blood glucose (mg/dL) | 76.4 ± 2.8 | 78.1 ± 3.4 | 0.7 |
| HbA1c % | 36.8 ± 1.3 | 35.8 ± 1.1 | 0.6 |
| Serum tryglicerides (mg/dL) | 149.4 ± 22.1 | 134.7 ± 20.6 | 0.6 |
| HDL cholesterol (mg/dL) | 53.9 ± 3.9 | 58 ± 5.2 | 0.6 |
| LDL cholesterol (mg/dL) | 101.7 ± 6.6 | 97.5 ± 10.4 | 0.8 |
| Total cholesterol mg/dL | 174.4 ± 8.4 | 184.4 ± 11.2 | 0.5 |
| Serum calcium (mg/dL) | 9.2 ± 0.2 | 9.2 ± 0.1 | 1.0 |
| Serum phosphorus (mg/dL) | 3.4 ± 0.3 | 3.5 ± 0.1 | 0.8 |
| Serum potassium (mEq/L) | 4.7 ± 0.1 | 4.5 ± 0.1 | 0.3 |
| Serum sodium (mEq/L) | 141 ± 0.6 | 142.1 ± 0.6 | 0.2 |
| CaxP (mg2/dL2) | 31.6 ± 2.7 | 32.3 ± 1.4 | 0.8 |
| Total serum proteins (g/dL) | 7.2 ± 0.1 | 7.2 ± 0.1 | 1.0 |
| CRP (mg/L) | 4.1 ± 1.1 | 3 ± 0.1 | 0.3 |
| Rrinary creatinine (mg/dL) | 66.7 ± 9.5 | 53.7 ± 3.6 | 0.2 |
| Proteinuria (mg/dL) | 577 ± 211 | 500.9 ± 117.9 | 0.8 |
| P | S | Difference at Baseline (p Value) | |
|---|---|---|---|
| Gender | 4 M, 10 F | 5 M, 5 F | - |
| Age | 42.7 ± 2.7 | 41.6 ± 3.0 | 0.8 |
| BMI | 23.5 ± 0.8 | 26 ± 1.6 | 0.1 |
| Serum creatinine mg/dl | 0.79 ± 0.0 | 0.83 ± 0.0 | 0.5 |
| sys BP (mmHg) | 109.2 ± 2.6 | 115.6 ± 3.5 | 0.2 |
| dia BP (mmHg) | 68.8 ± 2.1 | 78.3 ± 2.2 | 0.01 |
| Serum albumin (g/dL) | 4.2 ± 0.1 | 4.1 ± 0.1 | 0.4 |
| Azotemia (mg/dL) | 32.6 ± 1.1 | 36.4 ± 3.1 | 0.2 |
| BUN (mg/dL) | 15.3 ± 0.3 | 17.0 ± 1.4 | 0.2 |
| Blood glucose (mg/dL) | 71.5 ± 2.5 | 75.2 ± 3.2 | 0.4 |
| HbA1c % | 33.3 ± 1.3 | 34.6 ± 1.0 | 0.5 |
| Serum tryglicerides (mg/dL) | 65.8 ± 6.8 | 91 ± 9.8 | 0.04 |
| HDL cholesterol (mg/dL) | 72.7 ± 4.1 | 72.2 ± 7.4 | 1.0 |
| LDL cholesterol (mg/dL) | 100.2 ± 5.1 | 113.4 ± 8.5 | 0.2 |
| Total cholesterol mg/dL | 186.8 ± 6.3 | 204 ± 10.0 | 0.1 |
| Serum calcium (mg/dL) | 9.3 ± 0.1 | 9.2 ± 0.1 | 0.1 |
| Serum phosphorus (mg/dL) | 3.4 ± 0.1 | 3.2 ± 0.2 | 0.4 |
| Serum potassium (mEq/L) | 4.1 ± 0.1 | 4.1 ± 0.1 | 1.0 |
| CaxP (mg2/dL2) | 31.9 ± 1.4 | 29.6 ± 1.9 | 0.3 |
| Total serum proteins (g/dL) | 7.5 ± 0.2 | 7.4 ± 0.1 | 0.8 |
| CRP (mg/L) | 2.9 ± 0.0 | 4.2 ± 1.3 | 0.3 |
| Urinary creatinine (mg/dL) | 103.2 ± 11.0 | 157.1 ± 29.4 | 0.1 |
| Proteinuria (mg/dL) | 89 ± 10.6 | 125.9 ± 17.9 | 0.1 |
| Treatment | T0 | T2 | T3 | Difference Between Time Points (p-Value) | Difference Between Arms (p-Value) | |
|---|---|---|---|---|---|---|
| BMI | P | 25.8 ± 0.9 | 26.0 ± 0.9 | 26.0 ± 0.9 | - | - |
| S | 27.2 ± 1.0 | 27.0 ± 1.1 | 27.4 ± 1.0 | - | ||
| eGFR (mL/min) | P | 23.9 ± 3.6 | 22.7 ± 3.0 | 23.3 ± 3.3 | - | - |
| S | 31.5 ± 2.8 | 31.1 ± 2.4 | 31.4 ± 2.5 | - | ||
| Serum creatinine (mg/dL) | P | 3.2 ± 0.4 | 3.1 ± 0.3 | 3.1 ± 0.3 | - | p = 0.02 |
| S | 2.3 ± 0.2 $ | 2.3 ± 0.2 $ | 2.3 ± 0.2 $ | - | ||
| Serum albumin (g/dL) | P | 3.9 ± 0.1 | 3.9 ± 0.1 | 3.8 ± 0.1 | - | - |
| S | 3.9 ± 0.1 | 4.0 ± 0.1 | 4.0 ± 0.1 | - | ||
| Azotemia (mg/dL) | P | 97.0 ± 16.0 | 95.2 ± 16.4 | 97.5 ± 15.2 | - | - |
| S | 94.9 ± 7.2 | 94.1 ± 7.0 | 87.1 ± 6.2 § | 0.01 | ||
| Serum calcium (mg/dL) | P | 9.2 ± 0.1 | 9.2 ± 0.2 | 9.0 ± 0.1 | - | - |
| S | 9.2 ± 0.1 | 9.2 ± 0.1 | 9.1 ± 0.2 | - | ||
| Serum phosphorus (mg/dL) | P | 3.4 ± 0.3 | 3.42 ± 0.2 | 3.2 ± 0.2 | - | - |
| S | 3.5 ± 0.1 | 3.4 ± 0.1 | 3.3 ± 0.1 | - | ||
| Serum potassium (mEq/L) | P | 4.7 ± 0.1 | 4.5 ± 0.1 | 4.5 ± 0.1 | - | - |
| S | 4.5 ± 0.1 | 4.5 ± 0.1 | 4.5 ± 0.1 | - | ||
| Serum sodium (mEq/L) | P | 141.0 ± 0.6 | 140.5 ± 0.5 | 138.4 ± 0.8 *§ | p < 0.02 | p = 0.001 |
| S | 142.1 ± 0.6 | 142.7 ± 0.4 $ | 140.7 ± 0.8 | |||
| CaxP (mg2/dL2) | P | 31.6 ± 2.7 | 31.6 ± 2.5 | 19.7 ± 4.2 | - | - |
| S | 32.3 ± 1.4 | 31.8 ± 1.1 | 23.7 ± 3.2 § | 0.03 | ||
| Total serum proteins (g/dL) | P | 7.2 ± 0.1 | 7.2 ± 0.2 | 7.1 ± 0.1 | - | - |
| S | 7.2 ± 0.1 | 7.1 ± 0.1 | 7.1 ± 0.1 | - | ||
| CRP (mg/L) | P | 4.1 ± 1.1 | 3.1 ± 0.1 | 3.0 ± 0.1 | - | - |
| S | 3.0 ± 0.1 | 4.2 ± 1.2 | 3.3 ± 0.4 | - | ||
| Urinary creatinine (mg/dL) | P | 66.7 ± 9.5 | 59.4 ± 8.7 | 58.3 ± 7.6 | - | - |
| S | 53.7 ± 3.6 | 51.8 ± 4.5 | 48.9 ± 3.7 | - | ||
| Urinary proteins (mg/dL) | P | 577.0 ± 211.0 | 414.2 ± 139.6 | 431.8 ± 135.4 | - | - |
| S | 500.9 ± 117.9 | 497.8 ± 108.8 | 502.1 ± 116.3 | - |
| Treatment | T0 | T2 | T3 | Difference Between Time Points (p-Value) | Difference Between Arms (p-Value) | |
|---|---|---|---|---|---|---|
| BMI | P | 23.5 ± 0.8 | 23.3 ± 0.8 | 23.4 ± 0.8 | - | - |
| S | 26.0 ± 1.6 | 26.0 ± 1.6 | 26.3 ± 1.6 | - | ||
| Serum creatinine (mg/dL) | P | 0.79 ± 0.03 | 0.83 ± 0.04 | 0.77 ± 0.03 | - | - |
| S | 0.83 ± 0.05 | 0.90 ± 0.06 | 0.84 ± 0.05 | - | ||
| Serum albumin (g/dL) | P | 4.2 ± 0.1 | 4.1 ± 0.1 | 4.1 ± 0.1 | - | - |
| S | 4.1 ± 0.1 | 3.6 ± 0.4 | 4.0 ± 0.1 | - | ||
| Azotemia (mg/dL) | P | 32.6 ± 1.1 | 33.4 ± 2.1 | 31.2 ± 1.7 | - | - |
| S | 36.4 ± 3.1 | 33.3 ± 3.1 | 33.8 ± 3.0 | - | ||
| Serum calcium (mg/dL) | P | 9.4 ± 0.1 | 9.3 ± 0.2 | 7.1 ± 1.1 | - | - |
| S | 9.2 ± 0.1 | 9.1 ± 0.1 | 8.0 ± 1.0 | - | ||
| Serum phosphorus (mg/dL) | P | 3.4 ± 0.1 | 3.4 ± 0.2 | 3.4 ± 0.1 | - | - |
| S | 3.2 ± 0.2 | 3.3 ± 0.2 | 3.4 ± 0.2 | - | ||
| Serum potassium (mEq/L) | P | 4.1 ± 0.1 | 4.0 ± 0.1 | 4.1 ± 0.1 | - | - |
| S | 4.1 ± 0.1 | 4.0 ± 0.1 | 4.1 ± 0.1 | - | ||
| CaxP (mg2/dL2) | P | 31.9 ± 1.4 | 31.8 ± 1.5 | 23.8 ± 3.9 | - | - |
| S | 29.6 ± 1.9 | 29.9 ± 1.9 | 26.4 ± 3.9 | - | ||
| Total serum proteins (g/dL) | P | 7.5 ± 0.2 | 7.2 ± 0.1 | 7.3 ± 0.1 | - | |
| S | 7.4 ± 0.1 | 6.9 ± 0.4 | 7.2 ± 0.1 | - | ||
| CRP (mg/L) | P | 2.90 ± 0.04 | 2.9 ± 0.04 | 2.9 ± 0.00 | - | - |
| S | 4.2 ± 1.3 | 3.5 ± 0.4 | 3.2 ± 0.2 | - | ||
| Urinary creatinine (mg/dL) | P | 103.2 ± 11.0 | 112.2 ± 13.7 | 101.1 ± 17.5 | - | - |
| S | 157.1 ± 29.4 | 154.0 ± 43.8 | 136.2 ± 20.5 | - | ||
| Urinary proteins (mg/dL) | P | 89.0 ± 10.6 | 85.1 ± 9.0 | 76.2 ± 8.1 | - | - |
| S | 125.9 ± 17.9 | 109.2 ± 23.8 | 103.2 ± 13.1 | - |
| Healthy Subjects | CKD III B | CKD IV | p | |
|---|---|---|---|---|
| Number | 27 | 11 | 11 | |
| Stomach (sucrose recovery, %) | 0.031 ± 0.006 | 0.019 ± 0.007 | 0.021 ± 0.007 | ns |
| Median | 0.02 | 0.02 | 0.02 | |
| Range | 0.01–0.16 | 0.01–0.03 | 0.01–0.05 | |
| Small intestine (lactulose/mannitol, ratio) | 0.029 ± 0.01 | 0.019 ± 0.01 | 0.03 ± 0.01 | ns |
| Median | 0.017 | 0.018 | 0.017 | |
| Range | 0.005–0.31 | 0.012–0.032 | 0.01–0.1 | |
| Colon (sucralose recovery, %) | 1.03 ± 0.08 | 0.40 ± 0.09 * | 0.30 ± 0.09 * | <0.00001 |
| Median | 0.97 | 0.4 | 0.31 | |
| Range | 0.01–1.95 | 0.25–0.65 | 0.16–0.51 |
| Placebo | Baseline | T2 | T3 | p-Value |
| Number | 9 | 9 | 9 | |
| Stomach (sucrose recovery, %) | 0.02 ± 0.003 | 0.018 ± 0.003 | 0.021 ± 0.003 | ns |
| Median | 0.02 | 0.02 | 0.02 | |
| Range | 0.01–0.05 | 0.01–0.03 | 0.01–0.04 | |
| Small intestine (lactulose/mannitol, ratio) | 0.027 ± 0.006 | 0.022 ± 0.006 | 0.02 ± 0.006 | ns |
| Median | 0.018 | 0.02 | 0.02 | |
| Range | 0.012–0.10 | 0.014–0.035 | 0.01–0.035 | |
| Colon (sucralose recovery, %) | 0.36 ± 0.06 | 0.37 ± 0.07 | 0.34 ± 0.06 | ns |
| Median | 0.32 | 0.37 | 0.36 | |
| Range | 0.16–0.56 | 0–0.08 | 0.14–0.57 | |
| Synbiotic | Baseline | T2 | T3 | p-Value |
| Number | 12 | 12 | 12 | |
| Stomach (sucrose recovery, %) | 0.017 ± 0.002 | 0.017 ± 0.002 | 0.015 ± 0.002 | ns |
| Median | 0.02 | 0.015 | 0.01 | |
| Range | 0.01–0.03 | 0.01–0.03 | 0.01–0.03 | |
| Small intestine (lactulose/mannitol, ratio) | 0.019 ± 0.01 | 0.015 ± 0.001 * | 0.015 ± 0.001 * | 0.040 |
| Median | 0.017 | 0.015 | 0.014 | |
| Range | 0.01–0.032 | 0.008–0.022 | 0.01–0.023 | |
| Colon (sucralose recovery, %) | 0.35 ± 0.04 | 0.41 ± 0.05 | 0.36 ± 0.05 | ns |
| Median | 0.34 | 0.4 | 0.31 | |
| Range | 0.2–0.65 | 0.2–0.7 | 0–0.82 |
| Placebo | Baseline | T2 | T3 | p-Value |
| Number | 14 | 14 | 14 | |
| Stomach (sucrose recovery, %) | 0.027 ± 0.004 | 0.028 ± 0.004 | 0.025 ± 0.002 | ns |
| Median | 0.02 | 0.025 | 0.03 | |
| Range | 0.01–0.06 | 0.01–0.06 | 0.01–0.04 | |
| Small intestine (lactulose/mannitol, ratio) | 0.018 ± 0.003 | 0.18 ± 0.002 | 0.016 ± 0.001 | ns |
| Median | 0.016 | 0.016 | 0.017 | |
| Range | 0.009–0.047 | 0.01–0.05 | 0.009–0.025 | |
| Colon (sucralose recovery, %) | 1.03 ± 0.07 | 1.14 ± 0.09 | 1.04 ± 0.1 | ns |
| Median | 1.04 | 1.12 | 0.98 | |
| Range | 0.64–1.45 | 0.63–1.64 | 0.58–2.15 | |
| Synbiotic | Baseline | T2 | T3 | p-Value |
| Number | 10 | 10 | 10 | |
| Stomach (sucrose recovery, %) | 0.037 ± 0.012 | 0.035 ± 0.007 | 0.045 ± 0.016 | ns |
| Median | 0.02 | 0.02 | 0.02 | |
| Range | 0.01–0.16 | 0.02–0.07 | 0.01–0.17 | |
| Small intestine (lactulose/mannitol, ratio) | 0.042 ± 0.025 | 0.015 ± 0.002 | 0.017 ± 0.002 | ns |
| Median | 0.018 | 0.015 | 0.015 | |
| Range | 0.005–0.31 | 0–0.022 | 0.011–0.031 | |
| Colon (sucralose recovery, %) | 1.04 ± 0.17 | 0.98 ± 0.07 | 1.13 ± 0.13 | ns |
| Median | 0.94 | 0.92 | 1.17 | |
| Range | 0.01–1.95 | 0.76–1.44 | 0.45–1.68 |
| T0 | T2 | T3 | p-Value Between Time Points | ||
|---|---|---|---|---|---|
| GSRS 6 | P | 2.40 ± 0.41 | 2.20 ± 0.51 | 2.00 ± 0.32 | - |
| S | 2.38 ± 0.34 | 2.08 ± 0.41 | 1.46 ± 0.23 * | 0.04 | |
| GSRS 13 | P | 2.20 ± 0.76 | 1.40 ± 0.38 | 1.40 ± 0.38 | - |
| S | 2.23 ± 0.27 | 1.15 ± 0.15 * | 1.31 ± 0.20 * | <0.004 | |
| Abdominal pain | P | 4.00 ± 0.58 | 4.40 ± 0.75 | 3.40 ± 0.25 | - |
| S | 4.85 ± 0.51 | 3.54 ± 0.28 * | 3.62 ± 0.34 | 0.03 * | |
| Constipation syndrome | P | 5.00 ± 0.69 | 4.00 ± 0.58 | 4.40 ± 0.64 | - |
| S | 6.08 ± 0.80 | 3.77 ± 0.41* | 4.23 ± 0.71 | 0.02 * |
| T0 | T2 | T3 | p-Value Between Time Points | ||
|---|---|---|---|---|---|
| GSRS 3 | P | 2.08 ± 0.28 | 1.15 ± 0.15 * | 1.15 ± 0.15 | 0.01 |
| S | 1.67 ± 0.32 | 1.00 ± 0.00 | 1.44 ± 0.28 | - | |
| GSRS 9 | P | 3.15 ± 0.26 | 1.77 ± 0.27 * | 2.08 ± 0.35 | 0.01 |
| S | 2.33 ± 0.32 | 3.00 ± 0.55 | 2.11 ± 0.46 | - | |
| Abdominal pain | P | 8.69 ± 0.81 | 5.92 ± 0.42 * | 6.23 ± 0.51 * | <0.01 |
| S | 7.33 ± 0.98 | 5.33 ± 0.22 | 6.56 ± 1.26 | - | |
| Indigestion syndrome | P | 9.69 ± 0.95 | 6.31 ± 0.53 * | 6.46 ± 0.66 * | <0.01 |
| S | 7.56 ± 0.99 | 8.44 ± 1.17 | 6.89 ± 1.15 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosola, C.; Rocchetti, M.T.; di Bari, I.; Acquaviva, P.M.; Maranzano, V.; Corciulo, S.; Di Ciaula, A.; Di Palo, D.M.; La Forgia, F.M.; Fontana, S.; et al. An Innovative Synbiotic Formulation Decreases Free Serum Indoxyl Sulfate, Small Intestine Permeability and Ameliorates Gastrointestinal Symptoms in a Randomized Pilot Trial in Stage IIIb-IV CKD Patients. Toxins 2021, 13, 334. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13050334
Cosola C, Rocchetti MT, di Bari I, Acquaviva PM, Maranzano V, Corciulo S, Di Ciaula A, Di Palo DM, La Forgia FM, Fontana S, et al. An Innovative Synbiotic Formulation Decreases Free Serum Indoxyl Sulfate, Small Intestine Permeability and Ameliorates Gastrointestinal Symptoms in a Randomized Pilot Trial in Stage IIIb-IV CKD Patients. Toxins. 2021; 13(5):334. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13050334
Chicago/Turabian StyleCosola, Carmela, Maria Teresa Rocchetti, Ighli di Bari, Paola Maria Acquaviva, Valentina Maranzano, Simone Corciulo, Agostino Di Ciaula, Domenica Maria Di Palo, Flavia Maria La Forgia, Sergio Fontana, and et al. 2021. "An Innovative Synbiotic Formulation Decreases Free Serum Indoxyl Sulfate, Small Intestine Permeability and Ameliorates Gastrointestinal Symptoms in a Randomized Pilot Trial in Stage IIIb-IV CKD Patients" Toxins 13, no. 5: 334. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13050334





