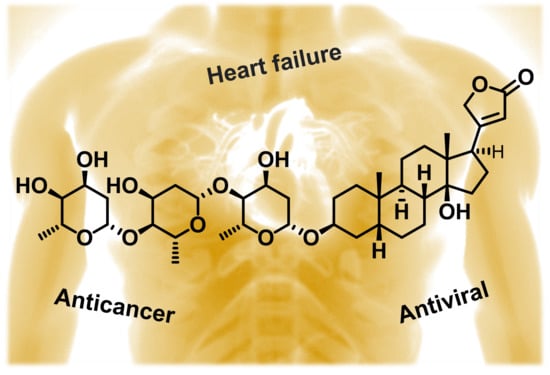
Quo vadis Cardiac Glycoside Research?
Abstract
:1. Introduction
2. Occurrence of Cardiac Glycosides
3. Production of Cardiac Glycosides
3.1. Precursor Feeding and Elicitation
3.2. Cultivation Techniques
3.3. Genetic Engineering
3.4. Physical Factors
4. Structure of Cardiac Glycosides
5. Na+/K+-ATPase Binding of Cardiac Glycosides
6. Biological Activity of the Most Important Cardiac Glycosides
- (i)
- SrcK/EGFR—in this case, activated SrcK transactivates EGFR, which in turn activates the Ras/Raf/MEK/MAPK pathway [88,89,90]. This activates transcription factors and generates reactive oxygen species. Reactive oxygen species subsequently interact with the NKA signalosome, which activates other SrcK molecules and, thus, amplifies the SrcK/EGFR pathway signal [91,92].
- (ii)
- SrcK/phospholipase C—activated phospholipase C hydrolyzes the ester bond of phosphatidylinositol-4,5-bisphosphate and the released inositol-1,4,5-triphosphate subsequently interacts with inositol triphosphate receptors on the ER, the opening of which causes Ca2+ oscillation [93,94]. Ca2+ oscillations subsequently induce the activation of the antiapoptotic subunit p65 of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which serves as a transcription factor and increases the production of the antiapoptotic factor Bcl-xL from the Bcl-2 family of proteins [95].
- (iii)
6.1. Heart Disease and Blood Pressure
6.2. Cardiac Glycosides and Cancer
6.3. Antiviral Activity of Cardiac Glycosides
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMPK | Adenosine monophosphate-activated protein kinase |
| ATP | Adenosine triphosphate |
| CG | Cardiac glycoside |
| CLK | Cell division control protein 2-like kinases |
| Dg | Digoxin |
| Dgt | Digitoxin |
| DNA | Deoxyribonucleic acid |
| EGFR | Epidermal growth factor receptor |
| Env | Envelope proteins |
| ER | Endoplasmic reticulum |
| ErgCh | Ether-à-go-go related gene family K+ channels |
| Gag | Group-specific antigen |
| Hs68 | Primary human fibroblasts |
| HCMV | Human cytomegalovirus |
| HEK 293T | Human embryonic cells |
| HeLa-S3 | Human cervix carcinoma cells (subclone) |
| HIF-1α | Hypoxia-induced factor 1α |
| HIV | Human immunodeficiency virus |
| HSV-1 | Herpes simplex virus type 1 |
| HT-29 | Human cells from colorectal carcinoma |
| IC50 | Half-maximal inhibitory concentration |
| MAPK | Mitogen-activated protein kinase |
| MEK | Mitogen-activated protein kinase kinase |
| MERS-CoV | Middle East respiratory syndrome coronavirus |
| mTOR | Mammalian target of rapamycin |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NKA | Na+/K+-ATPase |
| PDK-1 | Phosphoinositide-dependent protein kinase-1 |
| Raf | Serine/threonine kinase |
| Ras | Rat sarcoma protein |
| RNA | Ribonucleic acid |
| siRNA | Short interfering ribonucleic acid |
| SRPK | Serine/arginine-rich protein kinases |
| SrcK | Non-receptor tyrosine kinase |
| SRC-1 | Steroid receptor coactivator 1 |
| SRC-3 | Steroid receptor coactivator 3 |
| ULK1 | Unc-51 like autophagy activating kinase 1 |
| VRAC | Volume-regulated anion channels |
References
- Lichman, B.R. The scaffold-forming steps of plant alkaloid biosynthesis. Nat. Prod. Rep. 2021, 38, 103–129. [Google Scholar] [CrossRef]
- Žuvela, P.; David, J.; Yang, X.; Huang, D.; Wong, M.W. Non-linear quantitative structure⁻activity relationships modelling, mechanistic study and in-silico design of flavonoids as potent antioxidants. Int. J. Mol. Sci. 2019, 20, 2328. [Google Scholar] [CrossRef]
- Son, N.; Thuy, P.T.; Trang, N.V. Antioxidative capacities of stilbenoid suaveolensone A and flavonoid suaveolensone B: A detailed analysis of structural-electronic properties and mechanisms. J. Mol. Struct. 2021, 1224, 129025. [Google Scholar] [CrossRef]
- Cui, S.; Jiang, H.; Chen, L.; Xu, J.; Sun, W.; Sun, H.; Xie, Z.; Xu, Y.; Yang, F.; Liu, W.; et al. Design, synthesis and evaluation of wound healing activity for β-sitosterols derivatives as potent Na+/K+-ATPase inhibitors. Bioorg. Chem. 2020, 98, 103150. [Google Scholar] [CrossRef] [PubMed]
- The Top 200 Drugs of 2018. Available online: https://clincalc.com/DrugStats/ (accessed on 26 April 2021).
- Zalucki, M.P.; Brower, L.P.; Alonso, M.A. Detrimental effects of latex and cardiac glycosides on survival and growth of first-instar monarch butterfly larvae Danaus plexippus feeding on the sandhill milkweed Asclepias Humistrata. Ecol. Entomol. 2001, 26, 212–224. [Google Scholar] [CrossRef]
- Dobler, S.; Petschenka, G.; Wagschal, V.; Flacht, L. Convergent adaptive evolution–how insects master the challenge of cardiac glycoside-containing host plants. Entomol. Exp. Appl. 2015, 157, 30–39. [Google Scholar] [CrossRef]
- Goldberger, Z.D.; Goldberger, A.L. Therapeutic ranges of serum digoxin concentrations in patients with heart failure. Am. J. Cardiol. 2012, 109, 1818–1821. [Google Scholar] [CrossRef]
- Withering, W. An Account of the Foxglove, and Some of Its Medical Uses: With Practical Remarks on Dropsy, and Other Diseases; Cambridge University Press: Cambridge, UK, 2014; ISBN 978-11-0770-613-2. [Google Scholar]
- Turumtay, H.; Turumtay, E.A.; Selvi, E.K.; Sahin, H.; Sandallı, C.; Yazıcı, Z.A. Three seasonal comprehensive evaluation process of Digitalis trojana Ivan’s phenolics. Ind. Crop. Prod. 2016, 94, 160–166. [Google Scholar] [CrossRef]
- Van Wietmarschena, E.H.A.; Hagels, H.; Peters, R.; Heisteke, J.; Greef, J.; Wang, M. Optimizing growth conditions for digoxin production in Digitalis lanata Ehrh. World J. Tradit. Chin. Med. 2016, 2, 24–35. [Google Scholar] [CrossRef]
- Grosa, G.; Allegrone, G.; Del Grosso, E. LC-ESI-MS/MS characterization of strophanthin-K. J. Pharm. Biomed. Anal. 2005, 38, 79–86. [Google Scholar] [CrossRef]
- Makarevich, I.F.; Kovalev, S.V. Cardiac glycosides from Strophanthus kombe. Chem. Nat. Compd. 2006, 42, 189–193. [Google Scholar] [CrossRef]
- Hammerstein, F.; Kaiser, F. Quantitative direct fluorometric determination of extracts of medicinal plants on thin-layer-chromatograms. Planta Med. 1972, 21, 5–15. [Google Scholar] [CrossRef]
- Pellati, F.; Bruni, R.; Bellardi, M.G.; Bertaccini, A.; Benvenuti, S. Optimization and validation of a high-performance liquid chromatography method for the analysis of cardiac glycosides in Digitalis lanata. J. Chromatogr. A 2009, 1216, 3260–3269. [Google Scholar] [CrossRef] [PubMed]
- Usai, M.; Atzei, A.D.; Marchetti, M. Cardenolides content in wild Sardinian Digitalis purpurea L. populations. Nat. Prod. Res. 2007, 21, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Fujii, Y.; Nakaya, I.; Yamazaki, M. Quantitative HPLC analysis of cardiac glycosides in Digitalis purpurea leaves. J. Nat. Prod. 1995, 58, 897–901. [Google Scholar] [CrossRef]
- Fujii, Y.; Ikeda, Y.; Yamazaki, M. Separation and determination of purpurea glycosides in Digitalis purpurea leaves by micro-HPLC. J. High Resolut. Chromatogr. 1987, 10, 137–140. [Google Scholar] [CrossRef]
- Bai, L.; Zhao, M.; Toki, A.; Hasegawa, T.; Sakai, J.I.; Yang, X.Y.; Bai, Y.; Ogura, H.; Mitsui, T.; Kataoka, T.; et al. Polar cardenolide monoglycosides from stems and twigs of Nerium oleander and their biological activities. J. Wood Sci. 2011, 57, 47–55. [Google Scholar] [CrossRef]
- Turkmen, Z.; Mercan, S.; Cengiz, S. An HPTLC method for the determination of oleandrin in Nerium plant extracts and its application to forensic toxicology. J. Planar Chromatogr. 2013, 26, 279–283. [Google Scholar] [CrossRef]
- Opletal, L.; Vokac, K.; Hanus, V.; Sovova, M.; Blunden, G.; Patel, A.; Dacke, C. Simultaneous determination of cardenolides and coumarins in the seeds of Coronilla varia L. Folia Pharm. Univ. Carol. 1998, 21–22, 89–94. [Google Scholar]
- Welsh, K.J.; Huang, R.S.P.; Actor, J.K.; Dasgupta, A. Rapid detection of the active cardiac glycoside convallatoxin of lily of the valley using LOCI digoxin assay. Am. J. Clin. Pathol. 2014, 142, 307–312. [Google Scholar] [CrossRef]
- Higano, T.; Kuroda, M.; Sakagami, H.; Mimaki, Y. Convallasaponin A, a new 5β-spirostanol triglycoside from the rhizomes of Convallaria majalis. Chem. Pharm. Bull. 2007, 55, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Saxena, V.K.; Chaturvedi, P.K. Novel cardenolide, canarigenin-3-O-α-l-rhamnopyranosyl-(I→5)-O-β-d-xylofuranoside, from rhizomes of Convallaria majalis. J. Nat. Prod. 1992, 55, 39–42. [Google Scholar] [CrossRef]
- Krenn, L.; Schlifelner, L.; Stimpfl, T.; Kopp, B. A new HPLC method for the quantification of cardenolides in Convallaria majalis. Pharmazie 1996, 51, 906–909. [Google Scholar]
- Fumiko, A.B.E.; Yamauchi, T. Cardenolide glycosides from the roots of Apocynum cannabinum. Chem. Pharm. Bullet. 1994, 42, 2028–2031. [Google Scholar] [CrossRef]
- Radenkova-Saeva, J.; Atanasov, P. Cardiac glycoside plants self-poisoning. Acta Med. Bulg. 2014, 41, 99–104. [Google Scholar] [CrossRef]
- Oerther, S.E. Plant poisonings: Common plants that contain cardiac glycosides. J. Emerg. Nurs. 2011, 37, 102–103. [Google Scholar] [CrossRef] [PubMed]
- Maffè, S.; Cucchi, L.; Zenone, F.; Bertoncelli, C.; Beldì, F.; Colombo, M.L.; Bielli, M.; Paino, A.M.; Parravicini, U.; Paffoni, P.; et al. Digitalis must be banished from the table: A rare case of acute accidental Digitalis intoxication of a whole family. J. Cardiovasc. Med. 2009, 10, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Yang, C.C.; Phua, D.H.; Deng, J.F.; Lu, L.H. An outbreak of foxglove leaf poisoning. J. Chin. Med. Assoc. 2010, 73, 97–100. [Google Scholar] [CrossRef]
- Keppel, M.H.; Piecha, G.; März, W.; Cadamuro, J.; Auer, S.; Felder, T.K.; Mrazek, C.; Oberkofler, H.; Trummer, C.; Grübler, M.R.; et al. The endogenous cardiotonic steroid Marinobufagenin and decline in estimated glomerular filtration rate at follow-up in patients with arterial hypertension. PLoS ONE 2019, 14, e0212973. [Google Scholar] [CrossRef] [PubMed]
- Bauer, N.; Müller-Ehmsen, J.; Krämer, U.; Hambarchian, N.; Zobel, C.; Schwinger, R.H.; Neu, H.; Kirch, U.; Grünbaum, E.G.; Schoner, W. Ouabain-like compound changes rapidly on physical exercise in humans and dogs: Effects of beta-blockade and angiotensin-converting enzyme inhibition. Hypertension 2005, 45, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Nesher, M.; Dvela, M.; Igbokwe, V.U.; Rosen, H.; Lichtstein, D. Physiological roles of endogenous ouabain in normal rats. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H2026–H2034. [Google Scholar] [CrossRef] [PubMed]
- Lenaerts, C.; Wells, M.; Hambÿe, S.; Blankert, B. Marinobufagenin extraction from Rhinella marina toad glands: Alternative approaches for a systematized strategy. J. Sep. Sci. 2019, 42, 1384–1392. [Google Scholar] [CrossRef]
- Meng, Q.; Yau, L.F.; Lu, J.G.; Wu, Z.Z.; Zhang, B.X.; Wang, J.R.; Jiang, Z.H. Chemical profiling and cytotoxicity assay of bufadienolides in toad venom and toad skin. J. Ethnopharmacol. 2016, 187, 74–82. [Google Scholar] [CrossRef]
- El-Masri, M.A.; Clark, B.J.; Qazzaz, H.M.; Valdes, R., Jr. Human adrenal cells in culture produce both ouabain-like and dihydroouabain-like factors. Clin. Chem. 2002, 48, 1720–1730. [Google Scholar] [CrossRef]
- Bozorgi, M.; Amin, G.; Kasebzade, S.; Shekarchi, M. Development and validation of a HPLC-UV method for determination of proscillaridin A in Drimia maritima. Res. J. Pharm. 2016, 3, 1–7. [Google Scholar]
- Steyn, P.S.; van Heerden, F.R. Bufadienolides of plant and animal origin. Nat. Prod. Rep. 1998, 15, 397–413. [Google Scholar] [CrossRef]
- Schmiedeberg, O. Pharmacologically active ingredients of Digitalis purpurea L. Chem. Zent. 1875, 46, 262. [Google Scholar]
- Smith, S. LXXI1.-digoxin, a new digitalis glucoside. J. Chem. Soc. 1930, 508–510. [Google Scholar] [CrossRef]
- Hagimori, M.; Matsumoto, T.; Obi, Y. Studies on the production of Digitalis cardenolides by plant tissue culture III. Effects of nutrients on digitoxin formation by shoot-forming cultures of Digitalis purpurea L. grown in liquid media. Plant Cell Physiol. 1982, 23, 1205–1211. [Google Scholar] [CrossRef]
- Patil, J.G.; Ahire, M.L.; Nitnaware, K.M.; Panda, S.; Bhatt, V.P.; Kishor, P.B.; Nikam, T.D. In vitro propagation and production of cardiotonic glycosides in shoot cultures of Digitalis purpurea L. by elicitation and precursor feeding. Appl. Microbiol. Biotechnol. 2013, 97, 2379–2393. [Google Scholar] [CrossRef]
- Groeneveld, H.W.; van Tegelen, L.J.; Versluis, K. Cardenolide and neutral lipid biosynthesis from malonate in Digitalis lanata. Planta Med. 1992, 58, 239–244. [Google Scholar] [CrossRef]
- Haussmann, W.; Kreis, W.; Stuhlemmer, U.; Reinhard, E. Effects of various pregnanes and two 23-nor-5-cholenic acids on cardenolide accumulation in cell and organ cultures of Digitalis lanata. Planta Med. 1997, 63, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Alonso, N.; Capote, A.; Gerth, A.; Jiménez, E. Increased cardenolides production by elicitation of Digitalis lanata shoots cultured in temporary immersion systems. Plant Cell Tissue Organ Cult. 2012, 110, 153–162. [Google Scholar] [CrossRef]
- Paranhos, A.; Fernández-Tárrago, J.; Corchete, P. Relationship between active oxygen species and cardenolide production in cell cultures of Digitalis thapsi: Effect of calcium restriction. New Phytol. 1999, 141, 51–60. [Google Scholar] [CrossRef]
- Etienne, H.; Berthouly, M. Temporary immersion systems in plant micropropagation. Plant Cell Tissue Organ Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Pérez-Alonso, N.; Wilken, D.; Gerth, A.; Jähn, A.; Nitzsche, H.M.; Kerns, G.; Capote-Perez, A.; Jiménez, E. Cardiotonic glycosides from biomass of Digitalis purpurea L. cultured in temporary immersion systems. Plant Cell Tissue Organ Cult. 2009, 99, 151–156. [Google Scholar] [CrossRef]
- Hagimori, M.; Matsumoto, T.; Obi, Y. Studies on the production of Digitalis cardenolides by plant tissue culture: II. Effect of light and plant growth substances on digitoxin formation by undifferentiated cells and shoot-forming cultures of Digitalis purpurea L. grown in liquid media. Plant Physiol. 1982, 69, 653–656. [Google Scholar] [CrossRef]
- Nester, E.W. Agrobacterium: Nature’s genetic engineer. Front. Plant Sci. 2015, 5, 730. [Google Scholar] [CrossRef]
- Chandra, S. Natural plant genetic engineer Agrobacterium rhizogenes: Role of T-DNA in plant secondary metabolism. Biotechnol. Lett. 2012, 34, 407–415. [Google Scholar] [CrossRef]
- Saito, K.; Yamazaki, M.; Shimomura, K.; Yoshimatsu, K.; Murakoshi, I. Genetic transformation of foxglove (Digitalis purpurea) by chimeric foreign genes and production of cardioactive glycosides. Plant Cell Rep. 1990, 9, 121–124. [Google Scholar] [CrossRef]
- Lehmann, U.; Moldenhauer, D.; Thomar, S.; Dietrich, B.; Luckner, M. Regeneration of plants from Digitalis Lanata cells transformed with Agrobacterium Tumefaciens carrying bacterial genes encoding neomycin phosphotransferase II and {j-glucuronidase. J. Plant Physiol. 1995, 147, 53–57. [Google Scholar] [CrossRef]
- Pradel, H.; Dumke-Lehmann, U.; Diettrich, B.; Luckner, M. Hairy root cultures of Digitalis lanata. Secondary metabolism and plant regeneration. J. Plant Physiol. 1997, 151, 209–215. [Google Scholar] [CrossRef]
- Koga, M.; Hirashima, K.; Nakahara, T. The transformation system in foxglove (Digitalis purpurea L.) using Agrobacterium rhizogenes and traits of the regenerants. Plant Biotechnol. 2000, 17, 99–104. [Google Scholar] [CrossRef]
- Pérez-Alonso, N.; Chong-Pérez, B.; Capote, A.; Pérez, A.; Izquierdo, Y.; Angenon, G.; Jiménez, E. Agrobacterium tumefaciens-mediated genetic transformation of Digitalis purpurea L. Plant Biotechnol. Rep. 2014, 8, 387–397. [Google Scholar] [CrossRef]
- Kairuz, E.; Pérez-Alonso, N.; Capote-Pérez, A.; Pérez-Pérez, A.; Espinosa-Antón, A.A.; Angenon, G.; Jiménez, A.; Chong-Pérez, B. Enhancement of cardenolide production in transgenic Digitalis purpurea L. by expressing a progesterone-5β-reductase from Arabidopsis thaliana L. Ind. Crop. Prod. 2020, 146, 112166. [Google Scholar] [CrossRef]
- Wu, B.; Li, Y.; Yan, H.; Ma, Y.; Luo, H.; Yuan, L.; Chen, S.; Lu, S. Comprehensive transcriptome analysis reveals novel genes involved in cardiac glycoside biosynthesis and mlncRNAs associated with secondary metabolism and stress response in Digitalis purpurea. BMC Genom. 2012, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Rieck, C.; Geiger, D.; Munkert, J.; Messerschmidt, K.; Petersen, J.; Strasser, J.; Meitinger, N.; Kreis, W. Biosynthetic approach to combine the first steps of cardenolide formation in Saccharomyces cerevisiae. Microbiol. Open 2019, 8, e925. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Gantait, S.; Jeong, B.R.; Hwang, S.J. Enhanced growth and cardenolides production in Digitalis purpurea under the influence of different LED exposures in the plant factory. Sci. Rep. 2018, 8, 18009. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Shinoda, T.; Cornelius, F.; Toyoshima, C. Crystal structure of the sodium-potassium pump (Na,K-ATPase) with bound potassium and ouabain. Proc. Natl. Acad. Sci. USA 2009, 106, 13742–13747. [Google Scholar] [CrossRef] [PubMed]
- Laursen, M.; Yatime, L.; Nissen, P.; Fedosova, N.U. Crystal structure of the high-affinity Na+K+-ATPase-ouabain complex with Mg2+ bound in the cation binding site. Proc. Natl. Acad. Sci. USA 2013, 110, 10958–10963. [Google Scholar] [CrossRef]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; López-Lázaro, M. The in vivo antitumor activity of cardiac glycosides in mice xenografted with human cancer cells is probably an experimental artifact. Oncogene 2014, 33, 2947–2948. [Google Scholar] [CrossRef]
- O’Brien, W.J.; Wallick, E.T.; Lingrel, J.B. Amino acid residues of the Na,K-ATPase involved in ouabain sensitivity do not bind the sugar moiety of cardiac glycosides. J. Biol. Chem. 1993, 268, 7707–7712. [Google Scholar] [CrossRef]
- Dalla, S.; Swarts, H.G.; Koenderink, J.B.; Dobler, S. Amino acid substitutions of Na,K-ATPase conferring decreased sensitivity to cardenolides in insects compared to mammals. Insect Biochem. Mol. Biol. 2013, 43, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Croyle, M.L.; Woo, A.L.; Lingrel, J.B. Extensive random mutagenesis analysis of the Na+/K+-ATPase alpha subunit identifies known and previously unidentified amino acid residues that alter ouabain sensitivity implications for ouabain binding. Eur. J. Biochem. 1997, 248, 488–495. [Google Scholar] [CrossRef]
- Magpusao, A.N.; Omolloh, G.; Johnson, J.; Gascón, J.; Peczuh, M.W.; Fenteany, G. Cardiac glycoside activities link Na+/K+ ATPase ion-transport to breast cancer cell migration via correlative SAR. ACS Chem. Biol. 2015, 10, 561–569. [Google Scholar] [CrossRef]
- Manunta, P.; Hamilton, B.P.; Hamlyn, J.M. Structure-activity relationships for the hypertensinogenic activity of ouabain role of the sugar and lactone ring. Hypertension 2001, 37, 472–477. [Google Scholar] [CrossRef]
- Ren, Y.; Ribas, H.T.; Heath, K.; Wu, S.; Ren, J.; Shriwas, P.; Chen, X. Na+/K+-ATPase-targeted cytotoxicity of (+)-digoxin and several semisynthetic derivatives. J. Nat. Prod. 2020, 83, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Pessôa, M.T.C.; Alves, S.L.G.; Taranto, A.G.; Villar, J.A.F.P.; Blanco, G.; Barbosa, L.A. Selectivity analyses of γ-benzylidene digoxin derivatives to different Na,K-ATPase α isoforms: A molecular docking approach. J. Enzym. Inhib. Med. Chem. 2018, 33, 85–97. [Google Scholar] [CrossRef]
- Syeda, S.S.; Sánchez, G.; Hong, K.H.; Hawkinson, J.E.; Georg, G.I.; Blanco, G. Design, synthesis, and in vitro and in vivo evaluation of ouabain analogues as potent and selective Na,K-ATPase α4 isoform inhibitors for male contraception. J. Med. Chem. 2018, 61, 1800–1820. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.L.; Xin, W.; Zhou, M.; Stueckle, T.A.; Rojanasakul, Y.; O’Doherty, G.A. Stereochemical survey of digitoxin monosaccharides. ACS Med. Chem. Lett. 2011, 2, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Petschenka, G.; Fei, C.S.; Araya, J.J.; Schröder, S.; Timmermann, B.N.; Agrawal, A.A. Relative selectivity of plant cardenolides for Na+/K+-ATPases from the monarch butterfly and non-resistant insects. Front. Plant Sci. 2018, 9, 1424. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.; Lifshitz, Y.; Bab-Dinitz, E.; Kapri-Pardes, E.; Goldshleger, R.; Tal, D.M.; Karlish, S.J. Selectivity of digitalis glycosides for isoforms of human Na,K-ATPase. J. Biol. Chem. 2010, 285, 19582–19592. [Google Scholar] [CrossRef] [PubMed]
- Reuter, H.; Henderson, S.A.; Han, T.; Ross, R.S.; Goldhaber, J.I.; Philipson, K.D. The Na+-Ca2+ exchanger is essential for the action of cardiac glycosides. Circ. Res. 2002, 90, 305–308. [Google Scholar] [CrossRef]
- Peterková, L.; Kmoníčková, E.; Ruml, T.; Rimpelová, S. Sarco/endoplasmic reticulum calcium ATPase inhibitors: Beyond anticancer perspective. J. Med. Chem. 2020, 63, 1937–1963. [Google Scholar] [CrossRef]
- Peterková, L.; Rimpelová, S.; Kmoníčková, E.; Ruml, T. Sesquiterpene Lactones: From Weed to Remedy. Chem. Listy 2019, 113, 149–155. [Google Scholar]
- Bygrave, F.L.; Benedetti, A. What is the concentration of calcium ions in the endoplasmic reticulum? Cell Calcium 1996, 19, 547–551. [Google Scholar] [CrossRef]
- McConkey, D.J.; Lin, Y.; Nutt, L.K.; Ozel, H.Z.; Newman, R.A. Cardiac glycosides stimulate Ca2+ increases and apoptosis in androgen-independent, metastatic human prostate adenocarcinoma cells. Cancer Res. 2000, 60, 3807–3812. [Google Scholar] [PubMed]
- Pan, L.; Zhang, Y.; Zhao, W.; Zhou, X.; Wang, C.; Deng, F. The cardiac glycoside oleandrin induces apoptosis in human colon cancer cells via the mitochondrial pathway. Cancer Chemother. Pharmacol. 2017, 80, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.E.; Didelot, C.; Kroemer, G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Hughes, F.M., Jr.; Cidlowski, J.A. Potassium is a critical regulator of apoptotic enzymes in vitro and in vivo. Adv. Enzym. Regul. 1999, 39, 157–171. [Google Scholar] [CrossRef]
- Cain, K.; Langlais, C.; Sun, X.M.; Brown, D.G.; Cohen, G.M. Physiological concentrations of K+ inhibit cytochrome c-dependent formation of the apoptosome. J. Biol. Chem. 2001, 276, 41985–41990. [Google Scholar] [CrossRef] [PubMed]
- Andersson, B.; Janson, V.; Behnam-Motlagh, P.; Henriksson, R.; Grankvist, K. Induction of apoptosis by intracellular potassium ion depletion: Using the fluorescent dye PBFI in a 96-well plate method in cultured lung cancer cells. Toxicol. In Vitro 2006, 20, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Haas, M.; Liang, M.; Cai, T.; Tian, J.; Li, S.; Xie, Z. Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J. Biol. Chem. 2004, 279, 17250–17259. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Tian, J.; Liu, L.; Pierre, S.; Liu, J.; Shapiro, J.; Xie, Z.J. Identification of a pool of non-pumping Na/K-ATPase. J. Biol. Chem. 2007, 282, 10585–10593. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Bai, F.; Chaudhry, M.A.; Pratt, R.; Shapiro, J.I.; Liu, J. The Na/K-ATPase α1 and c-Src form signaling complex under native condition: A crosslinking approach. Sci. Rep. 2020, 10, 6006. [Google Scholar] [CrossRef]
- Kometiani, P.; Li, J.; Gnudi, L.; Kahn, B.B.; Askari, A.; Xie, Z. Multiple signal transduction pathways link Na+/K+-ATPase to growth-related genes in cardiac myocytes. The roles of Ras and mitogen-activated protein kinases. J. Biol. Chem. 1998, 273, 15249–15256. [Google Scholar] [CrossRef]
- Haas, M.; Askari, A.; Xie, Z. Involvement of Src and epidermal growth factor receptor in the signal-transducing function of Na+/K+-ATPase. J. Biol. Chem. 2000, 275, 7832–7837. [Google Scholar] [CrossRef]
- Haas, M.; Wang, H.; Tian, J.; Xie, Z. Src-mediated inter-receptor cross-talk between the Na+/K+-ATPase and the epidermal growth factor receptor relays the signal from ouabain to mitogen-activated protein kinases. J. Biol. Chem. 2002, 277, 18694–18702. [Google Scholar] [CrossRef]
- Xie, Z.; Kometiani, P.; Liu, J.; Li, J.; Shapiro, J.I.; Askari, A. Intracellular reactive oxygen species mediate the linkage of Na+/K+-ATPase to hypertrophy and its marker genes in cardiac myocytes. J. Biol. Chem. 1999, 274, 19323–19328. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, Q.; Liu, C.; Xie, J.X.; Yan, Y.; Lai, F.; Duan, Q.; Li, X.; Tian, J.; Xie, Z. Involvement of Na/K-ATPase in hydrogen peroxide-induced activation of the Src/ERK pathway in LLC-PK1 cells. Free Radic. Biol. Med. 2014, 71, 415–426. [Google Scholar] [CrossRef]
- Miyakawa-Naito, A.; Uhlén, P.; Lal, M.; Aizman, O.; Mikoshiba, K.; Brismar, H.; Zelenin, S.; Aperia, A. Cell signaling microdomain with Na,K-ATPase and inositol 1,4,5-trisphosphate receptor generates calcium oscillations. J. Biol. Chem. 2003, 278, 50355–50361. [Google Scholar] [CrossRef]
- Yuan, Z.; Cai, T.; Tian, J.; Ivanov, A.V.; Giovannucci, D.R.; Xie, Z. Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex. Mol. Biol. Cell 2005, 16, 4034–4045. [Google Scholar] [CrossRef]
- Burlaka, I.; Liu, X.L.; Rebetz, J.; Arvidsson, I.; Yang, L.; Brismar, H.; Karpman, D.; Aperia, A. Ouabain protects against Shiga toxin-triggered apoptosis by reversing the imbalance between Bax and Bcl-xL. J. Am. Soc. Nephrol. 2013, 24, 1413–1423. [Google Scholar] [CrossRef]
- Wu, J.; Akkuratov, E.E.; Bai, Y.; Gaskill, C.M.; Askari, A.; Liu, L. Cell signaling associated with Na+/K+-ATPase: Activation of phosphatidylinositide 3-kinase IA/Akt by ouabain is independent of Src. Biochemistry 2013, 52, 9059–9067. [Google Scholar] [CrossRef]
- Wick, M.J.; Dong, L.Q.; Riojas, R.A.; Ramos, F.J.; Liu, F. Mechanism of phosphorylation of protein kinase B/Akt by a constitutively active 3-phosphoinositide-dependent protein kinase-1. J. Biol. Chem. 2000, 275, 40400–40406. [Google Scholar] [CrossRef] [PubMed]
- Balendran, A.; Biondi, R.M.; Cheung, P.C.; Casamayor, A.; Deak, M.; Alessi, D.R. A 3-phosphoinositide-dependent protein kinase-1 (PDK1) docking site is required for the phosphorylation of protein kinase Czeta (PKCzeta) and PKC-related kinase 2 by PDK1. J. Biol. Chem. 2000, 275, 20806–20813. [Google Scholar] [CrossRef] [PubMed]
- Jelliffe, R.W.; Buell, J.; Kalaba, R.; Sridhar, R.; Rockwell, R. A mathematical study of the metabolic conversion of digitoxin to digoxin in man. Math. Biosci. 1970, 6, 387–403. [Google Scholar] [CrossRef]
- Lee, T.H.; Smith, T.W. Serum digoxin concentration and diagnosis of Digitalis toxicity current concepts. Clin. Pharmacokinet. 1983, 8, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Ochs, H.R.; Pabst, J.; Greenblatt, D.J.; Hartlapp, J. Digitoxin accumulation. Br. J. Clin. Pharmacol. 1982, 14, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Roever, C.; Ferrante, J.; Gonzalez, E.C.; Pal, N.; Roetzheim, R.G. Comparing the toxicity of digoxin and digitoxin in a geriatric population: Should an old drug be rediscovered? South Med. J. 2000, 93, 199–202. [Google Scholar] [CrossRef]
- Hamlyn, J.M.; Blaustein, M.P.; Bova, S.; DuCharme, D.W.; Harris, D.W.; Mandel, F.; Mathews, W.R.; Ludens, J.H. Identification and characterization of a ouabain-like compound from human plasma. Proc. Natl. Acad. Sci. USA 1991, 88, 6259–6263. [Google Scholar] [CrossRef] [PubMed]
- Hamlyn, J.M.; Linde, C.I.; Gao, J.; Huang, B.S.; Golovina, V.A.; Blaustein, M.P.; Leenen, F.H. Neuroendocrine humoral and vascular components in the pressor pathway for brain angiotensin II: A new axis in long term blood pressure control. PLoS ONE 2014, 9, e108916. [Google Scholar] [CrossRef]
- Manunta, P.; Messaggio, E.; Ballabeni, C.; Sciarrone, M.T.; Lanzani, C.; Ferrandi, M.; Hamlyn, J.M.; Cusi, D.; Galletti, F.; Bianchi, G. Salt Sensitivity Study Group of the Italian Society of Hypertension. Plasma ouabain-like factor during acute and chronic changes in sodium balance in essential hypertension. Hypertension 2001, 38, 198–203. [Google Scholar] [CrossRef]
- Agunanne, E.; Horvat, D.; Uddin, M.N.; Puschett, J. The treatment of preeclampsia in a rat model employing. Digibind. Am. J. Perinatol. 2010, 27, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, M.P.; Hamlyn, J.M. Signaling mechanisms that link salt retention to hypertension: Endogenous ouabain, the Na+ pump, the Na+/Ca2+ exchanger and TRPC proteins. Biochim. Biophys. Acta 2010, 1802, 1219–1229. [Google Scholar] [CrossRef]
- Ferrandi, M.; Molinari, I.; Barassi, P.; Minotti, E.; Bianchi, G.; Ferrari, P. Organ hypertrophic signaling within caveolae membrane subdomains triggered by ouabain and antagonized by PST 2238. J. Biol. Chem. 2004, 279, 33306–33314. [Google Scholar] [CrossRef]
- Shiratori, O. Growth inhibitory effect of cardiac glycosides and aglycones on neoplastic cells: In vitro and in vivo studies. GANN Jpn. J. Cancer Res. 1967, 58, 521–528. [Google Scholar]
- Stenkvist, B.; Bengtsson, E.; Eklund, G.; Eriksson, O.; Holmquist, J.; Nordin, B.; Westman-Naeser, S. Evidence of a modifying influence of heart glucosides on the development of breast cancer. Anal. Quant. Cytol. 1980, 2, 49–54. [Google Scholar]
- Stenkvist, B.; Bengtsson, E.; Dahlqvist, B.; Eriksson, O.; Jarkrans, T.; Nordin, B. Cardiac glycosides and breast cancer, revisited. N. Engl. J. Med. 1982, 306, 484. [Google Scholar] [PubMed]
- Barwe, S.P.; Anilkumar, G.; Moon, S.Y.; Zheng, Y.; Whitelegge, J.P.; Rajasekaran, S.A.; Rajasekaran, A.K. Novel role for Na,K-ATPase in phosphatidylinositol 3-kinase signaling and suppression of cell motility. Mol. Biol. Cell 2005, 16, 1082–1094. [Google Scholar] [CrossRef]
- Shin, H.K.; Ryu, B.J.; Choi, S.W.; Kim, S.H.; Lee, K. Inactivation of Src-to-ezrin pathway: A possible mechanism in the ouabain-mediated inhibition of A549 cell migration. Biomed. Res. Int. 2015, 2015, 537136. [Google Scholar] [CrossRef]
- Kometiani, P.; Liu, L.; Askari, A. Digitalis-induced signaling by Na+/K+-ATPase in human breast cancer cells. Mol. Pharmacol. 2005, 67, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Hurwitz, J.; Massagué, J. Cell-cycle inhibition by independent CDK and PCNA binding domains in p21Cip1. Nature 1995, 375, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Dulić, V.; Stein, G.H.; Far, D.F.; Reed, S.I. Nuclear accumulation of p21Cip1 at the onset of mitosis: A role at the G2/M-phase transition. Mol. Cell Biol. 1998, 18, 546–557. [Google Scholar] [CrossRef]
- Harper, J.W.; Elledge, S.J.; Keyomarsi, K.; Dynlacht, B.; Tsai, L.H.; Zhang, P.; Dobrowolski, S.; Bai, C.; Connell-Crowley, L.; Swindell, E. Inhibition of cyclin-dependent kinases by p21. Mol. Biol. Cell 1995, 6, 387–400. [Google Scholar] [CrossRef]
- Yamakawa, M.; Liu, L.X.; Date, T.; Belanger, A.J.; Vincent, K.A.; Akita, G.Y.; Kuriyama, T.; Cheng, S.H.; Gregory, R.J.; Jiang, C. Hypoxia-inducible factor-1 mediates activation of cultured vascular endothelial cells by inducing multiple angiogenic factors. Circ. Res. 2003, 93, 664–673. [Google Scholar] [CrossRef]
- Zhang, H.; Qian, D.Z.; Tan, Y.S.; Lee, K.; Gao, P.; Ren, Y.R.; Rey, S.; Hammers, H.; Chang, D.; Pili, R.; et al. Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proc. Natl. Acad. Sci. USA 2008, 105, 19579–19586. [Google Scholar] [CrossRef]
- Lee, D.H.; Oh, S.C.; Giles, A.J.; Jung, J.; Gilbert, M.R.; Park, D.M. Cardiac glycosides suppress the maintenance of stemness and malignancy via inhibiting HIF-1α in human glioma stem cells. Oncotarget 2017, 8, 40233–40245. [Google Scholar] [CrossRef]
- Yang, X.S.; Xu, Z.W.; Yi, T.L.; Xu, R.C.; Li, J.; Zhang, W.B.; Zhang, S.; Sun, H.T.; Yu, Z.Q.; Xu, H.X.; et al. Ouabain suppresses the growth and migration abilities of glioma U-87MG cells through inhibiting the Akt/mTOR signaling pathway and downregulating the expression of HIF-1α. Mol. Med. Rep. 2018, 17, 5595–5600. [Google Scholar] [CrossRef]
- Bejček, J.; Spiwok, V.; Kmoníčková, E.; Ruml, T.; Rimpelová, S. Cardiac glycosides: On their therapeutic potential for cancer treatment. Chem. Listy 2021, 115, 4–12. [Google Scholar]
- Fujii, T.; Shimizu, T.; Yamamoto, S.; Funayama, K.; Fujita, K.; Tabuchi, Y.; Ikari, A.; Takeshima, H.; Sakai, H. Crosstalk between Na+,K+-ATPase and a volume-regulated anion channel in membrane microdomains of human cancer cells. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3792–3804. [Google Scholar] [CrossRef]
- Pommier, Y.; Sun, Y.; Huang, S.N.; Nitiss, J.L. Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nat. Rev. Mol. Cell Biol. 2016, 17, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Bielawski, K.; Winnicka, K.; Bielawska, A. Inhibition of DNA topoisomerases I and II, and growth inhibition of breast cancer MCF-7 cells by ouabain, digoxin and proscillaridin A. Biol. Pharm. Bull. 2006, 29, 1493–1497. [Google Scholar] [CrossRef]
- Bouras, T.; Southey, M.C.; Venter, D.J. Overexpression of the steroid receptor coactivator AIB1 in breast cancer correlates with the absence of estrogen and progesterone receptors and positivity for p53 and HER2/neu. Cancer Res. 2001, 61, 903–907. [Google Scholar]
- Fleming, F.J.; Myers, E.; Kelly, G.; Crotty, T.B.; McDermott, E.W.; O’Higgins, N.J.; Hill, A.D.; Young, L.S. Expression of SRC-1, AIB1, and PEA3 in HER2 mediated endocrine resistant breast cancer; a predictive role for SRC-1. J. Clin. Pathol. 2004, 57, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Liu, Z.; Chen, H.; Xu, J. The steroid receptor coactivator-1 regulates twist expression and promotes breast cancer metastasis. Cancer Res. 2009, 69, 3819–3827. [Google Scholar] [CrossRef]
- Gregory, C.W.; He, B.; Johnson, R.T.; Ford, O.H.; Mohler, J.L.; French, F.S.; Wilson, E.M. A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res. 2001, 61, 4315–4359. [Google Scholar] [PubMed]
- Zhou, H.J.; Yan, J.; Luo, W.; Ayala, G.; Lin, S.H.; Erdem, H.; Ittmann, M.; Tsai, S.Y.; Tsai, M.J. SRC-3 is required for prostate cancer cell proliferation and survival. Cancer Res. 2005, 65, 7976–7983. [Google Scholar] [CrossRef]
- Wang, Y.; Lonard, D.M.; Yu, Y.; Chow, D.C.; Palzkill, T.G.; Wang, J.; Qi, R.; Matzuk, A.J.; Song, X.; Madoux, F.; et al. Bufalin is a potent small-molecule inhibitor of the steroid receptor coactivators SRC-3 and SRC-1. Cancer Res. 2014, 74, 1506–1517. [Google Scholar] [CrossRef]
- Raghavendra, P.B.; Sreenivasan, Y.; Manna, S.K. Oleandrin induces apoptosis in human, but not in murine cells: Dephosphorylation of Akt, expression of FasL, and alteration of membrane fluidity. Mol. Immunol. 2007, 44, 2292–2302. [Google Scholar] [CrossRef]
- Manna, S.K.; Sreenivasan, Y.; Sarkar, A. Cardiac glycoside inhibits IL-8-induced biological responses by downregulating IL-8 receptors through altering membrane fluidity. J. Cell Physiol. 2006, 207, 195–207. [Google Scholar] [CrossRef]
- Laird, G.M.; Eisele, E.E.; Rabi, S.A.; Nikolaeva, D.; Siliciano, R.F. A novel cell-based high-throughput screen for inhibitors of HIV-1 gene expression and budding identifies the cardiac glycosides. J. Antimicrob. Chemother. 2014, 69, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.W.; Lingwood, C.A.; Ostrowski, M.A.; Cabral, T.; Cochrane, A. Cardiac glycoside/aglycones inhibit HIV-1 gene expression by a mechanism requiring MEK1/2-ERK1/2 signaling. Sci. Rep. 2018, 8, 850. [Google Scholar] [CrossRef]
- Wong, R.W.; Balachandran, A.; Ostrowski, M.A.; Cochrane, A. Digoxin suppresses HIV-1 replication by altering viral RNA processing. PLoS Pathog. 2013, 9, e1003241. [Google Scholar] [CrossRef]
- Kapoor, A.; Cai, H.; Forman, M.; He, R.; Shamay, M.; Arav-Boger, R. Human cytomegalovirus inhibition by cardiac glycosides: Evidence for involvement of the HERG gene. Antimicrob. Agents Chemother. 2012, 56, 4891–4899. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R.; Venkatadri, R.; Katsnelson, J.; Arav-Boger, R. Digitoxin suppresses human cytomegalovirus replication via Na+, K+/ATPase α1 subunit-dependent AMP-activated protein kinase and autophagy activation. J. Virol. 2018, 92, e01861. [Google Scholar] [CrossRef]
- Su, C.T.; Hsu, J.T.; Hsieh, H.P.; Lin, P.H.; Chen, T.C.; Kao, C.L.; Lee, C.N.; Chang, S.Y. Anti-HSV activity of digitoxin and its possible mechanisms. Antivir. Res. 2008, 79, 62–70. [Google Scholar] [CrossRef]
- Zhang, C.X.; Ofiyai, H.; He, M.; Bu, X.; Wen, Y.; Jia, W. Neuronal activity regulates viral replication of herpes simplex virus type 1 in the nervous system. J. Neurovirol. 2005, 11, 256–264. [Google Scholar] [CrossRef]
- Amarelle, L.; Katzen, J.; Shigemura, M.; Welch, L.C.; Cajigas, H.; Peteranderl, C.; Celli, D.; Herold, S.; Lecuona, E.; Sznajder, J.I. Cardiac glycosides decrease influenza virus replication by inhibiting cell protein translational machinery. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, L1094–L1106. [Google Scholar] [CrossRef]
- Burkard, C.; Verheije, M.H.; Haagmans, B.L.; van Kuppeveld, F.J.; Rottier, P.J.; Bosch, B.J.; de Haan, C.A. ATP1A1-mediated Src signaling inhibits coronavirus entry into host cells. J. Virol. 2015, 89, 4434–4448. [Google Scholar] [CrossRef]
- Rimpelová, S.; Zimmermann, T.; Drašar, P.B.; Dolenský, B.; Bejček, J.; Kmoníčková, E.; Cihlářová, P.; Gurská, S.; Kuklíková, L.; Hajdůch, M.; et al. Steroid glycosides hyrcanoside and deglucohyrcanoside: On isolation, structural identification, and anticancer activity. Foods 2021, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Škubník, J.; Pavlíčková, V.; Rimpelová, S. Cardiac glycosides as immune system modulators. Biomolecules 2021, 11, 659. [Google Scholar] [CrossRef]
- Bejček, J.; Spiwok, V.; Kmoníčková, E.; Rimpelová, S. Na+/K+-ATPase revisited: On its mechanism of action, role in cancer, and activity modulation. Molecules 2021, 26, 1905. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Xu, W.Q.; Zheng, Y.Y.; Omari-Siaw, E.; Zhu, Y.; Cao, X.; Tong, S.S.; Yu, J.N.; Xu, X.M. Octreotide-periplocymarin conjugate prodrug for improving targetability and anti-tumor efficiency: Synthesis, in vitro and in vivo evaluation. Oncotarget 2016, 7, 86326–86338. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Zhang, X.X.; Miao, Y.Q.; He, S.F.; Tian, D.M.; Yao, X.S.; Tang, J.S.; Gan, Y. Delivery of acetylthevetin B, an antitumor cardiac glycoside, using polymeric micelles for enhanced therapeutic efficacy against lung cancer cells. Acta Pharmacol. Sin. 2017, 38, 290–300. [Google Scholar] [CrossRef] [PubMed]

 ” highlight the configuration of linkage of sugar to C-3 hydroxyl of the steroid) and C-12 and C-16 (panel B) substituted cardiac glycosides (red dots highlight the acetylation on sugar moiety).
” highlight the configuration of linkage of sugar to C-3 hydroxyl of the steroid) and C-12 and C-16 (panel B) substituted cardiac glycosides (red dots highlight the acetylation on sugar moiety).
 ” highlight the configuration of linkage of sugar to C-3 hydroxyl of the steroid) and C-12 and C-16 (panel B) substituted cardiac glycosides (red dots highlight the acetylation on sugar moiety).
” highlight the configuration of linkage of sugar to C-3 hydroxyl of the steroid) and C-12 and C-16 (panel B) substituted cardiac glycosides (red dots highlight the acetylation on sugar moiety).
 ” highlight the configuration of linkage of sugar to C-3 hydroxyl of the steroid).
” highlight the configuration of linkage of sugar to C-3 hydroxyl of the steroid).
 ” highlight the configuration of linkage of sugar to C-3 hydroxyl of the steroid).
” highlight the configuration of linkage of sugar to C-3 hydroxyl of the steroid).







| Source | Cardiac Glycoside | Reference |
|---|---|---|
| Strophanthus kombe (Apocynaceae) | K-strophanthoside, cymarin, helveticoside, strophanthidin, erysimoside, k-strophanthin-β, neoglucoerysimoside | [12,13] |
| Strophanthus gratus (Apocynaceae) | G-strophanthin (ouabain) | [14] |
| Digitalis lanata (Scrophulariaceae) | Digoxigenin, deacetyllanatoside C, digoxigenin-bis-digitoxoside, gitoxigenin, digoxin, lanatoside C, digitoxigenin, α-acetyldigoxin, β-acetyldigoxin, lanatoside B, gitoxin, lanatoside A, digitoxin | [15] |
| Digitalis purpurea (Scrophulariaceae) | Digitoxin, digitoxigenin, gitoxin, gitoxigenin, gitaloxin, glucodigitoxin, glucogitoxin, glucogitaloxin | [16,17,18] |
| Nerium oleander (Apocynaceae) | Oleandrin, neritaloside, cardenolide B-1, oleagenin, odoroside H, oleaside A, neriaside | [19,20] |
| Coronilla varia (Fabaceae) | Hyrcanoside, deglucohyrcanoside | [21] |
| Convallaria majalis (Liliaceae) | Convallatoxin, perconval, canariengenin, rhodexin, periplorhamnoside, convallatoxol, peripalloside, strophalloside, strophanolloside, deglucocheirotoxin, lukondjoside, convalloside, deglucocheirotoxol, periguloside, rhodexoside | [22,23,24,25] |
| Apocynum cannabinum (Apocynaceae) | Strophanthidin, cymarin, cynocannoside, helveticoside, apobioside, apocannoside, cannogenol | [26] |
| Source | Cardiac Glycoside | Reference |
|---|---|---|
| Homo sapiens sapiens | Ouabain (endogenous), marinobufagenin | [31] |
| Canis lupus familiaris | Ouabain (endogenous) | [32] |
| Wistar rats | Ouabain (endogenous) | [33] |
| Rhinella marina | Marinobufagenin | [34] |
| Bufo bufo gargarizans | Bufotoxin | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bejček, J.; Jurášek, M.; Spiwok, V.; Rimpelová, S. Quo vadis Cardiac Glycoside Research? Toxins 2021, 13, 344. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13050344
Bejček J, Jurášek M, Spiwok V, Rimpelová S. Quo vadis Cardiac Glycoside Research? Toxins. 2021; 13(5):344. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13050344
Chicago/Turabian StyleBejček, Jiří, Michal Jurášek, Vojtěch Spiwok, and Silvie Rimpelová. 2021. "Quo vadis Cardiac Glycoside Research?" Toxins 13, no. 5: 344. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13050344