Photocatalytic Degradation of Deoxynivalenol Using Cerium Doped Titanium Dioxide under Ultraviolet Light Irradiation
Abstract
:1. Introduction
2. Results and Discussion
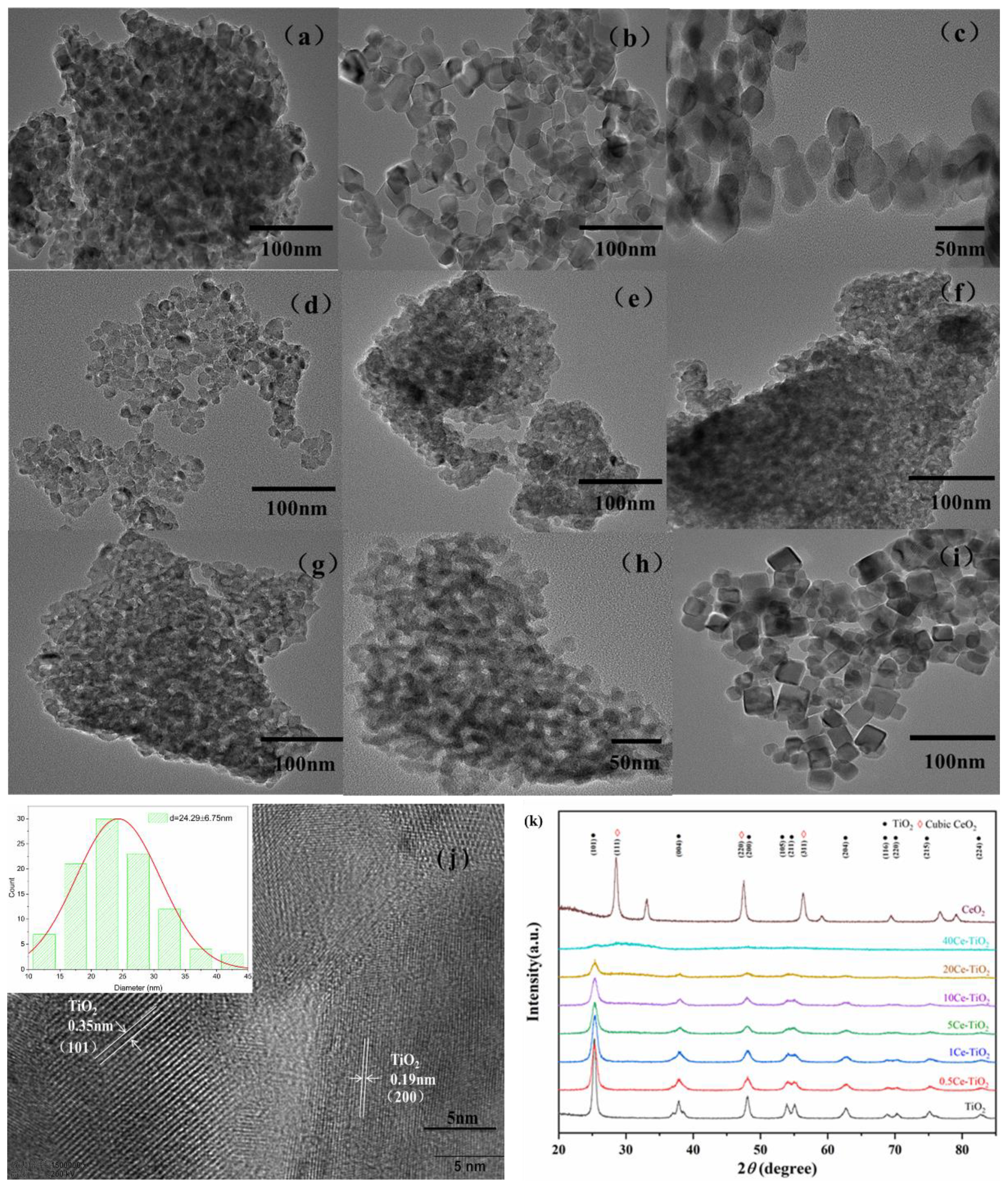
2.1. Structural Characterizations
2.1.1. Morphology Analyses and Crystal Phase
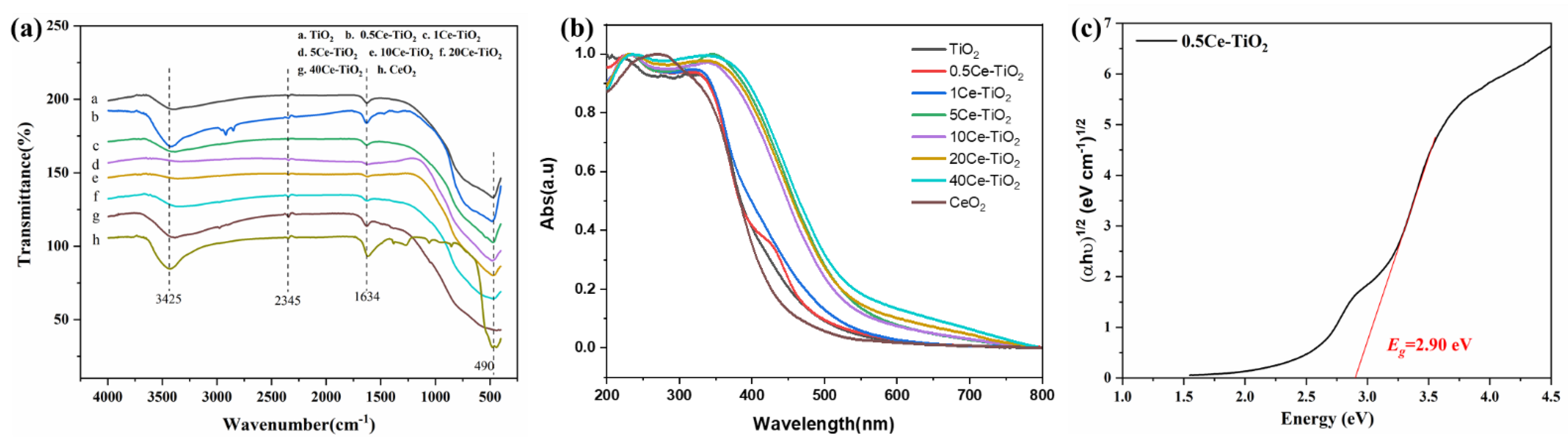
2.1.2. FT-IR and UV-Vis DRS Analysis
2.2. Photocatalytic Degradation of Deoxynivalenol
2.2.1. Evaluation of the Effectiveness of DON Degradation
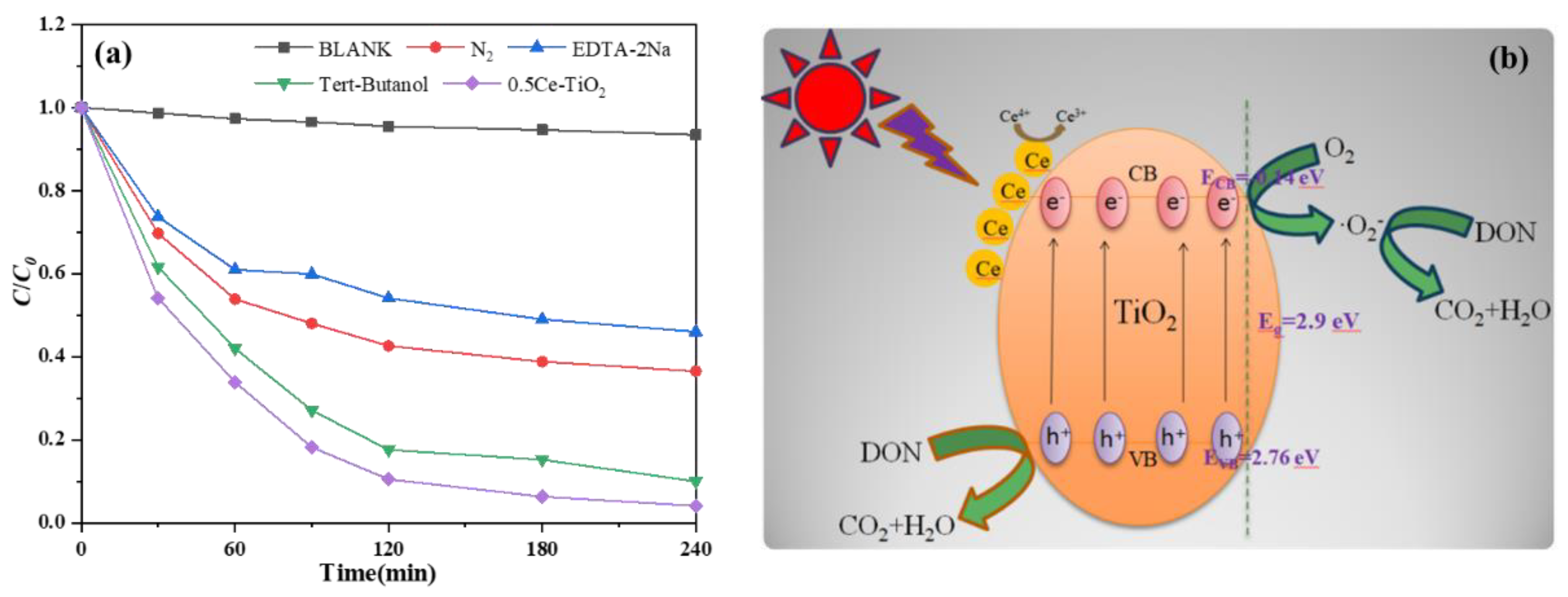
2.2.2. Free Radical Trapping Experiments and Photocatalytic Degradation Mechanism
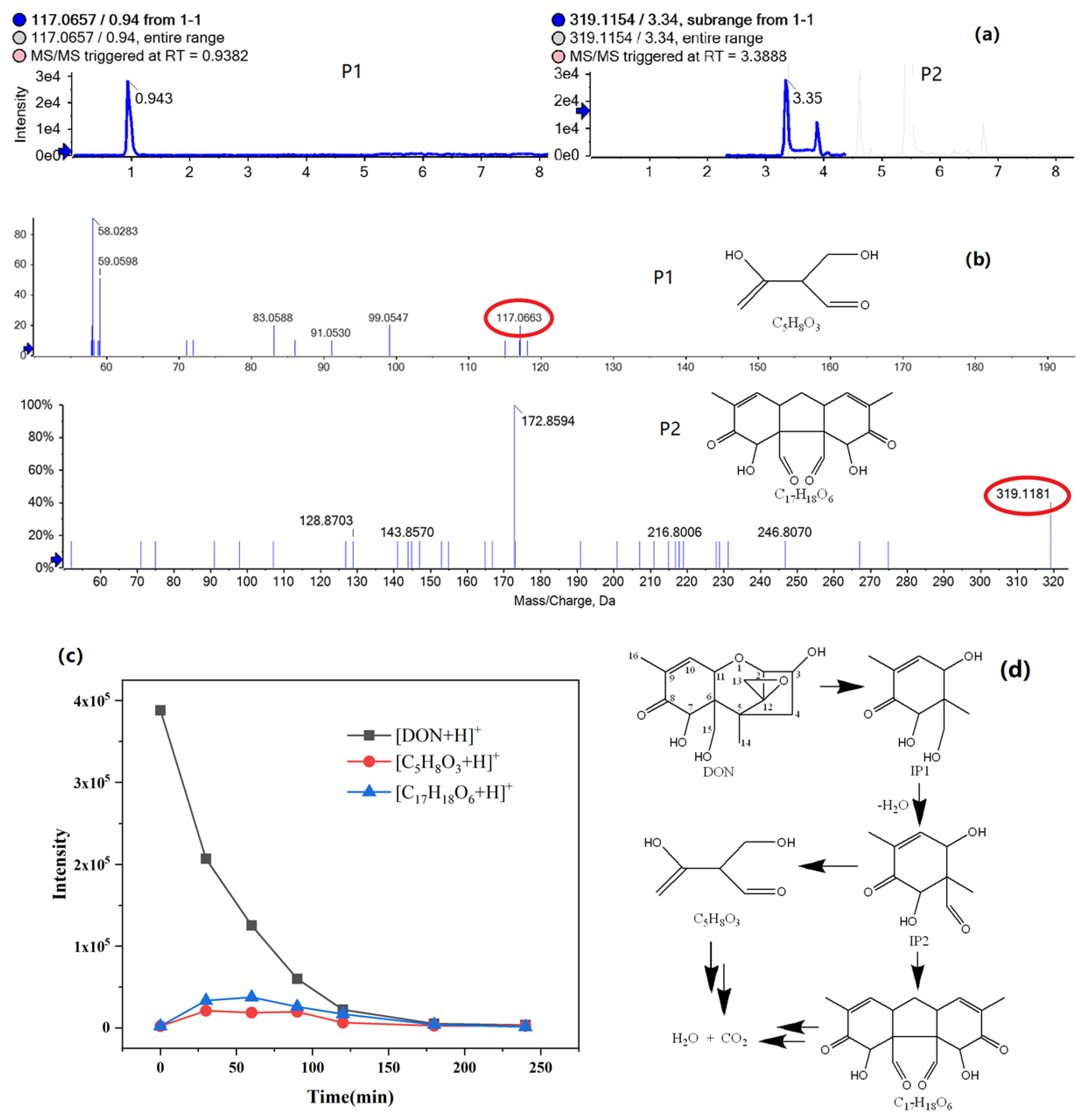
2.2.3. Intermediate Products of Don Degradation and Possible Photodegradation Pathway
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Ce-TiO2, TiO2 and CeO2
4.3. Characterization of Catalysts
4.4. DON Photocatalytic Tests
4.5. Degraded Intermediate Products Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Wu, S.; Wang, F.; Li, Q.; Zhou, Y.; He, C.; Duan, N. Detoxification of don by photocatalytic degradation and quality evaluation of wheat. RSC Adv. 2019, 9, 34351–34358. [Google Scholar] [CrossRef] [Green Version]
- Authority, E.F.S. Deoxynivalenol in food and feed: Occurrence and exposure. EFSA J. 2013, 11, 3379. [Google Scholar]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol 2010, 84, 663–679. [Google Scholar] [CrossRef]
- Zhao, Z.; Song, S.; Liu, N.; Rao, Q.; Hou, J.; De Saeger, S.; Wu, A. Large-scale preparation and multi-dimensional characterization of high-purity mycotoxin deoxynivalenol in rice culture inoculated with fusarium graminearum. Anal. Methods 2014, 6, 6651–6657. [Google Scholar] [CrossRef]
- Pronyk, C.; Cenkowski, S.; Abramson, D. Superheated steam reduction of deoxynivalenol in naturally contaminated wheat kernels. Food Control 2006, 17, 789–796. [Google Scholar] [CrossRef]
- Bretz, M.; Beyer, M.; Cramer, B.; Knecht, A.; Humpf, H.-U. Thermal degradation of the fusarium mycotoxin deoxynivalenol. J. Agric. Food Chem. 2006, 54, 6445–6451. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hassan, Y.I.; Lepp, D.; Shao, S.; Zhou, T. Strategies and methodologies for developing microbial detoxification systems to mitigate mycotoxins. Toxins 2017, 9, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, X.; Zhu, Z.; Zhou, Y.; Ji, F.; Yao, Z.; Shi, J.; Xu, J. Complete genome sequence of deoxynivalenol-degrading bacterium devosia sp. Strain a16. J. Biotechnol. 2016, 218, 21–22. [Google Scholar] [CrossRef]
- Bhatkhande, D.S.; Pangarkar, V.G.; Beenackers, A.A.C.M. Photocatalytic degradation for environmental applications—A review. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2002, 77, 102–116. [Google Scholar] [CrossRef]
- Wang, H.; Mao, J.; Zhang, Z.; Zhang, Q.; Zhang, L.; Zhang, W.; Li, P. Photocatalytic degradation of deoxynivalenol over dendritic-like α-fe2o3 under visible light irradiation. Toxins 2019, 11, 105. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Wu, S.; Wang, F.; Li, Q.; He, C.; Duan, N.; Wang, Z. Assessing the toxicity in vitro of degradation products from deoxynivalenol photocatalytic degradation by using upconversion nanoparticlesnanoparticles@TiO2 composite. Chemosphere 2020, 238, 124648. [Google Scholar] [CrossRef]
- Wang, F.; Feng, L.; Qin, Y.; Zhao, T.; Luo, H.; Zhu, J. Dual functional SiO2@TiO2 photonic crystals for dazzling structural colors and enhanced photocatalytic activity. J. Mater. Chem. C 2019, 7, 11972–11983. [Google Scholar] [CrossRef]
- Yan, N.; Zhu, Z.; Zhang, J.; Zhao, Z.; Liu, Q. Preparation and properties of ce-doped TiO2 photocatalyst. Mater. Res. Bull. 2012, 47, 1869–1873. [Google Scholar] [CrossRef]
- Siriwong, C.; Wetchakun, N.; Inceesungvorn, B.; Channei, D.; Samerjai, T.; Phanichphant, S. Doped-metal oxide nanoparticles for use as photocatalysts. Prog. Cryst. Growth Charact. Mater. 2012, 58, 145–163. [Google Scholar] [CrossRef]
- Ranjit, K.T.; Willner, I.; Bossmann, S.H.; Braun, A.M. Lanthanide oxide doped titanium dioxide photocatalysts: Effective photocatalysts for the enhanced degradation of salicylic acid and t-cinnamic acid. J. Catal. 2001, 204, 305–313. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, B.; Hong, L.; Jiang, H. Preparation and characterization of cerium oxide doped TiO2 nanoparticles. J. Phys. Chem. Solids 2005, 66, 161–167. [Google Scholar] [CrossRef]
- Gionco, C.; Paganini, M.C.; Agnoli, S.; Reeder, A.E.; Giamello, E. Structural and spectroscopic characterization of CeO2–TiO2 mixed oxides. J. Mater. Chem. A 2013, 1, 10918–10926. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, G.; Sun, Z.; Zheng, S.; Frost, R.L. A comparative study about the influence of metal ions (Ce, La and V) doping on the solar-light-induced photodegradation toward rhodamine B. J. Environ. Chem. Eng. 2015, 3, 1444–1451. [Google Scholar] [CrossRef]
- Anandan, S.; Rao, T.N.; Sathish, M.; Rangappa, D.; Honma, I.; Miyauchi, M. Superhydrophilic graphene-loaded TiO2 thin film for self-cleaning applications. ACS Appl. Mater. Interfaces 2013, 5, 207–212. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Zheng, H.; Zhang, P. Hierarchical z-scheme 1D/2D architecture with TiO2 nanowires decorated by MnO2 nanosheets for efficient adsorption and full spectrum photocatalytic degradation of organic pollutants. Catal. Sci. Technol. 2020, 10, 3603–3612. [Google Scholar] [CrossRef]
- Lu, H.; Zhuang, J.; Ma, Z.; Zhou, W.; Xia, H.; Xiao, Z.; Zhang, H.; Haimin, L. Crystal recombination control by using ce doped in mesoporous TiO2 for efficient perovskite solar cells. RSC Adv. 2019, 9, 1075–1083. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.-H.; Sasirekha, N.; Chen, Y.-W.; Wang, W.-J. Preferential oxidation of CO in H2 stream over au/MnO2–CeO2 catalysts. Ind. Eng. Chem. Res. 2006, 45, 4927–4935. [Google Scholar] [CrossRef]
- Li, F.B.; Li, X.Z.; Hou, M.F.; Cheah, K.W.; Choy, W.C.H. Enhanced photocatalytic activity of Ce3+–TiO2 for 2-mercaptobenzothiazole degradation in aqueous suspension for odour control. Appl. Catal. A Gen. 2005, 285, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Dong, G.; Chen, G. Synthesis, characterization and performance of ternary doped Cu–Ce–B/TiO2 nanotubes on the photocatalytic removal of nitrogen oxides. New J. Chem. 2015, 39, 6854–6863. [Google Scholar] [CrossRef]
- Tuyen, L.T.T.; Quang, D.A.; Tam Toan, T.T.; Tung, T.Q.; Hoa, T.T.; Mau, T.X.; Khieu, D.Q. Synthesis of CeO2/TiO2 nanotubes and heterogeneous photocatalytic degradation of methylene blue. J. Environ. Chem. Eng. 2018, 6, 5999–6011. [Google Scholar] [CrossRef]
- Huang, Z.; Sun, Q.; Lv, K.; Zhang, Z.; Li, M.; Li, B. Effect of contact interface between TiO2 and g-C3N4 on the photoreactivity of g-C3N4/TiO2 photocatalyst: (0 0 1) vs. (1 0 1) facets of TiO2. Appl. Catal. B Environ. 2015, 164, 420–427. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Xie, H.; Qu, A. Synthesis of g-C3N4/TiO2 with enhanced photocatalytic activity for H2 evolution by a simple method. Int. J. Hydrogen Energy 2014, 39, 6354–6363. [Google Scholar] [CrossRef]
- Pillai, S.C.; Periyat, P.; George, R.; McCormack, D.; Seery, M.; Hayden, H.; Colreavy, J.; Corr, D.; Hinder, S.J. Synthesis of high-temperature stable anatase TiO2 photocatalyst. J. Phys. Chem. C 2007, 111, 1605–1611. [Google Scholar] [CrossRef] [Green Version]
- Malik, A.; Hameed, S.; Siddiqui, M.; Haque, M.; Muneer, M. Influence of ce doping on the electrical and optical properties of TiO2 and its photocatalytic activity for the degradation of remazol brilliant blue R. Int. J. Photoenergy 2013, 2013, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.-h.; Zeng, Z.-x. The preparation, characterization, and photocatalytic activities of Ce-TiO2/SiO2. J. Mol. Catal. A Chem. 2008, 279, 77–81. [Google Scholar] [CrossRef]
- Hao, R.; Wang, G.; Jiang, C.; Tang, H.; Xu, Q. In situ hydrothermal synthesis of g-C3N4 /TiO2 heterojunction photocatalysts with high specific surface area for rhodamine b degradation. Appl. Surf. Sci. 2017, 411, 400–410. [Google Scholar] [CrossRef]
- Shayegan, Z.; Haghighat, F.; Lee, C.-S. Surface fluorinated ce-doped TiO2 nanostructure photocatalyst: A trap and remove strategy to enhance the voc removal from indoor air environment. Chem. Eng. J. 2020, 401, 125932. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, C.; Si, S.; Shi, Y.; Zhao, X. Study on the effect of edta on the photocatalytic reduction of mercury onto nanocrystalline titania using quartz crystal microbalance and differential pulse voltammetry. Electrochim. Acta 2011, 56, 2062–2067. [Google Scholar] [CrossRef]
- Hyunjoo Lee, W.C. Photocatalytic oxidation of arsenite in TiO2 suspension: Kinetics and mechanisms. Environ. Sci. Technol. 2002, 36, 3872–3878. [Google Scholar]
- Yang, B.; Zheng, J.; Li, W.; Wang, R.; Li, D.; Guo, X.; Rodriguez, R.D.; Jia, X. Engineering z-scheme TiO2-ov-biocl via oxygen vacancy for enhanced photocatalytic degradation of imidacloprid. Dalton Trans. 2020, 49, 11010–11018. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Sun, C.; Liu, D.; Luo, X.; Li, D.; Wang, J.; Wang, N.; Chang, X.; Zong, R.; Zhu, Y. Photocatalytic degradation of deoxynivalenol using graphene/zno hybrids in aqueous suspension. Appl. Catal. B Environ. 2017, 204, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Tahir, M.; Cao, C.; Butt, F.K.; Idrees, F.; Mahmood, N.; Ali, Z.; Aslam, I.; Tanveer, M.; Rizwan, M.; Mahmood, T. Tubular graphitic-C3N4: A prospective material for energy storage and green photocatalysis. J. Mater. Chem. A 2013, 1, 13949–13955. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.T.; Hoque, M.E.; Chandra Bhoumick, M. Facile one-pot synthesis of heterostructure SnO2/ZnO photocatalyst for enhanced photocatalytic degradation of organic dye. RSC Adv. 2020, 10, 23554–23565. [Google Scholar] [CrossRef]
- Xu, J.; Ao, Y.; Fu, D.; Yuan, C. Study on photocatalytic performance and degradation kinetics of x-3b with lanthanide-modified titanium dioxide under solar and uv illumination. J. Hazard. Mater. 2009, 164, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-X.; Li, X.-Z.; Li, F.-B. Enhanced photocatalytic activity of Ce3+-TiO2 hydrosols in aqueous and gaseous phases. Chem. Eng. J. 2010, 157, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Nagy, C.M.; Fejer, S.N.; Berek, L.; Molnar, J.; Viskolcz, B. Hydrogen bondings in deoxynivalenol (don) conformations—A density functional study. J. Mol. Struct. 2005, 726, 55–59. [Google Scholar] [CrossRef]
- Cheng, L.; Jiang, T.; Zhang, J. Photoelectrocatalytic degradation of deoxynivalenol on CuO-Cu2O/WO3 ternary film: Mechanism and reaction pathways. Sci. Total Environ. 2021, 776, 145840. [Google Scholar] [CrossRef] [PubMed]
- Periyat, P.; Laffir, F.; Tofail, S.A.M.; Magner, E. A facile aqueous sol–gel method for high surface area nanocrystalline CeO2. RSC Adv. 2011, 1, 1794–1798. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, P.; Zhao, Z.; Tan, Y.; E, H.; Zuo, M.; Wang, J.; Yang, J.; Cui, S.; Yang, X. Photocatalytic Degradation of Deoxynivalenol Using Cerium Doped Titanium Dioxide under Ultraviolet Light Irradiation. Toxins 2021, 13, 481. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13070481
He P, Zhao Z, Tan Y, E H, Zuo M, Wang J, Yang J, Cui S, Yang X. Photocatalytic Degradation of Deoxynivalenol Using Cerium Doped Titanium Dioxide under Ultraviolet Light Irradiation. Toxins. 2021; 13(7):481. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13070481
Chicago/Turabian StyleHe, Pengzhen, Zhiyong Zhao, Yanglan Tan, Hengchao E, Minghui Zuo, Jianhua Wang, Junhua Yang, Shuxin Cui, and Xianli Yang. 2021. "Photocatalytic Degradation of Deoxynivalenol Using Cerium Doped Titanium Dioxide under Ultraviolet Light Irradiation" Toxins 13, no. 7: 481. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13070481




