Effect of Popcorn (Zea mays var. everta) Popping Mode (Microwave, Hot Oil, and Hot Air) on Fumonisins and Deoxynivalenol Contamination Levels
Abstract
:1. Introduction
2. Results and Discussion
2.1. FUM and DON Contents of the Popcorn Samples (Unpopped Controls)
2.2. Impact of Popping Mode on FUM Contents
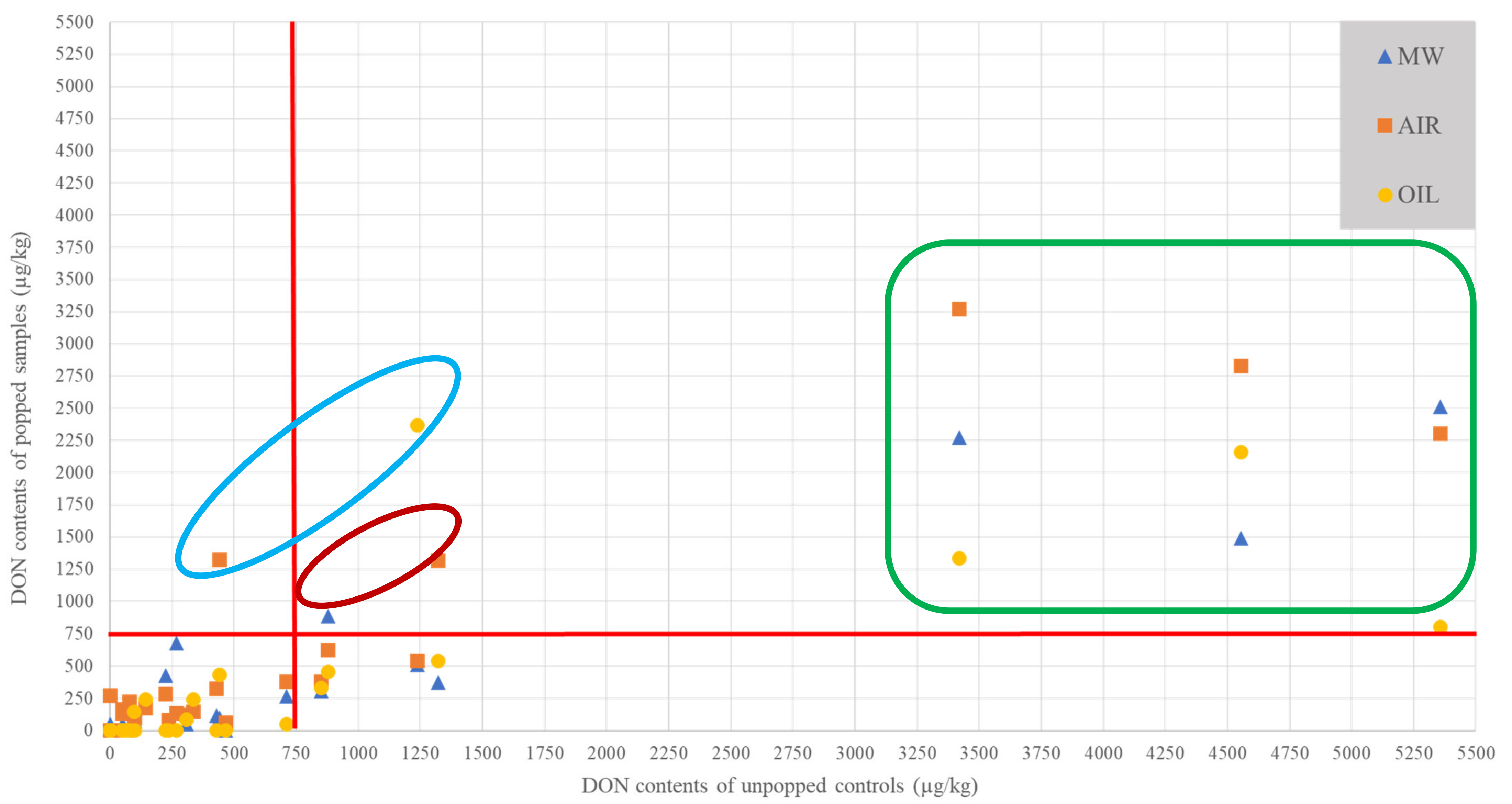
2.3. Impact of Popping Mode on DON Contents
3. Conclusions
4. Materials and Methods
4.1. Popcorn Samples
4.2. Popping
4.2.1. Popping with Hot Air (AIR)
4.2.2. Popping with Hot Oil (OIL)
4.2.3. Popping with Microwave (MW)
4.3. Determination of FUM and DON Contents
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| FUM | DON | |||||||
|---|---|---|---|---|---|---|---|---|
| MW vs. WP | AIR vs. WP | OIL vs. WP | ALL vs. WP | MW vs. WP | AIR vs. WP | OIL vs. WP | ALL vs. WP | |
| Butterfly | Yes *** | Yes *** | Yes *** | Yes *** | No | No | No | No |
| Mushroom | Yes *** | Yes *** | Yes *** | Yes *** | No | No | Yes * | No |
| Butterfly and Mushroom | Yes *** | Yes *** | Yes *** | Yes *** | No | No | No | No |
| MW vs AIR | OIL vs AIR | OIL vs MW | MW vs AIR | OIL vs AIR | OIL vs MW | |||
| Butterfly | No | Yes ** | Yes** | No | No | No | ||
| Mushroom | No | Yes ** | Yes ** | No | Yes * | No | ||
| Butterfly and Mushroom | No | Yes ** | Yes ** | No | No | No | ||
| Butterfly | Mushroom | Butterfly and Mushroom | ||||
|---|---|---|---|---|---|---|
| Type of Popping | Proportion > WP | Number > WP | Proportion > WP | Number > WP | Proportion > WP | Number > WP |
| AIR | 0% | 0 | 6% | 1 | 3% | 1 |
| OIL | 0% | 0 | 0% | 0 | 0% | 0 |
| MW | 14% | 3 | 6% | 1 | 10% | 4 |
| Total samples with at least one popped > WP | 14% | 3 | 11% | 2 | 13% | 5 |
| Total samples | 21 | 18 | 39 | |||
| Butterfly | Mushroom | Butterfly and Mushroom | ||||
|---|---|---|---|---|---|---|
| Type of Popping | Proportion > WP | Number > WP | Proportion > WP | Number > WP | Proportion > WP | Number > WP |
| AIR | 10% | 2 | 17% | 3 | 13% | 5 |
| OIL | 14% | 3 | 0% | 0 | 8% | 3 |
| MW | 14% | 3 | 28% | 5 | 21% | 8 |
| Total samples with at least one popped > WP | 19% | 4 | 33% | 6 | 26% | 10 |
| Total number of samples | 21 | 18 | 39 | |||
References
- Rodrigues, I.; Naehrer, K. A Three-Year Survey on the Worldwide Occurrence of Mycotoxins in Feedstuffs and Feed. Toxins 2012, 4, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [Green Version]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef]
- Pestka, J.J.; Smolinski, A.T. Deoxynivalenol: Toxicology and Potential Effects on Humans. J. Toxicol. Environ. Health Part B 2005, 8, 39–69. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef]
- Sun, G.; Wang, S.; Hu, X.; Su, J.; Huang, T.; Yu, J.; Tang, L.; Gao, W.; Wang, J.-S. Fumonisin B1contamination of home-grown corn in high-risk areas for esophageal and liver cancer in China. Food Addit. Contam. 2007, 24, 181–185. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006. Setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5. [Google Scholar]
- European Commission. Commission Regulation (EC) No 1126/2007 of 28 September 2007 Amending Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs as Regards Fusarium Toxins in Maize Products. Off. J. Eur. Union 2007, 225, 5–24. [Google Scholar]
- Suman, M. Last decade studies on mycotoxins’ fate during food processing: An overview. Curr. Opin. Food Sci. 2021, 41, 70–80. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, B. Fates of deoxynivalenol and deoxynivalenol-3-glucoside during bread and noodle processing. Food Control 2015, 50, 754–757. [Google Scholar] [CrossRef]
- Kamimura, H. Removal of Mycotoxins during Food Processing. Mycotoxins 1999, 1999, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Cano-Sancho, G.; Sanchis, V.; Ramos, A.J.; Marín, S. Effect of food processing on exposure assessment studies with mycotoxins. Food Addit. Contam. Part A 2013, 30, 867–875. [Google Scholar] [CrossRef] [PubMed]
- De Nijs, M.; Top, H.V.D.; De Stoppelaar, J.; Lopez, P.; Mol, H. Fate of enniatins and deoxynivalenol during pasta cooking. Food Chem. 2016, 213, 763–767. [Google Scholar] [CrossRef]
- Brera, C.; Peduto, A.; Debegnach, F.; Pannunzi, E.; Prantera, E.; Gregori, E.; De Giacomo, M.; De Santis, B. Study of the influence of the milling process on the distribution of deoxynivalenol content from the caryopsis to cooked pasta. Food Control 2013, 32, 309–312. [Google Scholar] [CrossRef]
- Visconti, A.; Haidukowski, E.M.; Pascale, M.; Silvestri, M. Reduction of deoxynivalenol during durum wheat processing and spaghetti cooking. Toxicol. Lett. 2004, 153, 181–189. [Google Scholar] [CrossRef]
- Jaukovic, M.; Stanisic, N.; Nikodijevic, B.; Krnjaja, V. Effects of temperature and time on deoxynivalenol (DON) and zearalenone (ZON) content in corn. Zb. Matice Srp. za Prir. Nauk. 2014, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Samar, M.; Resnik, S.; González, H.; Pacin, A.; Castillo, M. Deoxynivalenol reduction during the frying process of turnover pie covers. Food Control 2007, 18, 1295–1299. [Google Scholar] [CrossRef]
- Kabak, B. The fate of mycotoxins during thermal food processing. J. Sci. Food Agric. 2009, 89, 549–554. [Google Scholar] [CrossRef]
- Cirlini, M.; Hahn, I.; Varga, E.; Dall’Asta, M.; Falavigna, C.; Calani, L.; Berthiller, F.; Del Rio, D.; Dall’Asta, C. Hydrolysed fumonisin B1andN-(deoxy-D-fructos-1-yl)-fumonisin B1: Stability and catabolic fate under simulated human gastrointestinal conditions. Int. J. Food Sci. Nutr. 2014, 66, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.S.; Katta, S.K.; Fingerhut, D.D.; DeVries, J.W.; Bullerman, L.B. Effects of Baking and Frying on the Fumonisin B1Content of Corn-Based Foods. J. Agric. Food Chem. 1997, 45, 4800–4805. [Google Scholar] [CrossRef]
- Dupuy, J.; Le Bars, P.; Boudra, H. Thermostability of Fumonisin B 1, a Mycotoxin from Fusarium moniliforme, in Corn. Appl. Environ. Microbiol. 1993, 59, 2864–2867. [Google Scholar] [CrossRef] [Green Version]
- D’Ovidio, K.L.; Trucksess, M.W.; Devries, J.W.; Bean, G. Effects of irradiation on fungi and fumonisin B1in corn, and of microwave-popping on fumonisins in popcorn. Food Addit. Contam. 2007, 24, 735–743. [Google Scholar] [CrossRef]
- Hoseney, R.; Zeleznak, K.; Abdelrahman, A. Mechanism of popcorn popping. J. Cereal Sci. 1983, 1, 43–52. [Google Scholar] [CrossRef]
- Virot, E.; Ponomarenko, A. Popcorn: Critical temperature, jump and sound. J. R. Soc. Interface 2015, 12, 20141247. [Google Scholar] [CrossRef] [Green Version]
- Gökmen, S. Effects of moisture content and popping method on popping characteristics of popcorn. J. Food Eng. 2004, 65, 357–362. [Google Scholar] [CrossRef]
- Song, A.; Eckhoff, S.R. Optimum popping moisture content for popcorn kernels of different sizes. Cereal Chem. 1994, 71, 458–460. [Google Scholar]
- Mycotoxin Survey 2019: European Harvest Results at a Glance. Available online: https://www.biomin.net/science-hub/mycotoxin-survey-2019-european-harvest-results-at-a-glance/ (accessed on 23 March 2021).
- BIOMIN Mycotoxin Survey Preliminary European Corn Harvest Results 2020. Available online: https://www.biomin.net/science-hub/biomin-mycotoxin-survey-preliminary-european-corn-harvest-results-2020/ (accessed on 23 March 2021).
- BIOMIN World Mycotoxin Survey 2020. Available online: https://www.biomin.net/science-hub/world-mycotoxin-survey-impact-2021/ (accessed on 23 March 2021).
- Katta, S.K.; Cagampang, A.E.; Jackson, L.S.; Bullerman, L.B. Distribution ofFusariumMolds and Fumonisins in Dry-Milled Corn Fractions. Cereal Chem. J. 1997, 74, 858–863. [Google Scholar] [CrossRef]
- Vanara, F.; Reyneri, A.; Blandino, M. Fate of fumonisin B1 in the processing of whole maize kernels during dry-milling. Food Control 2009, 20, 235–238. [Google Scholar] [CrossRef]
- Rheeder, J.P.; Marasas, W.F.O.; Vismer, H.F. Production of Fumonisin Analogs by Fusarium Species. Appl. Environ. Microbiol. 2002, 68, 2101–2105. [Google Scholar] [CrossRef] [Green Version]
- Ingenbleek, L.; Sulyok, M.; Adegboye, A.O.; Hossou, S.; Koné, A.; Oyedele, A.; Kisito, C.; Dembélé, Y.; Eyangoh, S.; Verger, P.; et al. Regional Sub-Saharan Africa Total Diet Study in Benin, Cameroon, Mali and Nigeria Reveals the Presence of 164 Mycotoxins and Other Secondary Metabolites in Foods. Toxins 2019, 11, 54. [Google Scholar] [CrossRef] [Green Version]
- De Girolamo, A.; Solfrizzo, M.; Visconti, A. Effect of Processing on Fumonisin Concentration in Corn Flakes. J. Food Prot. 2001, 64, 701–705. [Google Scholar] [CrossRef]
- Ryu, D.; Bianchini, A.; Bullerman, L.B. Effects of processing on mycotoxins. Stewart Postharvest Rev. 2008, 4, 1–7. [Google Scholar] [CrossRef]
- Zentai, A.; Szeitzné-Szabó, M.; Mihucz, G.; Szeli, N.; Szabó, A.; Kovács, M. Occurrence and Risk Assessment of Fumonisin B1 and B2 Mycotoxins in Maize-Based Food Products in Hungary. Toxins 2019, 11, 709. [Google Scholar] [CrossRef] [Green Version]
- Schaafsma, A.W.; Frégeau-Reid, J.; Phibbs, T. Distribution of deoxynivalenol in Gibberella-infected food-grade corn kernels. Can. J. Plant Sci. 2004, 84, 909–913. [Google Scholar] [CrossRef] [Green Version]
- Kostelanska, M.; Dzuman, Z.; Malachova, A.; Capouchova, I.; Prokinova, E.; Skerikova, A.; Hajslova, J. Effects of Milling and Baking Technologies on Levels of Deoxynivalenol and its Masked Form Deoxynivalenol-3-Glucoside. J. Agric. Food Chem. 2011, 59, 9303–9312. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Marín, S.; Morales, H.; Ramos, A.J.; Sanchis, V. The fate of deoxynivalenol and ochratoxin A during the breadmaking process, effects of sourdough use and bran content. Food Chem. Toxicol. 2014, 68, 53–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savoie, J.M.; Martinez, C.; Richard-Forget, F. Des microorganismes capables de dégrader les mycotoxines: De nouveaux levains pour garantir la qualité sanitaire d’aliments à base de céréales? Innov. Agron. 2015, 44, 35. [Google Scholar]
- Eldredge, J.C.; Thomas, W.I. Popcorn, Its Production, Processing and Utilization; University of Science and Technology: Ames, IA, USA, 1959. [Google Scholar]
- COFRAC. Guide Technique D’accréditation. Dosage des Mycotoxines et des Phycotoxines dans les Denrées Alimentaires Destinées à L’homme ou aux Animaux; Document LAB GTA 21, Révision 01; COFRAC: Paris, France, 2014; p. 24. [Google Scholar]
- Lehotay, S.J.; Son, K.A.; Kwon, H.; Koesukwiwat, U.; Fu, W.; Mastovska, K.; Hoh, E.; Leepipatpiboon, N. Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J. Chromatogr. A 2010, 1217, 2548–2560. [Google Scholar] [CrossRef]

| Popcorn Types | FUM (FB1 + FB2) | DON | |
|---|---|---|---|
| All samples (n = 39) | Samples < LOD | 5 (13%) | 12 (30%) |
| Samples > EU regulation | 9 (23%) | 7 (18%) | |
| Average (µg/kg) | 1351 | 560 | |
| Average of positive samples (µg/kg) | 1550 | 809 | |
| Maximum (µg/kg) | 9315 | 5359 | |
| Butterfly (n = 21) | Samples < LOD | 5 (24%) | 4 (19%) |
| Samples > EU regulation | 6 (29%) | 6 (29%) | |
| Average (µg/kg) | 1570 | 943 | |
| Average of positive samples (µg/kg) | 2061 | 1165 | |
| Maximum (µg/kg) | 9315 | 5359 | |
| Mushroom (n = 18) | Samples < LOD | 0 (0%) | 8 (44%) |
| Samples > EU regulation | 3 (17%) | 1 (6%) | |
| Average (µg/kg) | 1097 | 113 | |
| Average of positive samples (µg/kg) | 1097 | 204 | |
| Maximum (µg/kg) | 7046 | 878 |
| Popping Method | FUM Average (µg/kg) | FUM Average Reduction | FUM Median (µg/kg) | FUM Median Reduction | FUM Interquartile Range (µg/kg) | |
|---|---|---|---|---|---|---|
| All samples | WP | 1351 | 552 b *** | 830 | ||
| MW | 192 | −86% | 54 a *** | −90% | 213 | |
| AIR | 150 | −89% | 37 a *** | −94% | 181 | |
| OIL | 34 | −98% | 0 a *** | −100% | 25 | |
| ALL | 126 | −91% | 25 a *** | −96% | 100 | |
| Butterfly | WP | 1570 | 264 b *** | 2059 | ||
| MW | 213 | −86% | 63 ab *** | −76% | 483 | |
| AIR | 83 | −95% | 0 a *** | −100% | 133 | |
| OIL | 24 | −99% | 0 a *** | −100% | 25 | |
| ALL | 107 | −93% | 0 a *** | −100% | 112 | |
| Mushroom | WP | 1097 | 765 b *** | 573 | ||
| MW | 167 | −85% | 39 a *** | −95% | 96 | |
| AIR | 229 | −79% | 47 a *** | −94% | 164 | |
| OIL | 47 | −96% | 0 a *** | −100% | 19 | |
| ALL | 148 | −87% | 25 a *** | −97% | 69 | |
| Popping Method | FB1 Average (µg/kg) | FB1 Average Reduction | FB1 Median (µg/kg) | FB1 Median Reduction | FB1 Interquartile Range | |
| All samples | WP | 1104 | 466 b *** | 655 | ||
| MW | 159 | −86% | 54 a *** | −88% | 213 | |
| AIR | 125 | −89% | 37 a *** | −92% | 181 | |
| OIL | 32 | −97% | 0 a *** | −100% | 25 | |
| ALL | 105 | −91% | 25 a *** | −95% | 100 | |
| Butterfly | WP | 1277 | 200 b *** | 1541 | ||
| MW | 182 | −86% | 63 ab *** | −69% | 399 | |
| AIR | 78 | −94% | 0 ab *** | −100% | 133 | |
| OIL | 24 | −98% | 0 a *** | −100% | 25 | |
| ALL | 95 | −93% | 0 a *** | −100% | 112 | |
| Mushroom | WP | 901 | 612 b *** | 453 | ||
| MW | 133 | −85% | 39 a *** | −94% | 96 | |
| AIR | 180 | −80% | 47 a *** | −92% | 180 | |
| OIL | 41 | −95% | 0 a *** | −100% | 19 | |
| ALL | 118 | −87% | 25 a *** | −96% | 69 | |
| Popping Method | FB2 Average (µg/kg) | FB2 Reduction | FB2 Median (µg/kg) | FB2 Median Reduction | FB2 Interquartile Range | |
| All samples | WP | 253 | 98 b *** | 182 | ||
| MW | 33 | −87% | 0 a *** | −100% | 15 | |
| AIR | 28 | −89% | 0 a *** | −100% | 0 | |
| OIL | 3 | −99% | 0 a *** | −100% | 0 | |
| ALL | 21 | −92% | 0 a *** | −100% | 0 | |
| Butterfly | WP | 296 | 64 b *** | 330 | ||
| MW | 32 | −89% | 0 ab *** | −100% | 63 | |
| AIR | 8 | −97% | 0 a *** | −100% | 0 | |
| OIL | 0 | −100% | 0 a *** | −100% | 0 | |
| ALL | 13 | −96% | 0 a *** | −100% | 0 | |
| Mushroom | WP | 202 | 121 b *** | 111 | ||
| MW | 34 | −83% | 0 a *** | −100% | 0 | |
| AIR | 52 | −74% | 0 a *** | −100% | 0 | |
| OIL | 6 | −97% | 0 a *** | −100% | 0 | |
| ALL | 31 | −85% | 0 a *** | −100% | 0 |
| Popping Method | DON Average (µg/kg) | DON Average Reduction | DON Median (µg/kg) | DON Median Reduction | DON Interquartile Range | |
|---|---|---|---|---|---|---|
| All samples | WP | 560 | 80 b * | 436 | ||
| MW | 284 | −49% | 50 ab * | −38% | 248 | |
| AIR | 398 | −29% | 98 ab * | +23% | 305 | |
| OIL | 236 | −58% | 0 a * | −100% | 192 | |
| ALL | 304 | −46% | 0 a * | −100% | 266 | |
| Butterfly | WP | 943 | 309 a * | 779 | ||
| MW | 432 | −54% | 117 a * | −62% | 325 | |
| AIR | 646 | −31% | 174 a * | −44% | 541 | |
| OIL | 416 | −56% | 89 a * | −71% | 430 | |
| ALL | 498 | −47% | 143 a * | −54% | 428 | |
| Mushroom | WP | 113 | 50 b * | 73 | ||
| MW | 112 | −1% | 0 ab ** | −100% | 70 | |
| AIR | 95 | −16% | 0 b * | −100% | 133 | |
| OIL | 25 | −78% | 0 a * | −100% | 0 | |
| ALL | 78 | −32% | 0 b * | −100% | 59 |
| Nature of Sample | Mass (kg) |
|---|---|
| Microwave popping (MW) | 0.084 |
| Oil popping (OIL) | 0.25 |
| Air popping (AIR) | 0.25 |
| Without popping (WP) | 0.25 |
| Time | Solvent A (%) | Solvent B (%) |
|---|---|---|
| 0.01 | 90 | 10 |
| 1.50 | 45 | 55 |
| 3.50 | 15 | 85 |
| 4.00 | 15 | 85 |
| 4.01 | 98 | 2 |
| Mycotoxin | m/z | Transition |
|---|---|---|
| DON | 355 →95.1 | Quantifiying |
| DON | 355 → 59 | Confirmation 1 |
| DON | 355 →265.1 | Confirmation 2 |
| FB1 | 722.35 → 334.4 | Quantifiying |
| FB1 | 722.35 → 352.4 | Confirmation 1 |
| FB1 | 722.25 → 141.3 | Confirmation 2 |
| FB2 | 706.2 → 336.4 | Quantifiying |
| FB2 | 706.2 → 318.4 | Confirmation 1 |
| Concentration (µg/L) | |||||
|---|---|---|---|---|---|
| Std 1 | Std 2 | Std 3 | Std 4 | Std 5 | |
| DON | 5 | 50 | 100 | 200 | 500 |
| FB1-FB2 | 2.5 | 25 | 50 | 100 | 250 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schambri, P.; Brunet, S.; Bailly, J.-D.; Kleiber, D.; Levasseur-Garcia, C. Effect of Popcorn (Zea mays var. everta) Popping Mode (Microwave, Hot Oil, and Hot Air) on Fumonisins and Deoxynivalenol Contamination Levels. Toxins 2021, 13, 486. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13070486
Schambri P, Brunet S, Bailly J-D, Kleiber D, Levasseur-Garcia C. Effect of Popcorn (Zea mays var. everta) Popping Mode (Microwave, Hot Oil, and Hot Air) on Fumonisins and Deoxynivalenol Contamination Levels. Toxins. 2021; 13(7):486. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13070486
Chicago/Turabian StyleSchambri, Pierre, Sophie Brunet, Jean-Denis Bailly, Didier Kleiber, and Cecile Levasseur-Garcia. 2021. "Effect of Popcorn (Zea mays var. everta) Popping Mode (Microwave, Hot Oil, and Hot Air) on Fumonisins and Deoxynivalenol Contamination Levels" Toxins 13, no. 7: 486. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13070486






