Critical Review and Conceptual and Quantitative Models for the Transfer and Depuration of Ciguatoxins in Fishes
Abstract
:1. Introduction
2. A Conceptual Model for a Ciguateric Food-Chain in Platypus Bay
2.1. Food Chain Links Leading to Ciguatera in Platypus Bay, Trophic Level 1
2.2. Food Chain Links Leading to Ciguatera in Platypus Bay, Trophic Level 2
2.3. Food Chain Links Leading to Ciguatera in Platypus Bay, Trophic Levels 3 and 4
- The spatial extent of the Cladophora layer varies over time, limiting the available substrate for benthic dinoflagellates;
- And/or, the population of ciguatoxin-producers are sometimes too low for significant amounts of toxin to enter food chains in the bay [77];
- And/or, the profile of the ciguatoxins being produced varies over time, with possibly P-CTX3C congeners less likely to be retained by fishes [71];
- And/or, ciguatoxins are produced, but are not concentrated/transferred into the invertebrate populations being eaten by small predatory fish; and
- And/or, ciguatoxins are not accumulated by small predatory fish (e.g., blotched-javelin) at high enough concentrations to increase their risk of predation.
2.4. Summarizing the Production and Food Chain Transfer of Ciguatoxins in Platypus Bay
- Identifying the resident Gambierdiscus/Fukuyoa species in Platypus Bay;
- Determining the profile and quantities of ciguatoxins produced by these species;
- Identifying the profile and quantities of ciguatoxins that bio-transfer across Platypus Bay trophic levels; and
- Determining the relationship between the spatial extent of the Cladophora substrate and the risk of ciguatera to allow the development of remote sensing imagery as a monitoring tool (e.g., [149]).
3. Model for the Dilution of Ciguatoxins in the Flesh of Spanish Mackerel (S. commerson) through Growth
4. Model for the Dilution of Ciguatoxins in the Flesh of Spanish Mackerel (S. commerson) through Growth and Depuration
5. A conceptual Model for Ciguateric Food-Chains on the Great Barrier Reef
5.1. Food Chain Links Leading to Ciguatera along the Great Barrier Reef, Trophic Level 1
5.2. The Food Chain Links Leading to Ciguatera along the Great Barrier Reef, Trophic Level 2
- Gambierdiscus are common epiphytes of turf algae on the Great Barrier Reef;
- Turf algae are ubiquitous on reefs; and
- Turf algae have high rates of productivity which support high grazing pressure by surgeonfishes on the Great Barrier Reef.
5.3. The Food Chain Links Leading to Ciguatera along the Great Barrier Reef, Trophic Levels 3 and 4
5.4. Summarizing the Production and Food Chain Transfer of Ciguatoxins from the Great Barrier Reef
- What are the Gambierdiscus/Fukuyoa species that produce ciguatoxins on the Great Barrier Reef?
- What is the profile of ciguatoxins produced by these species?
- What ciguatoxins are transferred/transformed along the food chain that leads to the ciguatoxin profile found in demersal reef fish such as coral trout?
- Which of the potential food chains (Figure 10) operate to produce demersal ciguatoxic fish such as coral trout?
6. Model for Dilution of Ciguatoxins in the Flesh of the Common Coral Trout (P. leopardus) through Growth
7. Comparative Risk of Ciguatera Estimated from Catches of Spanish Mackerel and Coral Trout (Plectropomus spp.) along the East Coast of Australia
8. Disturbance and the New Surface Hypothesis for Ciguatera
9. Mitigation of Ciguatera
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lehane, L.; Lewis, R.J. Ciguatera: Recent advances but the risk remains. Int. J. Food Microbiol. 2000, 61, 91–125. [Google Scholar] [CrossRef]
- Skinner, M.P.; Brewer, T.D.; Johnstone, R.; Fleming, L.E.; Lewis, R.J. Ciguatera fish poisoning in the Pacific Islands (1998 to 2008). PLoS Negl. Trop. Dis. 2011, 5, e1416. [Google Scholar] [CrossRef] [PubMed]
- Grant, C. The History of Mauritius, or the Isle of France, and the Neighbouring Islands, from Their First Discovery to the Present Time; Bulmer and Co.: London, UK, 1801; p. 571. Available online: https://books.google.com.au/books?id=0kpBAAAAcAAJ&pg=PR9&source=gbs_selected_pages&cad=2#v=onepage&q&f=false (accessed on 1 July 2021).
- Bagnis, R.; Kuberski, T.; Langier, S. Clinical observations of 3009 cases of ciguatera (fish poisoning) in the South Pacific. Am. J. Trop. Med. Hyg. 1979, 28, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, N.C.; Lewis, R.J.; Pearn, J.H.; Bourke, A.T.C.; Holmes, M.J.; Bourke, J.B.; Shields, W.J. Ciguatera in Australia: Occurrence, clinical features, pathophysiology and management. Med. J. Aust. 1986, 145, 584–590. [Google Scholar] [CrossRef]
- Lewis, R.J. Ciguatera: Australian perspectives on a global problem. Toxicon 2006, 48, 799–809. [Google Scholar] [CrossRef]
- Chinain, M.; Gatti, C.M.; Roué, M.; Darius, H.T. Ciguatera poisoning in French Polynesia: Insights into the novel trends of an ancient disease. New Microbes New Infect. 2019, 31, 565. [Google Scholar] [CrossRef]
- Vetter, I.; Touska, F.; Hess, A.; Hinsbey, R.; Sattler, S.; Lampert, A.; Sergejeva, M.; Sharov, A.; Collins, L.S.; Eberhardt, M.; et al. Ciguatoxins activate specific cold pain pathways to elicit burning pain from cooling. EMBO J. 2012, 31, 3795–3808. [Google Scholar] [CrossRef] [Green Version]
- Lewis, R.J. The changing face of ciguatera. Toxicon 2001, 39, 97–106. [Google Scholar] [CrossRef]
- Inserra, M.C.; Israel, M.R.; Caldwell, A.; Castro, J.; Deuis, J.R.; Harrington, A.M.; Keramidas, A.; Garcia-Caraballo, S.; Maddern, J.; Erickson, A.; et al. Multiple sodium channel isoforms mediate the pathological effects of Pacific ciguatoxin-1. Sci. Rep. 2017, 7, 42810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vetter, I.; Zimmermann, K.; Lewis, R.J. Ciguatera Toxins: Pharmacology, Toxicology, and Detection. In Seafood and Freshwater Toxins. Pharmacology, Physiology and Detection; Botana, L., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 925–950. [Google Scholar]
- Lombet, A.; Bidard, J.N.; Lazdunski, M. Ciguatoxin and brevetoxins share a common receptor site on the neuronal voltage-dependant Na+ channel. FEBS Lett. 1987, 219, 355–359. [Google Scholar] [CrossRef] [Green Version]
- L’Herondelle, K.; Pierre, O.; Fouyet, S.; Leschiera, R.; Le Gall-Ianotto, C.; Phillippe, R.; Buscaglia, P.; Mignen, O.; Talgas, M.; Lewis, R.J.; et al. PAR2, kearatinocytes, and cathepsin S mediate the sensory effects of ciguatoxins responsible for ciguatera poisoning. J. Investig. Dermatol. 2020, 140, 648–658. [Google Scholar] [CrossRef]
- Farrell, H.; Murray, S.A.; Zammit, A.; Edwards, A.W. Management of ciguatera risk in eastern Australia. Toxins 2017, 9, 367. [Google Scholar] [CrossRef] [Green Version]
- Sparrow, L. Key Factors Influencing the Occurrence and Frequency of Ciguatera. Ph.D. Thesis, James Cook University, Townsville, Australia, 2017. [Google Scholar]
- Palafox, N.A.; Jain, L.G.; Pinano, A.Z.; Gulick, T.M.; Williams, R.K.; Schatz, I.J. Successful treatment of ciguatera fish poisoning with intravenous mannitol. JAMA 1988, 259, 2740–2742. [Google Scholar] [CrossRef]
- Pearn, J.H.; Lewis, R.J.; Ruff, T.; Tait, M.; Quinn, J.; Murtha, W.; King, G.; Mallett, A.; Gillespie, N.C. Ciguatera and mannitol: Experience with a new treatment regimen. Med. J. Aust. 1989, 151, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Mullins, M.E.; Hoffman, R.S. Is mannitol the treatment of choice for patients with ciguatera poisoning? Clin. Toxicol. 2017, 55, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Pasinszki, T.; Lako, J.; Dennis, T.E. Advances in detecting ciguatoxins in fishes. Toxins 2020, 12, 494. [Google Scholar] [CrossRef]
- Scheuer, P.J.; Takahashi, W.; Tsutsumi, J.; Yoshida, T. Ciguatoxin: Isolation and chemical nature. Science 1967, 155, 1267–1268. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Legrand, A.M.; Ishibashi, Y.; Fukui, M.; Yasumoto, T. Structure and configurations of ciguatoxin from the moray eel Gymnothorax javanicus and its likely precursor from the dinoflagellate Gambierdiscus toxicus. J. Am. Chem. Soc. 1990, 112, 4380–4386. [Google Scholar] [CrossRef]
- FAO; WHO. Report of the Expert Meeting on Ciguatera Poisoning: Rome, Italy, 19–23 November 2018. Food Saf. Qual. 2020. [Google Scholar] [CrossRef]
- Satake, M.; Ishibashi, Y.; Legrand, A.-M.; Yasumoto, T. Isolation and structure of ciguatoxin-4A, a new ciguatoxin precursor from cultures of dinoflagellate Gambierdiscus toxicus and parrotfish Scarus gibbus. Biosci. Biotechnol. Biochem. 1997, 60, 2103–2105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasumoto, T.; Igarashi, T.; Legrand, A.-M.; Cruchet, P.; Chinain, M.; Fujita, T.; Naoki, H. Structural elucidation of ciguatoxin congeners by fast-atom bombardment tandem mass spectrometry. J. Am. Chem. Soc. 2000, 122, 4988–4989. [Google Scholar] [CrossRef]
- Ikehara, T.; Kuniyoshi, K.; Oshiro, N.; Yasumoto, T. Biooxidation of ciguatoxins leads to species-specific toxin profiles. Toxins 2017, 9, 205. [Google Scholar] [CrossRef] [Green Version]
- Lewis, R.J.; Sellin, M. Multiple ciguatoxins in the flesh of fishes. Toxicon 1992, 30, 915–919. [Google Scholar] [CrossRef]
- Lucas, R.E.; Lewis, R.J.; Taylor, J.M. Pacific ciguatoxin-1 associated with a large common-source outbreak of ciguatera in east Arnhem Land, Australia. Nat. Toxins 1997, 5, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B.; Whittle, N.; Shaw, G.; Eaglesham, G.; Moore, M.R.; Lewis, R.J. Human fatality associated with Pacific ciguatoxin contaminated fish. Toxicon 2010, 56, 668–673. [Google Scholar] [CrossRef]
- Kohli, G.S.; Haslauer, K.; Sarowar, C.; Kretzschmar, A.L.; Boulter, M.; Harwood, D.T.; Laczka, O.; Murray, S.M. Qualitative and quantitative assessment of the presence of ciguatoxin, P-CTX-1b, in Spanish mackerel (Scomberomorus commerson) from waters in New South Wales (Australia). Toxicol. Rep. 2017, 4, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Sellin, M.; Poli, M.A.; Norton, R.S.; MacLeod, J.K.; Sheil, M.M. Purification and characterization of ciguatoxins from moray eel (Lycodontis javanicus, Muraenidae). Toxicon 1991, 29, 1115–1127. [Google Scholar] [CrossRef]
- Yogi, K.; Oshiro, N.; Inafuku, Y.; Hirama, M.; Yasumoto, T. Detailed LC-MS/MS analysis of ciguatoxins revealing distinct regional and species characteristics in fish and causative alga from the Pacific. Anal. Chem. 2011, 83, 8886–8891. [Google Scholar] [CrossRef] [PubMed]
- Mak, Y.L.; Wai, T.-C.; Murphy, M.B.; Chan, W.H.; Wu, J.J.; Lam, J.C.W.; Chan, L.L.; Lam, P.K.S. Pacific ciguatoxins in food web components of coral reef systems in the Republic of Kiribati. Environ. Sci. Technol. 2013, 47, 14070–14079. [Google Scholar] [CrossRef]
- Lewis, R.J.; Holmes, M.J. Origin and transfer of toxins involved in ciguatera. Comp. Biochem. Physiol. 1993, 106, 615–628. [Google Scholar] [CrossRef]
- Vernoux, J.P.; Lewis, R.J. Isolation and characterisation of Caribbean ciguatoxins from the horse-eye jack (Caranx latus). Toxicon 1997, 35, 889–900. [Google Scholar] [CrossRef]
- Lewis, R.J.; Vernoux, J.-P.; Brereton, I.M. Structure of Caribbean ciguatoxin isolated from Caranx latus. J. Am. Chem. Soc. 1998, 120, 5914–5920. [Google Scholar] [CrossRef]
- Kryuchkov, F.; Robertson, A.; Miles, C.O.; Mudge, E.M.; Uhlig, S. LC-HRMS and chemical derivatization strategies for the structure elucidation of Caribbean ciguatoxins: Identification of C-CTX-3 and -4. Mar. Drugs 2020, 18, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, B.; Hurbungs, M.; Vernoux, J.P.; Jones, A.; Lewis, R.J. Isolation and characterisation of Indian Ocean ciguatoxin. Toxicon 2002, 40, 685–693. [Google Scholar] [CrossRef]
- Hamilton, B.; Hurbungs, M.; Jones, A.; Lewis, R.J. Multiple ciguatoxins present in Indian Ocean reef fish. Toxicon 2002, 40, 1347–1353. [Google Scholar] [CrossRef]
- Diogène, J.; Reverté, L.; Rambla-Alegre, M.; del Río, V.; de la Iglesia, P.; Campàs, M.; Palacios, O.; Flores, C.; Caixach, J.; Ralijaona, C.; et al. Identification of ciguatoxins in a shark involved in a fatal food poisoning in the Indian Ocean. Sci. Rep. 2017, 7, 8240. [Google Scholar] [CrossRef]
- Adachi, R.; Fukuyo, Y. The thecal structure of a marine toxic dinoflagellate Gambierdiscus toxicus gen. et sp. nov. collected in a ciguatera-endemic area. Bull. Jpn. Soc. Sci. Fish. 1979, 45, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Holmes, M.J. Gambierdiscus yasumotoi sp. nov. (Dinophyceae), a toxic benthic dinoflagellate from southeastern Asia. J. Phycol. 1998, 34, 661–668. [Google Scholar] [CrossRef]
- Yasumoto, T.; Nakajima, I.; Bagnis, R.; Adachi, R. Finding of a dinoflagellate as a likely culprit for ciguatera. Bull. Jpn. Soc. Sci. Fish. 1977, 43, 1021–1026. [Google Scholar] [CrossRef] [Green Version]
- Yasumoto, T.; Bagnis, R.; Thevenin, S.; Garcon, M. A survey of comparative toxicity in the food chain of ciguatera. Bull. Jpn. Soc. Sci. Fish. 1977, 43, 1015–1019. [Google Scholar] [CrossRef] [Green Version]
- Chinain, M.; Gatti, C.M.; Roué, M.; Darius, H.T. Ciguatera-Causing Dinoflagellates in the Genera Gambierdiscus and Fukuyoa: Distribution, Ecophysiology and Toxicology. In Dinoflagellates; Subba Rao, D.V., Ed.; Nova Science: New York, NY, USA, 2020; pp. 405–457. [Google Scholar]
- Chinain, M.; Darius, H.T.; Ung, A.; Cruchet, P.; Wang, Z.; Ponton, D.; Laurent, D.; Pauillac, S. Growth and toxin production in the ciguatera-causing dinoflagellate Gambierdiscus polynesiensis (Dinophyceae) in culture. Toxicon 2010, 56, 739–750. [Google Scholar] [CrossRef]
- Pisapia, F.; Holland, W.C.; Hardison, D.R.; Litaker, R.W.; Fraga, S.; Nishimura, T.; Adachi, M.; Nguyen-Ngoc, L.; Séchet, V.; Amzil, Z.; et al. Toxicity screening of 13 Gambierdiscus strains using neuro-2a and erythrocyte lysis bioassays. Harmful Algae 2017, 63, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Litaker, R.W.; Holland, W.C.; Hardison, D.H.; Pisapia, F.; Hess, P.; Kibler, S.R.; Tester, P.A. Ciguatoxicity of Gambierdiscus and Fukuyoa species from the Caribbean and Gulf of Mexico. PLoS ONE 2017, 12, e0185776. [Google Scholar] [CrossRef] [PubMed]
- Litaker, R.W.; Vandersea, M.W.; Faust, M.A.; Kibler, S.R.; Chinain, M.; Holmes, M.J.; Holland, W.C.; Tester, P.A. Taxonomy of Gambierdiscus including four new species, Gambierdiscus caribaeus, Gambierdiscus carpenteri and Gambierdiscus ruetzleri (Gonyaucales, Dinophyceae). Phycologia 2009, 48, 344–390. [Google Scholar] [CrossRef]
- Chinain, M.; Faust, M.A.; Pauillac, S. Morphology and molecular analyses of three toxic species of Gambierdiscus (Dinophyceae): G. pacificus, sp. nov., G. australes, sp. nov., and G. polynesiensis, sp. nov. J. Phycol. 1999, 35, 1282–1296. [Google Scholar] [CrossRef]
- Yasumoto, T.; Bagnis, R.; Vernoux, J.-P. Toxicity of the surgeonfishes –II Properties of the principal water-soluble toxin. Bull. Jpn. Soc. Sci. Fish. 1976, 42, 359–365. [Google Scholar] [CrossRef]
- Murata, M.; Naoki, H.; Iwashita, T.; Matsunaga, S.; Sasaki, M.; Yokoyama, A.; Yasumoto, T. Structure of maitotoxin. J. Am. Chem. Soc. 1993, 115, 2060–2062. [Google Scholar] [CrossRef]
- Holmes, M.J.; Lewis, R.J.; Gillespie, N.C. Toxicity of Australian and French Polynesian strains of Gambierdiscus toxicus (Dinophyceae) grown in culture: Characterization of a new type of maitotoxin. Toxicon 1990, 28, 1159–1172. [Google Scholar] [CrossRef]
- Munday, R.; Murray, S.; Rhodes, L.L.; Larsson, M.E.; Harwood, D.T. Ciguatoxins and maitotoxins in extracts of sixteen Gambierdiscus isolates and one Fukuyoa isolate from the South Pacific and their toxicity to mice by intraperitoneal and oral administration. Mar. Drugs 2017, 15, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, M.J.; Lewis, R.J. Purification and characterisation of large and small maitotoxins from cultured Gambierdiscus toxicus. Natural Toxins 1994, 2, 64–72. [Google Scholar] [CrossRef]
- Lewis, R.J.; Holmes, M.J.; Alewood, P.F.; Jones, A. Ionspray mass spectrometry of ciguatoxin-1, maitotoxin-2 and -3, and related marine polyether toxins. Nat. Toxins 1994, 2, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Pisapia, F.; Sibat, M.; Herrenknecht, C.; Lhaute, K.; Gaini, G.; Ferron, P.-J.; Fessard, V.; Fraga, S.; Nascimento, S.M.; Litaker, R.W.; et al. Maitotoxin-4, a novel MTX analog produced by Gambierdiscus excentricus. Mar. Drugs 2017, 15, 220. [Google Scholar] [CrossRef]
- Boente-Juncal, A.; Álvarez, M.; Antelo, Á.; Rodríguez, I.; Calabro, K.; Vale, C.; Thomas, O.P.; Botana, L.M. Structure elucidation and biological evaluation of maitotoxin-3, a homologue of gambierone, from Gambieridiscus belizeanus. Toxins 2019, 11, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, J.S.; Selwood, A.I.; Harwood, D.T.; van Ginkel, R.; Puddick, J.; Rhodes, L.L.; Rise, F. Wilkins Al 44-Methylgambierone, a new gambierone analogue isolated from Gambierdiscus australes. Tetrahedron Lett. 2019, 60, 621–625. [Google Scholar] [CrossRef]
- Longo, S.; Sibat, M.; Viallon, J.; Darius, H.T.; Hess, P.; Chinain, M. Intraspecific variability in the toxin production and toxin profiles of in vitro cultures of Gambierdiscus polynesiensis (Dinophyceae) from French Polynesia. Toxins 2019, 11, 735. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, L.L.; Smith, K.F.; Murray, J.S.; Nishimura, T.; Finch, S.C. Ciguatera fish poisoning: The risk from an Aotearoa/New Zealand perspective. Toxins 2020, 12, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, M.; Leung, P.T.Y.; Gu, J.; Lam, V.T.T.; Murray, J.S.; Harwood, D.T.; Wai, T.-C.; Lam, P.K.S. Hemolysis associated toxicities of benthic dinoflagellates from Hong Kong waters. Mar. Pol. Bull. 2020, 155, 111114. [Google Scholar] [CrossRef]
- Murray, J.S.; Nishimura, T.; Finch, S.C.; Rhodes, L.L.; Puddick, J.; Harwood, D.T.; Larsson, M.E.; Doblin, M.A.; Leung, P.; Yan, M.; et al. The role of 44-methylgambierone in ciguatera fish poisoning: Acute toxicity, production by marine microalgae and its potential as a biomarker for Gambierdiscus spp. Harmful Algae 2020, 97, 101853. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Finch, S.C.; Puddick, J.; Rhodes, L.L.; Harwood, D.T.; van Ginckel, R.; Prinsep, M. Acute Toxicity of gambierone and quantitative analysis of gambierones produced by cohabitating benthic dinoflagellates. Toxins 2021, 13, 333. [Google Scholar] [CrossRef]
- Estevez, P.; Sibat, M.; Leão-Martins, J.M.; Tudó, A.; Rambla-Alegre, M.; Aligizaki, K.; Diogène, J.; Gago-Martinez, A.; Hess, P. Use of mass spectrometry to determine the diversity of toxins produced by Gambierdiscus and Fukuyoa species from Balearic Islands and Crete (Mediterranean Sea) and the Canary Islands (Northeast Atlantic). Toxins 2020, 12, 305. [Google Scholar] [CrossRef]
- Yokoyama, A.; Murata, M.; Oshima, Y.; Iwashita, T.; Yasumoto, T. Some chemical properties of maitotoxin, a putative calcium channel agonist from a marine dinoflagellate. J. Biochem. 1988, 104, 184–187. [Google Scholar] [CrossRef]
- Holmes, M.J.; Lewis, R. Toxin-Producing Dinoflagellates. In Perspectives in Molecular Toxinology; Ménez, A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2002; pp. 39–65. [Google Scholar]
- Holmes, M.J.; Brust, A.; Lewis, R.J. Dinoflagellate Toxins: An overview. In Seafood and Freshwater Toxins. Pharmacology, Physiology and Detection; Botana, L., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 3–38. [Google Scholar]
- Holmes, M.J.; Lewis, R.J.; Poli, M.A.; Gillespie, N.C. Strain dependent production of ciguatoxin precursors (gambiertoxins) by Gambierdiscus toxicus (Dinophyceae) in culture. Toxicon 1991, 29, 761–775. [Google Scholar] [CrossRef]
- Mills, A.R. Poisonous fishes in the South Pacific. J. Trop. Med. Hyg. 1956, 59, 99–103. [Google Scholar]
- Randall, J.E. A review of ciguatera, tropical fish poisoning, with a tentative explanation of its cause. Bull. Mar. Sci. 1958, 8, 236–267. [Google Scholar]
- Ledreux, A.; Brand, H.; Chinain, M.; Bottein, M.-Y.D. Dynamics of ciguatoxins from Gambierdiscus polynesiensis in the benthic herbivore Mugil cephalus: Trophic transfer implications. Harmful Algae 2014, 39, 165–174. [Google Scholar] [CrossRef]
- Li, J.; Mak, Y.L.; Chang, Y.-H.; Xiao, C.; Chen, Y.-M.; Shen, J.; Wang, Q.; Ruan, Y.; Lam, P.K.S. Uptake and depuration kinetics of Pacific ciguatoxins in orange-spotted grouper (Epinephelus coioides). Environ. Sci. Technol. 2020, 54, 4475–4483. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Henao, A.; García-Àlvarez, N.; Padilla, D.; Ramos-Sosa, M.; Sergent, F.S.; Fernández, A.; Estévez, P.; Gago-Martínez, A.; Diogène, J.; Real, F. Accumulation of C-CTX1 in muscle tissue of goldfish (Carassius auratus) by dietary exposure. Animals 2021, 11, 242. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Endean, R. Purification of ciguatoxin-like material from Scomberomorus commersoni and its effect on the rat phrenic nerve-diaphragm. Toxicon Suppl. 1983, 3, 249–252. [Google Scholar] [CrossRef]
- Lewis, R.J.; Endean, R. Ciguatoxin from the flesh and viscera of the barracuda, Sphyraena jello. Toxicon 1984, 22, 805–810. [Google Scholar] [CrossRef]
- Lewis, R.J.; Chaloupka, M.Y.; Gillespie, N.C.; Holmes, M.J. An Analysis of the Human Response to Ciguatera in Australia. In Proceedings of the 6th International Coral Reef Symposium Executive Committee, Townsville, Australia, 8–12 August 1988; Choat, J.H., Barnes, D., Borowitzka, M.A., Coll, J.C., Davies, P.J., Flood, P., Hatcher, B.G., Hopley, D., Hutchings, P.A., Kinsey, D., et al., Eds.; Townsville, Australia, 1988; Volume 3, pp. 67–72. [Google Scholar]
- Holmes, M.; Lewis, R.J.; Sellin, M.; Street, R. The origin of ciguatera in Platypus Bay, Australia. Mem. Qld Mus. 1994, 34, 505–512. [Google Scholar]
- Lewis, R.J. Ciguatera in South-Eastern Queensland. In Toxic Plants and Animals: A Guide for Australia; Covacevich, J., Davie, P., Pearn, J., Eds.; Queensland Museum: Brisbane, Australia, 1987; pp. 181–187. [Google Scholar]
- Buckworth, R.C.; Newman, S.J.; Ovenden, J.R.; Lester, R.J.G.; McPherson, G.R. The Stock Structure of Northern and Western Australian Spanish Mackerel; Final Report, Fisheries Research & Development Corporation Project 1988/159; Fishery Report 88; Department of Primary Industry, Fisheries and Mines: Northern Territory Government, Australia, 2007; Volume i–vi, p. 225.
- Lewis, R.J. Ciguatera and Ciguatoxin-Like Substances in Fishes, Especially Scomberomorus commersoni from Southern Queensland. Ph.D. Thesis, University of Queensland, Brisbane, Australia, 1985. [Google Scholar]
- Chaloupka, M.Y.; Lewis, R.J.; Sellin, M. The changing face of ciguatera prevalence. Mem. Qld Mus. 1994, 34, 554. [Google Scholar]
- McPherson, G.R. Age and growth of the narrow-barred Spanish mackerel (Scomberomorus commerson Lacépède, 1800) in north-eastern Queensland waters. Aust. J. Mar. Freshw. Res. 1992, 43, 1269–1282. [Google Scholar] [CrossRef]
- O’Neill, M.F.; Langstreth, J.; Buckley, S.M.; Stewart, J. Stock Assessment of Australian East Coast Spanish Mackerel: Predictions of Stock Status and Reference Points; Queensland Government Report: Brisbane, Australia, 2018; p. 103.
- Lewis, R.J.; Holmes, M.J.; Sellin, M. Invertebrates implicated in the transfer of gambiertoxins to the benthic carnivore Pomadasys maculatus. Mem. Qld Mus. 1994, 34, 561–564. [Google Scholar]
- McKay, R.J. Classification of the grunters and javelin-fishes of Australia. Aust. Fish. 1984, 43, 37–40. [Google Scholar]
- Lewis, R.J.; Sellin, M.; Street, R.; Holmes, M.J.; Gillespie, N.C. Excretion of ciguatoxin from Moray eels (Muraenidae) of the central Pacific. In Proceedings of the Third International Conference on Ciguatera Fish Poisoning, La Parguera, Puerto Rico; Tosteson, T.R., Ed.; Polyscience Publications: Quebec City, QC, Canada, 1992; pp. 131–143. [Google Scholar]
- Chan, W.H.; Mak, Y.L.; Wu, J.J.; Jin, L.; Sit, W.H.; Lam, J.C.W.; de Mitcheson, Y.S.; Chan, L.L.; Lam, P.K.S.; Murphy, M.B. Spatial distribution of ciguateric fish in the Republic of Kiribati. Chemosphere 2011, 84, 117–123. [Google Scholar] [CrossRef]
- Holmes, M.J.; Lewis, R.J. Multiple Gambiertoxins (ciguatoxin precursors) from an Australian Strain of Gambierdiscus toxicus in Culture. In Recent Advances in Toxinology Research; Gopalakrishnakone, P., Tan, C.K., Eds.; National University of Singapore: Singapore, 1992; Volume 2, pp. 520–529. [Google Scholar]
- Holmes, M.J.; Lewis, R.J. The origin of ciguatera. Mem. Qld Mus. 1994, 34, 497–504. [Google Scholar]
- Gillespie, N.C.; Holmes, M.J.; Burke, J.B.; Doley, J. Distribution and Periodicity of Gambierdiscus toxicus in Queensland, Australia. In Toxic Dinoflagellates; Anderson, D.M., White, A.W., Baden, D.G., Eds.; Elsevier: Oxford, UK, 1985; pp. 183–188. [Google Scholar]
- Gillespie, N.C.; Lewis, R.J.; Burke, J.; Holmes, M. The Significance of the Absence of Ciguatoxin in a Wild Population of G. toxicus. In Proceedings of the Fifth International Coral Reef Congress, Tahiti, France, 27 May–1 June 1985; Gabrie, C., Salvat, B., Eds.; Antenne Museum-Ephe: Moorea, France, 1985; pp. 437–441. [Google Scholar]
- Lewis, R.J.; Gillespie, N.C.; Holmes, M.J.; Burke, J.B.; Keys, A.B.; Fifoot, A.T.; Street, R. Toxicity of Lipid-Soluble Extracts from Demersal Fishes at Flinders Reef, Southern Queensland. In Proceedings of the 6th International Coral Reef Symposium Executive Committee, Townsville, Australia, 8–12 August 1988; Volume 3, pp. 61–65. [Google Scholar]
- Kohli, G.S.; Murray, S.A.; Neilan, B.A.; Rhodes, L.L.; Harwood, T.; Smith, K.F.; Meyer, L.; Capper, A.; Brett, S.; Hallegraeff, G.M. High abundance of the potentially maitotoxic dinoflagellate Gambierdiscus carpenteri in temperate waters of New South Wales, Australia. Harmful Algae 2014, 39, 134–145. [Google Scholar] [CrossRef]
- Bagnis, R.; Bennett, J.; Prieur, C.; Legrand, A.M. The Dynamics of Three Benthic Dinoflagellates and the Toxicity of Ciguateric Surgeonfish in French Polynesia. In Toxic Dinoflagellates; Anderson, D.M., White, A.W., Baden, D.G., Eds.; Elsevier: Oxford, UK, 1985; pp. 177–182. [Google Scholar]
- Chinain, M.; Germain, M.; Deparis, X.; Pauillac, S.; Legrand, A.-M. Seasonal abundance and toxicity of the dinoflagellate Gambierdiscus spp. (Dinophyceae), the causative agent of ciguatera in Tahiti, French Polynesia. Mar. Biol. 1999, 135, 259–267. [Google Scholar] [CrossRef]
- Parsons, M.L.; Settlemier, C.J.; Bienfang, P.K. A simple model capable of simulating the population dynamics of Gambierdiscus, the benthic dinoflagellate responsible for ciguatera fish poisoning. Harmful Algae 2010, 10, 71–80. [Google Scholar] [CrossRef]
- Liefer, J.D.; Richlen, M.L.; Smith, T.B.; DeBose, J.L.; Xu, Y.; Anderson, D.M.; Robertson, A. Asynchrony of Gambierdiscus spp. abundance and toxicity in the U.S. Virgin Islands: Implications for monitoring and management of ciguatera. Toxins 2021, 13, 413. [Google Scholar] [CrossRef]
- Gaiani, G.; Toldrà, A.; Andree, K.B.; Rey, M.; Diogène, J.; Alcaraz, C.; O’Sullivan, C.K.; Campàs, M. Detection of Gambierdiscus and Fukuyoa single cells using recombinase polymerase amplification combined with a sandwich hybridization assay. J. App. Phycol. 2021. [Google Scholar] [CrossRef]
- Wu, Z.; Luo, H.; Yu, L.; Lee, W.H.; Li, L.; Mak, Y.L.; Lin, S.; Lam, P.K.S. Characterizing ciguatoxin (CTX)- and non-CTX-producing strains of Gambierdiscus balechii using comparative transcriptomics. Sci. Total Environ. 2020, 717, 137184. [Google Scholar] [CrossRef] [PubMed]
- Van Dolah, F.M.; Morey, J.S.; Milne, S.; Ung, A.; Anderson, P.E.; Chinain, M. Transcriptomic analysis of polyketide synthases in a highly ciguatoxic dinoflagellate, Gambierdiscus polynesiensis and low toxicity Gambierdiscus pacificus, from French Polynesia. PLoS ONE 2020, 15, e0231400. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Yao, M.; Zhou, J.; Tan, S.; Jin, H.; Zhang, F.; Mal, Y.L.; Wu, J.; Chan, L.L.; Cai, Z. Growth and toxin production of Gambierdiscus spp. can be regulated by quorum-sensing bacteria. Toxins 2018, 10, 257. [Google Scholar] [CrossRef] [Green Version]
- Clausing, R.J.; Losen, B.; Oberhaensli, F.R.; Darius, H.T.; Sibat, M.; Hess, P.; Swarzenski, P.W.; Chinain, M.; Bottein, M.-Y.D. Experimental evidence of dietary ciguatoxin accumulation in an herbivorous coral reef fish. Aquat. Toxicol. 2018, 200, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Yon, T.; Sibat, M.; Réveillon, D.; Bertrand, S.; Chinain, M.; Hess, P. Deeper insight into Gambierdiscus polynesiensis toxin production relies on specific optimization of high-performance liquid chromatography-high resolution mass spectrometry. Talanta 2021, 232, 122400. [Google Scholar] [CrossRef]
- Gräwe, U.; Wolff, J.-O.; Ribbe, J. Mixing, hypersalinity and gradients in Hervey Bay. Ocean Dyn. 2009, 59, 643–658. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, M.; Roder, C.A.; Roelofs, A.J.; Lee Long, W.J. Post-Flood Monitoring of Seagrasses in Hervey Bay and the Great Sandy Strait, 1999: Implications for Dugong, Turtle & Fisheries Management; DPI Information Series QI00059: Cairns, Australia, 2000; p. 46. [Google Scholar]
- Meager, J.J.; Limpus, C.J.; Sumpton, W. A Review of the Population Dynamics of Dugongs in Southern Queensland 1830–2012; Department of Environment and Heritage Protection, Queensland Government: Brisbane, Australia, 2013; p. 29.
- Larsson, M.E.; Laczka, O.F.; Harwood, D.T.; Lewis, R.J.; Himaya, S.W.A.; Murray, S.A.; Doblin, M.A. Toxicology of Gambierdiscus spp. (Dinophyceae) from tropical and temperate Australian waters. Mar. Drugs 2018, 16, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landsberg, J.H.; Steidinger, K.A. A Historical Review of Gymnodinium brevis Red Tide Implicated in Mass Mortalities of the Manatee (Trichechus manatus latirostris) in Florida, USA. In Harmful Algae, Proceedings of the VIII International Conference on Harmful Algae, Vigo, Spain, 25–29 June 1997; Reguera, B., Blanco, J., Fernández, M.L., Wyatt, T., Eds.; Xunta de Galicia and International Oceanographic Commission of UNESCO: Santiago de Compostela, Spain, 1998; pp. 97–100. [Google Scholar]
- Fire, S.E.; Flewelling, L.J.; Naar, J.; Twiner, M.J.; Henry, M.S.; Pierce, R.H.; Gannon, D.P.; Wang, Z.; Davidson, L.; Wells, R.S. Prevalence of brevetoxins in prey fish of bottlenose dolphins in Sarasota Bay, Florida. Mar. Ecol. Prog. Ser. 2008, 368, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Bottein, M.-Y.D.; Kashinsky, L.; Wang, Z.; Littnan, C.; Ramsdell, J.S. Identification of ciguatoxins in Hawaiian Monk seals Monachus schauinslandi from the northwestern and main Hawaiian islands. Env. Sci. Technol. 2011, 45, 5403–5409. [Google Scholar] [CrossRef] [PubMed]
- Brieva, D.; Ribbe, J.; Lemckert, C. Is the East Australian Current causing a marine ecological hot-spot and an important fisheries near Fraser Island, Australia? Est. Coast. Shelf. Sci. 2015, 153, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, C.; Lawrey, E. Circulation and Upwelling, Introduction to Circulation and Upwelling and Why It Is Important. 2018. Available online: https://eatlas.org.au/ne-aus-seascape-connectivity/circulation-upwelling (accessed on 20 July 2021).
- Preen, A.R.; Lee Long, W.J.; Coles, R.G. Flood and cyclone related loss, and partial recovery, of more than 1000 km2 of seagrass in Hervey Bay, Queensland, Australia. Aquat. Bot. 1995, 52, 3–17. [Google Scholar] [CrossRef]
- Waterhouse, J.; Schaffelke, B.; Bartley, R.; Eberhard, R.; Brodie, J.; Star, M.; Thorburn, P.; Rolfe, J.; Ronan, M.; Taylor, B.; et al. Scientific Consensus Statement: Land Use Impacts on Great Barrier Reef Water Quality and Ecosystem Condition; Queensland Government: Brisbane, Australia, 2017. Available online: https://www.reefplan.qld.gov.au/__data/assets/pdf_file/0029/45992/2017-scientific-consensus-statement-summary.pdf (accessed on 20 July 2021).
- Brodie, J.E.; Baird, M.; Mongin, M.; Skerrat, J.; Waterhouse, J.; Robillot, C.; Smith, R.; Mann, R.; Warne, M. Development of Basin Specific Ecologically Relevant Targets; Report Submitted to the Reef Plan Independent Science Panel; TropWater, James Cook University: Townsville, QLD, Australia, 2017; p. 53. [Google Scholar]
- Baird, M.E.; Mongin, M.; Skerratt, J.; Margvelashvili, N.; Tickell, S.; Steven, A.D.L.; Robillot, C.; Ellis, R.; Waters, D.; Kaniewska, P.; et al. Impact of catchment-derived nutrients and sediments on marine water quality on the Great Barrier Reef: An application of the eReefs marine modelling system. Mar. Pol. Bull. 2021, 167, 112297. [Google Scholar] [CrossRef]
- Loeffler, C.R.; Richlen, M.L.; Brandt, M.E.; Smith, T.B. Effects of grazing, nutrients, and depth on the ciguatera-causing dinoflagellate Gambierdiscus in the US Virgin Islands. Mar. Ecol. Prog. Ser. 2015, 531, 91–104. [Google Scholar] [CrossRef]
- McMahon, K.; Nash, S.B.; Eaglesham, G.; Müller, J.F.; Duke, N.C.; Winderlich, S. Herbicide contamination and the potential impact to seagrass meadows in Hervey Bay, Queensland, Australia. Mar. Poll. Bull. 2005, 51, 325–334. [Google Scholar] [CrossRef]
- Davis, A.M.; Lewis, S.E.; Bainbridge, Z.T.; Glendenning, L.; Turner, R.D.R.; Brodie, J.E. Dynamics of herbicide transport and portioning under event flow conditions in the lower Burdekin region, Australia. Mar. Poll. Bull. 2012, 65, 182–193. [Google Scholar] [CrossRef]
- Magnusson, M.; Heimann, K.; Ridd, M.; Negri, A.P. Chronic herbicide exposures affect the sensitivity and community structure of tropical benthic microalgae. Mar. Poll. Bull. 2012, 65, 363–372. [Google Scholar] [CrossRef]
- Wood, R.J.; Mitrovic, S.M.; Lim, R.P.; Warne, M.S.t.J.; Dunlop, J.; Kefford, B.J. Benthic diatoms as indicators of herbicide toxicity in rivers–A new Species at Risk (SPEARherbicides) index. Ecol. Indic. 2019, 99, 203–213. [Google Scholar] [CrossRef]
- Holmes, M.J.; Lewis, R.J.; Jones, A.; Hoy, A.W. Cooliatoxin, the first toxin from Coolia monotis (Dinophyceae). Nat. Toxins 1995, 3, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Mohammad-Noor, N.; Moestrup, Ø.; Lundholm, N.; Fraga, S.; Adam, A.; Holmes, M.J.; Saleh, E. Autecology and phylogeny of Coolia tropicalis and Coolia malayensis (Dinophyceae), with emphasis on taxonomy of C. tropicalis based on light microscopy, scanning electron microscopy and LSU rDNA(1). J. Phycol. 2013, 49, 536–545. [Google Scholar] [CrossRef]
- de Azevedo Tibiriçá, C.E.J.; Sibat, M.; Fernandes, L.F.; Bilien, G.; Chomerat, N.; Hess, P. Diversity and toxicity of the genus Coolia Meunier in Brazil, and detection of 44-methyl gambierone in Coolia tropicalis. Toxins 2020, 12, 327. [Google Scholar] [CrossRef]
- Momigliano, P.; Sparrow, L.; Blair, D.; Heimann, K. The diversity of Coolia spp. (Dinophyceae Ostreopsidaceae) in the Central Great Barrier Reef Region. PLoS ONE 2013, 8, e79278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsson, M.E.; Smith, K.F.; Dobin, M.A. First description of the environmental niche of the epibenthic dinoflagellate species Coolia palmyrensis, C. malayensis, and C. tropicalis (Dinophyceae) from Eastern Australia. J. Phycol. 2019, 55, 565–577. [Google Scholar] [CrossRef] [Green Version]
- Horn, M. Biology of marine herbivorous fishes. Oceanogr. Mar. Biol. Annu. Rev. 1989, 27, 167–172. [Google Scholar]
- Rongo, T.; van Woesik, R. Ciguatera poisoning in Rarotonga, southern Cook Islands. Harmful Algae 2011, 10, 345–355. [Google Scholar] [CrossRef]
- Kelly, A.M.; Kohler, C.C.; Tindall, D.R. Are crustaceans linked to the ciguatera food chain? Environ. Biol. Fishes 1992, 33, 275–286. [Google Scholar] [CrossRef]
- Yasumoto, T.; Kanno, K. Occurrence of toxins resembling ciguatoxin, scaritoxin and maitotoxin in a turban shell. Bull. Jpn. Soc. Sci. Fish. 1976, 42, 1399–1404. [Google Scholar] [CrossRef]
- Roué, M.; Darius, H.T.; Picot, S.; Ung, A.; Viallon, J.; Gaertner-Mazouni, N.; Sibat, M.; Amzil, Z.; Chinain, M. Evidence of the bioaccumulation of ciguatoxins in giant clams (Tridacna maxima) exposed to Gambierdiscus spp. Cells. Harmful Algae 2016, 57, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.; Rodriguez, I.; Barreiro, A.; Kaufmann, M.; Neto, A.I.; Hassouani, M.; Sabour, B.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. First report of ciguatoxins in two starfish species: Ophidiaster ophidianus and Marthasterias glacialis. Toxins 2015, 7, 3740–3757. [Google Scholar] [CrossRef] [Green Version]
- Darius, H.T.; Roue, M.; Sibat, M.; Viallon, J.; Gatti, C.M.; Vandersea, M.W.; Tester, P.A.; Litaker, R.W.; Amzil, Z.; Hess, P.; et al. Tectus niloticus (Teguilidae, Gastropod) as a novel vector of ciguatera poisoning: Detection of Pacific ciguatoxins in toxic samples from Nuku Hiva island (French Polynesia). Toxins 2018, 10, 2. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Ascenio, L.; Clausing, R.J.; Vandersea, M.; Chamero-Lago, D.; Gómez-Batista, M.; Hernández-Albernas, J.I.; Chomérat, N.; Rojas-Abrahantes, G.; Litaker, R.W.; Tester, P.; et al. Ciguatoxin occurrence in food-web components of a Cuban coral reef ecosystem: Risk-assessment implications. Toxins 2019, 11, 722. [Google Scholar] [CrossRef] [Green Version]
- Neves, R.A.F.; Fernandes, T.; dos Santos, L.N.; Nascimento, M. Toxicity of benthic dinoflagellates on grazing, behavior and survival of the brine shrimp Artemia salina. PLoS ONE 2017, 12, e0175168. [Google Scholar] [CrossRef] [Green Version]
- Chinain, M.; Darius, H.T.; Ung, A.; Fouc, M.T.; Revel, T.; Cruchet, P.; Paullac, S.; Laurent, D. Ciguatera risk management in French Polynesia: The case study of Raivavae Island (Australes Archipelago). Toxicon 2010, 56, 674–690. [Google Scholar] [CrossRef]
- Gaboriau, M.; Ponton, D.; Darius, H.T.; Chinain, M. Ciguatera fish toxicity in French Polynesia: Size does not always matter. Toxicon 2014, 84, 41–50. [Google Scholar] [CrossRef]
- Capra, M.F.; Cameron, J.; Flowers, A.E.; Coombe, I.F.; Blanton, C.G.; Hahn, S.T. The Effects of Ciguatoxin on Teleosts. In Proceedings of the 6th International Coral Reef Symposium, Townsville, Australia, 8–12 August 1988; Volume 3, pp. 37–41. [Google Scholar]
- Davin, W.T.; Kohler, C.C.; Tindall, D.R. Ciguatera toxins adversely affect piscivorous fishes. Trans. Am. Fish. Soc. 1988, 117, 374–384. [Google Scholar] [CrossRef]
- Lewis, R.J. Ciguatoxins are potent ichthyotoxins. Toxicon 1992, 30, 207–211. [Google Scholar] [CrossRef]
- QFish. Available online: http://qfish.fisheries.qld.gov.au/ (accessed on 20 July 2021).
- Tonge, J.I.; Battey, Y.; Forbes, J.J.; Grant, E.M. Ciguatera poisoning: A report of two outbreaks and a probable fatal case in Queensland. Med. J. Aust. 1967, 2, 1088–1090. [Google Scholar] [CrossRef]
- Tobin, A.; Heupel, M.; Simpfendorfer, C.; Buckley, S.; Thustan, R.; Pandolfi, J. Utilizing Innovative Technology to Better Understand Spanish Mackerel Spawning Aggregations and the Protection Offered by Marine Protected Areas; Centre for Sustainable Tropical Fisheries and Aquaculture, James Cook University: Townsville, Australia, 2014; p. 70. [Google Scholar]
- Tobin, A.; Maplestone, A. Exploitation Dynamics and Biological Characteristics of the Queensland East Coast Spanish Mackerel (Scomberomorus commerson) Fishery; CRC Reef Research Centre Technical Report No 51; CRC Reef Research Centre: Townsville, Australia, 2004; p. 61. [Google Scholar]
- Champion, C.; Brodie, S.; Coleman, M.A. Climate-driven range shifts are rapid yet variable among recreationally important coastal-pelagic fisheries. Front. Mar. Sci. 2021, 8, 159. [Google Scholar] [CrossRef]
- Espinoza, M.; Matley, J.; Heupel, M.R.; Tobin, A.J.; Fisk, A.T.; Simpfendorfer, C.A. Multi-tissue stable isotope analysis reveals resource partitioning and trophic relationships of large reef-associated predators. Mar. Ecol. Prog. Ser. 2019, 615, 159–176. [Google Scholar] [CrossRef]
- Wortmann, J.; O’Neill, M.F.; Sumpton, W.; Campbell, M.J.; Stewart, J. Stock Assessment of Australian east Coast Snapper, Chrysophrys auratus: Predictions of Stock Status and Reference Points for 2016; Queensland Government Report: Brisbane, Australia, 2018; p. 122.
- Leigh, G.M.; Yang, W.-H.; O’Neill, M.F.; McGilvray, J.G.; Wortmann, J. Stock Assessments of Bream, Whiting and Flathead (Acanthopagrus australis, Sillago ciliata and Platycephalus fuscus) in South East Queensland; Fisheries Queensland, Department of Agriculture and Fisheries: Brisbane, Australia, 2019; p. 197.
- Roelfsema, C.M.; Kovacs, E.M.; Ortiz, J.C.; Callaghan, D.P.; Hock, K.; Mongin, M.; Johansen, K.; Mumby, P.J.; Wettle, M.; Ronan, M.; et al. Habitat maps to enhance monitoring and management of the Great Barrier Reef. Coral Reefs 2020, 39, 1039–1054. [Google Scholar] [CrossRef]
- Yang, Z.; Luo, Q.; Liang, Y.; Mazumder, A. Processes and pathways of ciguatoxin in aquatic food webs and fish poisoning of seafood consumers. Environ. Rev. 2016, 150, 144–150. [Google Scholar] [CrossRef] [Green Version]
- Halstead, B.W.; Bunker, N.C. A survey of the poisonous fishes of the Phoenix Islands. Copeia 1954, 1, 1–11. [Google Scholar] [CrossRef]
- Banner, A.H.; Helfrich, P.; Piyakarnchana, T. Retention of ciguatera toxin by the red snapper, Lutjanus bohar. Copeia 1966, 2, 297–301. [Google Scholar]
- Cooper, M.J. 1964 Ciguatera and other marine poisoning in the Gilbert Islands. Pac. Sci. 1964, 4, 411–440. [Google Scholar]
- USFDA. 2021 Natural Toxins. In Fish and Fishery Product Hazards and Controls Guidance, 4th ed.; 2021; Table A-5; p. A5-11. Available online: http://www.fda.gov/media/80637/download (accessed on 20 July 2021).
- Ferreira, B.P.; Russ, G.R. Age validation and estimation of growth rate of the coral trout, Plectropomus leopardus, (Lacepede 1802) from Lizard Island, norther Great Barrier Reef. Fish. Bull. 1994, 92, 46–57. [Google Scholar]
- Begg, G.A.; Chen, C.C.-M.; O’Neill, M.F.O.; Rose, D.B. Stock Assessment of the Torres Strait Spanish Mackerel Fishery; CRC Reef Research Centre Technical Report No. 66; CRC Reef Research Centre: Townsville, Australia, 2006; p. 81. [Google Scholar]
- Campbell, A.B.; O’Neill, M.F.; Staunton-Smith, J.; Atfield, J.; Kirkwood, J. Stock Assessment of the Australian East Coast Spanish Mackerel (Scomberomorus commerson) Fishery; Department of Employment, Economic Development and Innovation, Queensland Government: Brisbane, Australia, 2012; p. 138.
- Oshiro, N.; Nagaswa, H.; Kuniyoshi, K.; Kobayashi, N.; Sugita-Konishi, Y.; Asakura, H.; Yasumoto, T. Characteristic distribution of ciguatoxins in the edible parts of a grouper, Variola louti. Toxins 2021, 13, 218. [Google Scholar] [CrossRef]
- Vernoux, J.-P.; Gaign, M.; Riyeche, N.; Tagmouti, F.; Magras, L.P.; Nolen, J. Mise en evidence d’une toxine liposoluble de type ciguatérique chez Caranx bartholomaei pêché aux Antilles françaises. Biochimie 1982, 64, 933–939. [Google Scholar] [CrossRef]
- Helfrich, P.; Piyakarnchana, T.; Miles, P. Ciguatera fish poisoning I: The ecology of ciguateric reef fishes in the Line Islands. Occ. Pap. Bernice P. Bishop Mus. 1968, 23, 305–370. [Google Scholar]
- Banner, A.H. Ciguatera: A Disease from Coral Reef Fish. In Biology and Geology of Coral Reefs; Jones, A.O., Endean, R., Eds.; Academic Press: London, UK, 1976; Volume 3, pp. 177–212. [Google Scholar]
- Mackie, M.; Gaughan, D.J.; Buckworth, R.C. Stock assessment of narrow-barred Spanish mackerel (Scomberomorus commerson) in Western Australia. FRDC Proj. 2003, 151, 242. [Google Scholar]
- O’Toole, A.C.; Bottein, M.-Y.D.; Danylchuk, A.J.; Ramsdell, J.S.; Cooke, S.J. Linking ciguatera poisoning to spatial ecology of fish: A novel approach to examining the distribution of biotoxin levels in the great barracuda by combining non-lethal blood sampling and biotelemetry. Sci. Total Environ. 2012, 427–428, 98–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helfrich, P.; Banner, A.H. Experimental induction of ciguatera toxicity in fish through diet. Nature 1963, 197, 1025–1026. [Google Scholar] [CrossRef]
- Chan, T.Y.K. Regional variations in the risk and severity of ciguatera caused by eating moray eels. Toxins 2017, 9, 201. [Google Scholar] [CrossRef] [Green Version]
- Hahn, S.T.; Capra, M.F.; Walsh, T.P. Ciguatoxin-protein association in skeletal muscle of Spanish mackerel (Scomberomorus commersoni). Toxicon 1992, 30, 843–852. [Google Scholar] [CrossRef]
- Jiang, X.-W.; Li, X.; Lam, P.K.S.; Cheng, S.H.; Schlenk, D.; de Mitcheson, Y.S.; Li, Y.; Gu, J.-D.; Chan, L.L. Proteomic analysis of hepatic tissue of ciguatoxin (CTX) contaminated coral reef fish Cephalopholis argus and the moray eel Gymnothorax undulatus. Harmful Algae 2012, 13, 65–71. [Google Scholar] [CrossRef]
- Hossen, V.; Soliño, L.; Leroy, P.; David, E.; Velge, P.; Dragacci, S.; Krys, S.; Quintana, H.F.; Diogène, J. Contribution to the risk characterization of ciguatoxins: LOAEL estimated from eight ciguatera fish poisoning events in Guadeloupe (French West Indies). Environ. Res. 2015, 143, 100–108. [Google Scholar] [CrossRef]
- Dalzell, P. Management of ciguatera fish poisoning in the South Pacific. Mem. Qld. Mus. 1994, 34, 471–479. [Google Scholar]
- Tosteson, T.R.; Ballantine, D.L.; Durst, H.D. Seasonal frequency of ciguatoxic barracuda in southwest Puerto Rico. Toxicon 1988, 26, 795–801. [Google Scholar] [CrossRef]
- Ginsberg, G.; Hattis, D.; Sonawane, B.; Russ, A.; Banati, P.; Kozlak, M.; Smolenski, S.; Goble, R. Evaluation of child/adult pharmacokinetic differences from a database derived from the therapeutic drug literature. Toxicol. Sci. 2002, 66, 185–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovett, R.A.; Prosser, A.; Leigh, G.M.; O’Neill, M.F.; Stewart, J. Stock Assessment of the Australian East Coast Sea Mullet (Mugil cephalus) Fishery; Queensland Department of Agriculture and Fisheries Report: Brisbane, Australia, 2018; p. 80.
- Bell, P.A.; O’Neill, M.F.; Leigh, G.M.; Courtney, A.J.; Peel, S.L. Stock Assessment of the Queensland-New South Wales Sea Mullet Fishery (Mugil Cephalus); Queensland Department of Primary Industries and Fisheries: Brisbane, Australia, 2005; p. 93.
- Yogi, K.; Sakugawa, S.; Oshiro, N.; Ikehara, T.; Sugiyama, K.; Yasumoto, T. Determination of toxins involved in ciguatera fish poisoning in the Pacific by LC/MS. J. AOAC 2014, 97, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Sellin, M.; Gillespie, N.C.; Holmes, M.J.; Keys, A.; Street, R.; Smythe, H.; Thaggard, H.; Bryce, S. Ciguatera and herbivores: Uptake and accumulation of ciguatoxins in Ctenochaetus striatus on the Great Barrier Reef. Mem. Qld Mus. 1994, 34, 565–570. [Google Scholar]
- Colman, J.R.; Bottein, M.-Y.D.; Dickey, R.W.; Ramsdell, J.S. Characterization of the developmental toxicity of Caribbean ciguatoxins in finfish embryos. Toxicon 2004, 44, 59–66. [Google Scholar] [CrossRef]
- Yan, M.; Mak, M.Y.L.; Cheng, J.; Li, J.; Gu, J.R.; Leung, P.T.Y.; Lam, P.K.S. Effects of dietary exposure to ciguatoxin P-CTX-1 on the reproductive performance in marine medaka (Oryzias melastigma). Mar. Poll. Bull. 2020, 152, 110837. [Google Scholar] [CrossRef]
- Mak, Y.L.; Li, J.; Liu, C.-N.; Cheng, S.H.; Lam, P.K.S.; Cheng, J.; Chan, L.L. Physiological and behavioural impacts of Pacific ciguatoxin-1 (P-CTX-1) in marine medaka (Oryzias melastigma). J. Hazard. Mater. 2017, 321, 782–790. [Google Scholar] [CrossRef]
- Yan, M.; Leung, P.T.Y.; Ip, J.C.H.; Cheng, J.-P.; Wu, J.-J.; Gu, J.-R.; Lam, P.K.S. Developmental toxicity and molecular responses of marine medaka (Oryzias melastigma) embryos to ciguatoxin P-CTX-1 -exposure. Aquat. Toxicol. 2017, 185, 149–159. [Google Scholar] [CrossRef]
- Emslie, M.J.; Cheal, A.J.; Logan, M. The distribution and abundance of reef-associated predatory fishes on the Great Barrier Reef. Coral Reefs 2017, 36, 829–846. [Google Scholar] [CrossRef]
- Gillespie, N.C. Possible origins of ciguatera. In Toxic Plants and Animals: A Guide for Australia; Covacevich, J., Davie, P., Pearn, J., Eds.; Queensland Museum: Brisbane, Austrilia, 1987; pp. 170–179. [Google Scholar]
- Bagnis, R. Naissance et développment d’une flambée de ciguatera dans un aoll des Tuamotu. Revue Corps Santé 1969, 10, 783–795. [Google Scholar]
- Cheal, A.J.; MacNeill, M.A.; Cripps, E.; Emslie, M.J.; Jonker, M.; Schaffelke, B.; Sweatman, H. Coral-macroalgal phase shifts or reef resilience: Links with diversity and functional roles of herbivorous fishes on the Great Barrier Reef. Coral Reefs 2010, 29, 1005–1015. [Google Scholar] [CrossRef]
- Webley, J.; McInnes, K.; Teixeira, D.; Lawson, A.; Quinn, R. Statewide Recreational Fishing Survey 2013–2014; Queensland Government Report: Brisbane, Australia, 2015; p. 127.
- Skinner, M.P.; Lewis, R.J.; Morton, S. Ecology of the ciguatera causing dinoflagellates from the Northern Great Barrier Reef: Changes in community distribution and coastal eutrophication. Mar. Poll. Bull. 2013, 77, 210–219. [Google Scholar] [CrossRef]
- Davies, C.H.; Coughlan, A.; Hallegraeff, G.; Ajani, P.; Armbrecht, L.; Atikins, N.; Bonham, P.; Brett, S.; Brinkman, R.; Burford, M.; et al. A database of marine phytoplankton abundance, biomass and species composition in Australian waters. Sci. Data 2016, 3, 160043. [Google Scholar] [CrossRef]
- Larsson, M.E.; Harwood, T.D.; Lewis, R.J.; Himaya, S.W.A.; Doblin, M.A. Toxicological characterization of Fukuyoa paulensis (Dinophyceae) from temperate Australia. Phycol. Res. 2019, 67, 65–71. [Google Scholar] [CrossRef]
- Catania, D.; Richlen, M.L.; Mak, Y.L.; Morton, S.L.; Laban, E.H.; Xu, Y.; Anderson, D.M.; Chan, L.L.; Berumen, M.L. The prevalence of benthic dinoflagellates associated with ciguatera fish poisoning in the central Red Sea. Harmful Algae 2017, 68, 206–216. [Google Scholar] [CrossRef]
- Gaiani, G.; Leonardo, S.; Tudó, À.; Toldrà, A.; Rey, M.; Andree, K.B.; Tsumuraya, T.; Hirama, M.; Diogène, J.; O’Sullivan, C.K.; et al. Rapid detection of ciguatoxins in Gambierdiscus and Fukuyoa with immunosensing tools. Ecotoxicol. Environ. Saf. 2020, 204, 111004. [Google Scholar] [CrossRef]
- Kretzschmar, A.L.; Verma, A.; Harwood, D.T.; Hoppenrath, M.; Murray, S. Characterization of Gambierdiscus lapillus sp. nov. (Gonyaucales, Dinophyceae): A new toxic dinoflagellate from the Great Barrier Reef (Australia). J. Phycol. 2017, 53, 283–297. [Google Scholar] [CrossRef]
- Kretzschmar, A.L.; Larsson, M.E.; Hoppenrath, M.; Doblin, M.A.; Murray, S.A. Characterisation of two toxic Gambierdiscus spp. (Gonyaulacales, Dinophyceae) from the Great Barrier Reef (Australia): G. lewisii sp. nov. and G. holmesii sp. nov. Protist 2019, 170, 125699. [Google Scholar] [CrossRef]
- Yasumoto, T.; Inoue, A.; Bagnis, R.; Garcon, M. Ecological survey on a dinoflagellate possibly responsible for the induction of ciguatera. Bull. Jpn. Soc. Sci. Fish. 1979, 45, 395–399. [Google Scholar] [CrossRef]
- Yasumoto, T.; Inoue, A.; Ochi, T.; Fujimoto, K.; Oshima, Y.; Fukuyo, Y.; Adachi, R.; Bagnis, R. Environmental studies on a toxic dinoflagellate responsible for ciguatera. Bull. Jpn. Soc. Sci. Fish. 1980, 46, 1397–1404. [Google Scholar] [CrossRef] [Green Version]
- Lobel, P.S.; Anderson, D.M.; Durand-Clement, M. Assessment of ciguatera dinoflagellate populations: Sample variability and algal substrate selection. Biol. Bull. 1988, 175, 95–101. [Google Scholar] [CrossRef]
- Caire, J.F.; Raymond, A.; Bagnis, R. Ciguatera: Study of Setting Up and the Evolution of Gambiediscus Toxicus Population on an Artificial Substrate Introduced in an Atoll Lagoon with Follow up of Associated Environmental Factors. In Proceedings of the Fifth International Coral Reef Congress: French Polynesian Coral Reefs, Tahiti, France, 27 May–1 June 1985; Delsalle, B., Galzin, R., Salvat, B., Eds.; Antenne Museum-Ephe: Moorea, France, 1985; Volume 1, pp. 429–435. [Google Scholar]
- Tester, P.A.; Kibler, S.R.; Holland, W.C.; Usup, G.; Vandersea, M.W.; Leaw, C.P.; Teen, L.P.; Larsen, J.; Mohammad-Noor, N.; Faust, M.A.; et al. Sampling harmful benthic dinoflagellates: Comparison of artificial and natural substrate methods. Harmful Algae 2014, 39, 8–25. [Google Scholar] [CrossRef]
- Yong, H.L.; Mustapa, N.I.; Lee, L.K.; Lim, Z.F.; Tan, T.H.; Usup, G.; Gu, H.; Litaker, R.W.; Tester, P.A.; Lim, P.T.; et al. Habitat complexity affects benthic harmful dinoflagellate assemblages in the fringing reef of Rawa Island, Malaysia. Harmful Algae 2018, 78, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.K.; Lim, Z.F.; Gu, H.; Chan, L.L.; Litaker, R.W.; Tester, P.A.; Leaw, C.P.; Lim, P.T. Effects of substratum and depth on benthic harmful dinofagellate assemblages. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Jauzein, C.; Açaf, L.; Accoroni, S.; Asnaghi, V.; Fricke, A.; Hachani, M.A.; abboud-Abi Saab, M.; Chiantore, M.; Mangialajo, L.; Totti, C.; et al. Optimization of sampling, cell collection and counting for the monitoring of benthic harmful algal blooms: Application to Ostreopsis spp. blooms in the Mediterranean Sea. Ecol. Indic. 2018, 91, 116–127. [Google Scholar] [CrossRef] [Green Version]
- Parsons, M.L.; Brandt, A.L.; Ellsworth, A.; Leyne, A.L.; Rains, L.K.; Anderson, D.M. Assessing the use of artificial substrates to monitor Gambierdiscus populations in the Florida Keys. Harmful Algae 2017, 68, 52–66. [Google Scholar] [CrossRef]
- Parsons, M.L.; Richlen, M.L.; Smith, T.B.; Solow, A.R.; Anderson, D.M. Evaluation of 24-h screen deployments as a standardized platform to monitor Gambierdiscus populations in the Florida Keys and U.S. Virgin Islands. Harmful Algae 2021, 103, 101998. [Google Scholar] [CrossRef]
- Diaz-Pulido, G.; McCook, L.J.; Larkum, A.W.D.; Lotze, H.K.; Raven, J.A.; Schaffelke, B.; Smith, J.E.; Steneck, R.S. Chapter 7: Vulnerability of Macroalgae of the Great Barrier Reef to Climate Change. In Climate Change and the Great Barrier Reef: A Vulnerability Assessment; Johnson, J.E., Marshall, P.A., Eds.; Great Barrier Reef Marine Park Authority: Townsville, Australia, 2007; pp. 153–192. [Google Scholar]
- Cruz-Rivera, E.; Villareal, T.A. Macroalgal palatability and the flux of ciguatera toxins through marine food webs. Harmful Algae 2006, 5, 497–525. [Google Scholar] [CrossRef]
- Madin, E.M.P.; Gaines, S.D.; Warner, R.R. Field evidence for pervasive indirect effects of fishing on prey foraging behaviour. Ecology 2010, 91, 3563–3571. [Google Scholar] [CrossRef]
- Madin, E.M.P.; Madin, J.S.; Harmer, A.M.T.; Barrett, N.S.; Booth, D.J.; Caley, M.J.; Cheal, A.J.; Edgar, G.J.; Emslie, M.J.; Gaines, S.D.; et al. Latitude and protection affect decadal trends in reef trophic structure over a continental scale. Ecol. Evol. 2020, 10, 6954–6966. [Google Scholar] [CrossRef]
- Rasher, D.B.; Hoey, A.S.; Hay, M.E. Cascading predator effects in a Fijian coral reef ecosystem. Nat. Sci. Rep. 2017, 7, 15684. [Google Scholar] [CrossRef] [Green Version]
- Rongo, T.; van Woesik, R. The effects of natural disturbances, reef state, and herbivorous fish densities on ciguatera poisoning in Rarotonga, southern Cook Islands. Toxicon 2013, 64, 87–95. [Google Scholar] [CrossRef]
- Kelly, E.L.A.; Eynaud, Y.; Williams, I.D.; Sparks, R.T.; Dailer, M.L.; Sandin, S.A.; Smith, J.E. A budget of algal production and consumption by herbivore fisheries management area, Maui Hawaii. Ecosphere 2017, 8, e01899. [Google Scholar] [CrossRef]
- GBRMPA. Great Barrier Reef Outlook Report 2019; Great Barrier Reef Marine Park Authority: Townsville, Australia, 2019; Available online: http://hdl.handle.net/11017/3474 (accessed on 20 July 2021).
- Yasumoto, T.; Hashimoto, Y.; Bagnis, R.; Randall, J.E.; Banner, A.H. Toxicity of the surgeonfishes. Bull. Jpn. Soc. Sci. Fish. 1971, 37, 724–734. [Google Scholar] [CrossRef]
- Caillaud, A.; de la Iglesia, P.; Barber, E.; Eixarch, H.; Mohammad-Noor, N.; Yasumoto, T.; Diogène, J. Monitoring of dissolved ciguatoxin and maitotoxin using solid-phase adsorption toxin tracking devices: Application to Gambierdiscus pacificus in culture. Harmful Algae 2011, 10, 433–446. [Google Scholar] [CrossRef]
- Roué, M.; Darius, H.T.; Viallon, J.; Ung, A.; Gatti, C.; Harwood, D.T.; Chinain, M. Application of solid phase adsorption toxin tracking (SPATT) devices for the field detection of Gambierdiscus toxins. Harmful Algae 2018, 71, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Roué, M.; Smith, K.F.; Sibat, M.; Viallon, J.; Henry, K.; Ung, A.; Biessy, L.; Hess, P.; Darius, H.T.; Chinain, M. Assessment of ciguatera and other phycotoxin-related risks in Anaho Bay (Nuku Hiva Island, French Polynesia): Molecular, toxicological, and chemical analyses of passive samplers. Toxins 2020, 12, 321. [Google Scholar] [CrossRef] [PubMed]
- Furnas, M.; Alongi, A.; McKinnon, D.; Trott, L.; Skuza, M. Regional-scale nitrogen and phosphorus budgets for the northern (14° S) and central (17° S) Great Barrier Reef shelf ecosystem. Cont. Shelf Res. 2011, 31, 1967–1990. [Google Scholar] [CrossRef]
- Brodie, J.E.; Lewis, S.E.; Collier, C.J.; Woolridge, S.; Bainbridge, Z.T.; Waterhouse, J.; Rasheed, M.A.; Honchin, C.; Holmes, G.; Fabricus, K. Setting ecologically relevant targets for river pollutant loads to meet marine water quality requirements for the Great Barrier Reef, Australia: A preliminary methodology and analysis. Ocean Coast. Manag. 2017, 143, 136–147. [Google Scholar] [CrossRef]
- Devlin, M.J.; McKinna, L.W.; Álvarez-Romero, J.G.; Petus, C.; Abott, B.; Harkenss, P.; Brodie, J. Mapping the pollutants in surface riverine flood plume waters in the Great Barrier Reef, Australia. Mar. Poll. Bull. 2012, 65, 224–235. [Google Scholar] [CrossRef]
- Williams, S.L.; Carpenter, R.C. Nitrogen-limited primary productivity of coral reef algal turfs: Potential contribution of ammonium excreted by Diadema antillarum. Mar. Ecol. Prog. Ser. 1988, 47, 145–152. [Google Scholar] [CrossRef]
- Karcher, D.B.; Roth, F.; Carvalho, S.; El-Khaled, Y.C.; Tilstra, A.; Kürten, B.; Struck, U.; Jones, B.H.; Wild, C. Nitrogen eutrophication particularly promotes turf algae in coral reefs of the central Red Sea. PeerJ 2020, 8, e8737. [Google Scholar] [CrossRef] [Green Version]
- Faust, M.A. Mixotrophy in tropical benthic dinoflagellates. In Harmful Algae, Proceedings of the VIII International Conference on Harmful Algae, Vigo, Spain, 25–29 June 1997; Reguera, B., Blanco, J., Fernández, M.L., Wyatt, T., Eds.; Xunta de Galicia and International Oceanographic Commission of UNESCO: Santiago de Compostela, Spain, 1998; pp. 390–393. [Google Scholar]
- Price, D.C.; Farinholt, N.; Gates, C.; Shumaker, A.; Wagner, N.E.; Bienfang, P.; Bhattacharya, D. Analysis of Gambierdiscus transcriptome data supports ancient origins of mixotrophic pathways in dinoflagellates. Environ. Microbiol. 2016, 18, 4501–4510. [Google Scholar] [CrossRef]
- Clausing, R.J.; Annunziata, C.; Baker, G.; Lee, C.; Bittick, S.J.; Fong, P. Effects of sediment depth on algal turf height are mediated by interactions with fish herbivory on a fringing reef. Mar. Ecol. Prog. Ser. 2014, 517, 121–129. [Google Scholar] [CrossRef]
- Tebbett, S.B.; Goatley, C.H.R.; Bellwood, D.R. Fine sediments suppress detritivory on coral reefs. Mar. Poll. Bull. 2017, 114, 934–940. [Google Scholar] [CrossRef]
- Tebbett, S.B.; Bellwood, D.R.; Purcell, S.W. Sediment addition drives declines in algal turf yield to herbivorous coral reef fishes: Implications for reefs and reef fisheries. Coral Reefs 2018, 37, 929–937. [Google Scholar] [CrossRef]
- Tebbett, S.B.; Goatley, C.H.R.; Streit, R.P.; Bellwood, D.R. Algal turf sediments limit the spatial extent of function delivery on coral reefs. Sci. Total Environ. 2020, 734, 139422. [Google Scholar] [CrossRef]
- Tebbett, S.B.; Goatley, C.H.R.; Bellwood, D.R. The effects of algal turf sediments and organic loads on feeding by coral reef surgeonfishes. PLoS ONE 2017, 12, e0169479. [Google Scholar] [CrossRef] [Green Version]
- Tebbett, S.B.; Bellwood, D.R. Sediments ratchet-down coral reef algal turf productivity. Sci. Total Environ. 2020, 713, 136709. [Google Scholar] [CrossRef]
- Tebbett, S.B.; Goatley, C.H.R.; Bellwood, D.R. Algal turf sediments across the Great Barrier Reef: Putting coastal reefs in perspective. Mar. Poll. Bull. 2018, 137, 518–525. [Google Scholar] [CrossRef]
- Gordon, S.E.; Goatley, C.H.R.; Bellwood, D.R. Low-quality sediments deter grazing by the parrotfish Scarus rivulatus on inner-shelf reefs. Coral Reefs 2016, 35, 285–291. [Google Scholar] [CrossRef]
- Lewis, S.E.; Schaffelke, B.; Shaw, M.; Bainbridge, Z.T.; Rohde, K.W.; Kennedy, K.; Davis, A.M.; Masters, B.L.; Devlin, M.J.; Mueller, J.F.; et al. Assessing the additive risks of PSII herbicide exposure to the Great Barrier Reef. Mar. Poll. Bull. 2012, 65, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.M.; Brodie, J.; Mueller, J.F. Phytotoxicity induced in isolated zooxanthellae by herbicides extracted from Great Barrier Reef flood waters. Mar. Poll. Bull. 2012, 65, 335–362. [Google Scholar] [CrossRef]
- McNeil, M.A.; Webster, J.M.; Beaman, R.J.; Graham, T.L. New constraints on the spatial distribution and morphology of the Halimeda bioherms of the Great Barrier Reef, Australia. Coral Reefs 2016, 35, 1343–1355. [Google Scholar] [CrossRef] [Green Version]
- Sih, T.L.; Daniell, J.J.; Bridge, T.C.L.; Beaman, R.J.; Cappo, M.; Kingsford, M.J. Deep-reef communities of the Great Barrier Reef shelf-break: Trophic structure and habitat associations. Diversity 2019, 11, 26. [Google Scholar] [CrossRef] [Green Version]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, S.T.; Capra, M.F. The cyanobacterium Oscillatoria erythraea—A potential source of the toxin in the ciguatera food-chain. Food Addit. Contam. 1992, 9, 351–355. [Google Scholar] [CrossRef]
- Kerbrat, A.-S.; Darius, H.T.; Pauillac, S.; Chinain, M.; Laurent, D. Detection of ciguatoxin-like and paralysing toxins in Trichodesmium spp. from New Caledonia lagoon. Mar. Pol. Bull. 2010, 61, 360–366. [Google Scholar] [CrossRef]
- Clements, K.D.; German, D.P.; Piché, J.; Tribollet, A.; Choat, J.H. Integrating ecological roles and trophic diversification on coral reefs: Multiple lines of evidence identify parrotfishes as microphages. Biol. J. Linn. Soc. 2017, 120, 729–751. [Google Scholar] [CrossRef]
- Nicholson, G.M.; Clments, K.D. Resolving resource partitioning in parrotfishes (Scarini) using microhistology of feeding substrata. Coral Reefs 2020, 39, 1313–1327. [Google Scholar] [CrossRef]
- Laurent, D.; Kerbrat, A.-S.; Darius, H.T.; Cirarad, E.; Golubic, S.; Benoit, E.; Sauviat, M.-P.; Chinain, M.; Molgo, J.; Pauillac, S. Are cyanobacteria involved in ciguatera fish poisoning-like outbreaks in New Caledonia. Harmful Algae 2008, 7, 827–838. [Google Scholar] [CrossRef]
- Bakus, G.J. Chemical defense mechanisms on the Great Barrier Reef, Australia. Science 1981, 211, 497–499. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.J.; Gillespie, N.C.; Lewis, R.J. Toxicity and morphology of Ostreopsis cf. siamensis cultured from a ciguatera endemic region of Queensland, Australia. In Proceedings of the 6th International Coral Reef Symposium Executive Committee, Townsville, Australia, 8–12 August 1988; Choat, J.H., Barnes, D., Borowitzka, M.A., Coll, J.C., Davies, P.J., Flood, P., Hatcher, B.G., Hopley, D., Hutchings, P.A., Kinsey, D., et al., Eds.; Townsville, Australia, 1988; Volume 3, pp. 49–54. [Google Scholar]
- Murakami, M.; Oshima, Y.; Yasumoto, T. Identification of okadaic acid as a toxic component of a marine dinoflagellate Prorocentrum lima. Bull. Jpn. Soc. Sci. Fish. 1982, 48, 69–72. [Google Scholar] [CrossRef]
- Reguera, B.; Velo-Suárez, L.; Raine, R.; Park, M.G. Harmful Dinophysis species. Harmful Algae 2012, 14, 87–106. [Google Scholar] [CrossRef]
- Murata, M.; Shimatani, M.; Sugitani, H.; Oshima, Y.; Yasumoto, T. Isolation and structural elucidation of the causative toxin of the diarrhetic shellfish poisoning. Bull. Jpn. Soc. Sci. Fish. 1982, 48, 549–552. [Google Scholar] [CrossRef] [Green Version]
- Gamboa, P.M.; Park, D.L.; Fremy, J.-M. Extraction and Purification of Toxic Fractions from Barracuda (Sphyraena barracuda) Implicated in Ciguatera Poisoning. In Proceedings of the Third International Conference on Ciguatera Fish Poisoning, La Parguera, Puerto Rico, 30 April–5 May 1990; Tosteson, T.R., Ed.; Polyscience Publications: Quebec, QC, Canada, 1992; pp. 13–24. [Google Scholar]
- Corriere, M.; Soliño, L.; Costa, P.R. Effects of the marine biotoxins okadaic acid and dinophysistoxins on fish. J. Mar. Sci. Eng. 2021, 9, 293. [Google Scholar] [CrossRef]
- Usami, M.; Satake, M.; Ishida, S.; Inoue, A.; Kan, Y.; Yasumoto, T. Palytoxin analogs from the dinoflagellate Ostreopsis siamensis. J. Am. Chem. Soc. 1995, 117, 5389–5390. [Google Scholar] [CrossRef]
- Parsons, M.L.; Aligizaki, K.; Bottein, M.-Y.D.; Fraga, S.; Morton, S.L.; Penna, A.; Rhodes, L. Gambierdiscus and Ostreopsis: Reassessment of the state of knowledge of their taxonomy, geography, ecophysiology, and toxicology. Harmful Algae 2012, 14, 107–129. [Google Scholar] [CrossRef]
- Katikou, P.; Vlamis, A. Palytoxin and Analogs: Ecobiology and Origin, Chemistry, and Chemical Analysis. In Seafood and freshwater Toxins. Pharmacology, Physiology and Detection; Botana, L., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 695–740. [Google Scholar]
- Kerbrat, A.S.; Amzil, Z.; Pawlowiez, R.; Golubic, S.; Sibat, M.; Darius, H.T.; Chinain, M.; Laurent, D. First evidence of palytoxin and 42-hydroxy-palytoxin in the marine cyanobacterium Trichodesmium. Mar. Drugs 2011, 9, 543–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onuma, Y.; Satake, M.; Ukena, T.; Roux, J.; Chanteau, S.; Rasolofonirina, N.; Ratsimaloto, M.; Naoki, H.; Yasumoto, T. Identification of putative palytoxin as the cause of clupeotoxism. Toxicon 1999, 37, 55–65. [Google Scholar] [CrossRef]
- Tubaro, A.; Durando, P.; Favero, D.G.; Ansaldi, F.; Icardi, G.; Deeds, J.R.; Sosa, S. Case definitions for human poisonings postulated to palytoxins exposure. Harmful Algae 2011, 57, 478–495. [Google Scholar] [CrossRef]
- Verma, A.; Hoppenrath, M.; Harwood, T.; Brett, S.; Rhodes, L.; Murray, S. Molecular phylogeny, morphology and toxicgenecity of Ostreopsis c.f. siamensis (Dinophyceae) from temperate south-east Australia. Phycol. Res. 2016, 64, 146–159. [Google Scholar] [CrossRef]
- Verma, A.; Hughes, D.J.; Harwood, D.T.; Suggett, D.J.; Ralph, P.J.; Murray, S.A. Functional significance of phylogeographic structure in a toxic benthic marine microbial eukaryote over a latitudinal gradient along the East Australian Current. Ecol. Evol. 2020, 10, 6257–6273. [Google Scholar] [CrossRef]
- Cvitanovic, C.; Fox, R.J.; Bellwood, D.R. Herbivory by Fishes on the Great Barrier Reef: A Review of Knowledge and Understanding; Unpublished Report to the Marine and Tropical Sciences Research Facility; Reef and Rainforest Research Centre Limited: Cairns, Australia, 2007; p. 33. [Google Scholar]
- Cheal, A.; Emslie, M.; Miller, I.; Sweatman, H. The distribution of herbivorous fishes on the Great Barrier Reef. Mar. Biol. 2012, 159, 1143–1154. [Google Scholar] [CrossRef]
- Purcell, S.W.; Bellwood, D.R. A functional analysis of food procurement in two surgeonfish species, Acanthurus nigrofuscus and Ctenochaetus striatus (Acanthuridae). Env. Biol. Fish. 1993, 37, 139–159. [Google Scholar] [CrossRef]
- Tebbett, S.B.; Goatley, C.H.R.; Bellwood, D.R. Clarifying functional roles: Algal removal by the surgeonfishes Ctenochaetus striatus and Acanthurus nigrofuscus. Coral Reefs 2017, 36, 803–813. [Google Scholar] [CrossRef]
- Bagnis, R. Natural versus anthropogenic disturbances to coral reefs: Comparison in epidemiological patterns of ciguatera. Mem. Qld Mus. 1994, 34, 455–460. [Google Scholar]
- Yasumoto, T. Chemistry, etiology, and food chain dynamics of marine toxins. Proc. Jpn. Acad. 2005, 81, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Tebbett, S.B.; Goatley, C.H.R.; Huertas, V.; Mihalitsis, M.; Bellwood, D.R. A functional evaluation of feeding in the surgeonfish Ctenochaetus striatus: The role of soft tissues. R. Soc. Open Sci. 2018, 5, 171111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, B.; Nakagawa, L.K.; Kobayashi, M.N.; Hokama, Y. Gambierdiscus toxicus in gut content of the surgeonfish Ctenochaetus strigosus (herbivore) and its relationship to toxicity. Toxicon 1987, 25, 1125–1127. [Google Scholar]
- Magnelia, S.J.; Kohler, C.C.; Tindall, D.R. Acanthurids do not avoid consuming cultured toxic dinoflagellates yet do not become ciguatoxic. Trans. Am. Fish. Soc. 1992, 121, 737–745. [Google Scholar] [CrossRef]
- Marshell, A.; Mumby, P.J. The role of surgeonfish (Acanthuridae) in maintaining algal turf biomass on coral reefs. J. Exp. Mar. Biol. Ecol. 2015, 473, 152–160. [Google Scholar] [CrossRef]
- Russ, G.R. Grazer biomass correlates more strongly with production than with biomass of algal turfs on a coral reef. Coral Reefs 2003, 22, 63–67. [Google Scholar] [CrossRef]
- Vacarizas, J.; Benico, G.; Austero, N.; Azanza, R. Taxonomy and toxin production of Gambierdiscus carpenteri (Dinophyceae) in a tropical marine ecosystem: The first record from the Philippines. Mar. Pol. Bull. 2018, 137, 430–443. [Google Scholar] [CrossRef]
- Bomber, J.W.; Guillard, R.R.L.; Nelson, W.G. Rôles of temperature, salinity, and light in seasonality, growth, and toxicity of ciguatera-causing Gambierdiscus toxicus Adachi et Fukuyo (Dinophyceae). J. Exp. Mar. Biol. Ecol. 1988, 115, 53–65. [Google Scholar] [CrossRef]
- Kibler, S.R.; Litaker, R.W.; Holland, W.C.; Vandersea, M.W.; Tester, P.A. Growth of eight Gambierdiscus (Dinophyceae) species: Effects of temperature, salinity and irradiance. Harmful Algae 2012, 19, 1–14. [Google Scholar] [CrossRef]
- Xu, Y.; Richlen, M.L.; Liefer, J.D.; Robertson, A.; Kullis, D.; Smith, T.B.; Parsons, M.L.; Andersen, D.M. Influence of environmental variables on Gambierdiscus sp. (Dinophyceae) growth and distribution. PLoS ONE 2016, 11, e0153197. [Google Scholar] [CrossRef]
- Sparrow, L.; Momigliano, P.; Russ, G.R.; Heimann, K. Effects of temperature, salinity and composition of the dinoflagellate assemblage on the growth of Gambierdiscus carpenteri isolated from the Great Barrier Reef. Harmful Algae 2017, 65, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Mustapa, N.I.; Yong, H.L.; Lee, L.K.; Lim, Z.F.; Lim, H.C.; Teng, S.T.; Luo, Z.; Gu, H.; Leaw, C.P.; Lim, P.T. Growth and epiphytic behaviour of three Gambierdiscus species (Dinophyceae) associated with various macroalgal substrates. Harmful Algae 2019, 89, 101671. [Google Scholar] [CrossRef] [PubMed]
- Russ, G.R.; Cheal, A.J.; Dolman, A.M.; Emslie, M.J.; Evans, R.D.; Miller, I.; Sweatman, H.; Williamson, D.H. Rapid increase in fish numbers follows creation of world’s largest marine reserve network. Curr. Biol. 2008, 18, R514–R515. [Google Scholar] [CrossRef] [Green Version]
- McCook, L.J.; Ayling, T.; Cappo, M.; Choat, J.H.; Evans, R.D.; De Freitas, D.M.; Heupel, M.; Hughes, T.; Jones, G.P.; Mapstone, B.; et al. Adaptive management of the Great Barrier Reef: A globally significant demonstration of the benefits of networks of marine reserves. Proc. Natl. Acad. Sci. USA 2010, 107, 18278–18285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzari, J.R.; Bergseth, B.J.; Frisch, A.J. Impact of conservation areas on trophic interactions between apex predators and herbivores on coral reefs. Conserv. Biol. 2015, 29, 418–429. [Google Scholar] [CrossRef]
- Casey, J.M.; Baird, A.H.; Brandl, S.J.; Hoogenboom, M.O.; Rizzari, J.R.; Frisch, A.J.; Mirbach, C.E.; Connolly, S.R. A test of trophic cascade theory: Fish and benthic assemblages across a predator density gradient on coral reefs. Oceologia 2017, 183, 161–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madin, E.M.P.; Madin, J.S.; Booth, D.J. Landscape of fear visible from space. Nature Sci. Rep. 2011, 1, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, G.B.; Taylor, B.M.; Robbins, W.D.; Franklin, E.C.; Toonen, R.; Bowen, B.; Choat, J.H. Diversity and structure of parrotfish assemblages across the northern Great Barrier Reef. Diversity 2019, 11, 14. [Google Scholar] [CrossRef] [Green Version]
- Mellin, C.; McNeil, M.A.; Cheal, A.J.; Emslie, M.J.; Caley, M.J. Marine protected areas increase resilience among coral reef communities. Ecol. Lett. 2016, 19, 629–637. [Google Scholar] [CrossRef]
- Newton, K.; Côté, I.M.; Pilling, G.M.; Jennings, S.; Dulvy, N.K. Current and future sustainability of island coral reef fisheries. Curr. Biol. 2007, 17, 655–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, T.P.; Rodrigues, M.J.; Bellwood, D.R.; Ceccarelli, D.; Hoegh-Guldberg, O.; McCook, L.; Moltschaniwskyj, N.; Pratchett, M.S.; Steneck, R.S.; Willis, B. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr. Biol. 2007, 17, 360–365. [Google Scholar] [CrossRef] [Green Version]
- Steneck, R.S.; Arnold, S.N.; Mumby, P.J. Experiment mimics fishing on parrotfish: Insights on coral reef recovery and alternative attractors. Mar. Ecol. Prog. Ser. 2014, 506, 115–127. [Google Scholar] [CrossRef] [Green Version]
- Russ, G.R.; Questel, S.-L.A.; Rizzari, J.R.; Alcala, A.C. The parrotfish-coral relationship: Refuting the ubiquity of a prevailing paradigm. Mar. Biol. 2015, 162, 2029–2045. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Hughes, T.P.; Hoey, A.S. Sleeping functional group drives coral-reef recovery. Curr. Biol. 2006, 16, 2434–2439. [Google Scholar] [CrossRef] [Green Version]
- Fox, R.J.; Bellwood, D.R. Remote video bioassays reveal the potential feeding impact of the rabbitfish Siganus canaliculatus (f: Siganidae) on an inner-shelf reef of the Great Barrier Reef. Coral Reefs 2008, 27, 605–615. [Google Scholar] [CrossRef]
- Tebbett, S.B.; Hoey, A.S.; Depczynski, M.; Wismer, S.; Bellwood, D.R. Macroalgae removal on coral reefs: Realised ecosystem functions transcend biogeographic locations. Coral Reefs 2020, 39, 203–214. [Google Scholar] [CrossRef]
- Morais, R.A.; Depczynski, M.; Fulton, C.; Marnane, M.; Narvaez, P.; Huertas, V.; Brandl, S.J.; Bellwood, D. Severe coral loss shifts energetic dynamics on a coral reef. Funct. Ecol. 2020, 34, 1507–1518. [Google Scholar] [CrossRef]
- Leigh, G.M.; Campbell, A.B.; Lunow, C.P.; O’Neill, M.F. Stock Assessment of the Queensland East Coast Common Coral Trout (Plectropomus leopardus) Fishery; Queensland Department of Agriculture, Fisheries and Forestry: Brisbane, Australia, 2014; p. 113.
- Heaven, C. Queensland Fisheries Summary, October 2018; Queensland Department of Agriculture and Fisheries: Brisbane, Australia, 2018; p. 62.
- Vermeij, M.J.A.; van der Heijden, R.A.; Olthuis, J.G.; Marhaver, K.L.; Smith, J.E.; Visser, P.M. Survival and dispersal of turf algae and macroalage consumed by herbivorous coral reef fishes. Oecologia 2013, 171, 417–425. [Google Scholar] [CrossRef]
- Goatley, C.H.R.; Bellwood, D.R. Biologically mediated sediment fluxes on coral reefs: Sediment removal and off-reef transportation by the surgeonfish Ctenochaetus striatus. Mar. Ecol. Prog. Ser. 2010, 415, 237–245. [Google Scholar] [CrossRef]
- Kramer, M.J.; Bellwood, O.; Bellwood, D.R. The trophic importance of algal turfs for coral reef fishes: The crustacean link. Coral Reefs 2013, 32, 575–583. [Google Scholar] [CrossRef]
- Brandl, S.J.; Goatley, C.H.R.; Bellwood, D.R.; Tornabene, L. The hidden half: Ecology and evolution of cryptobenthic fishes on coral reefs. Biol. Rev. 2018, 93, 1846–1873. [Google Scholar] [CrossRef] [PubMed]
- Brandl, S.J.; Tornabene, L.; Goatley, C.H.R.; Casey, J.M.; Morais, R.A.; Côte, I.M.; Baldwin, C.C.; Parravicini, V.; Schiettekatte, N.M.D.; Bellwood, D.R. Demographic dynamics of the smallest marine vertebrates fuel coral-reef ecosystem functioning. Science 2019, 364, 1189–1192. [Google Scholar] [CrossRef]
- Gillespie, N.C. Ciguatera poisoning. In Toxic Plants and Animals: A Guide for Australia; Covacevich, J., Davie, P., Pearn, J., Eds.; Queensland Museum: Brisbane, Australia, 1987; pp. 160–169. [Google Scholar]
- Sumpton, W.; Mayer, D.; Brown, I.; Sawynok, B.; McLennan, M.; Butcher, A.; Kirkwood, J. Investigation of movement and factors influencing post-release survival of line-caught coral reef fish using recreational tag-recapture data. Fish. Res. 2008, 92, 189–195. [Google Scholar] [CrossRef]
- Currey, L.M.; Heupel, M.R.; Simpfendorfer, C.A.; Williams, A.J. Sedentary or mobile? Variability in space and depth use of an exploited coral reed fish. Mar Biol. 2014, 161, 2155–2166. [Google Scholar] [CrossRef]
- Frisch, A.J.; Ireland, M.; Baker, R. Trophic ecology of large predatory reef fishes: Energy pathways, trophic level, and implications for fisheries in a changing climate. Mar. Biol. 2014, 161, 61–73. [Google Scholar] [CrossRef]
- Holmes, B.; Leslie, M.; Keag, M.; Roelofs, A.; Winning, M.; Zeller, B. Stock Status of Queensland’s Fisheries Resources 2012; Queensland Department of Agriculture, Fisheries and Forestry: Brisbane, Australia, 2013; p. 120.
- Campbell, A.; Leigh, G.; Bessell-Browne, P.; Lovett, R. Stock Assessment of the Queensland East Coast Common Coral Trout (Plectropomus leopardus) Fishery; Department of Employment, Economic Development and Innovation, Queensland Government: Brisbane, Australia, 2019; p. 61.
- Campbell, A.B.; Northrop, A.R. Stock Assessment of the Common Coral Trout (Plectropomus leopardus) in Queensland, Australia; Department of Employment, Economic Development and Innovation, Queensland Government: Brisbane, Australia, 2020; p. 45.
- Kingsford, M.J. Spatial and temporal variation in predation on reef fishes by coral trout (Plectropomus leopardus, Serranidae). Coral Reefs 1992, 11, 193–198. [Google Scholar] [CrossRef]
- St John, J.; Russ, G.R.; Brown, I.W.; Squire, L.C. The diet of the large coral reef serranid Plectropomus leopardus in two fishing zones on the Great Barrier Reef, Australia. Fish. Bull. 2001, 99, 180–192. [Google Scholar]
- Matley, J.K.; Maes, G.E.; Devloo-Delva, F.; Huerlimann, R.; Chua, G.; Tobin, A.J.; Fisk, A.T.; Simpfendorfer, C.A.; Heupel, M.R. Integrating complementary methods to improve diet analysis in fishery-targeted species. Ecol. Evol. 2018, 8, 9503–9515. [Google Scholar] [CrossRef]
- St John, J. Temporal variation in the diet of a coral reef piscivore (Pisces: Serranidae) was not seasonal. Coral Reefs 2001, 20, 163–170. [Google Scholar] [CrossRef]
- Streit, R.P.; Cumming, G.S.; Bellwood, D.R. Patchy delivery of functions undermines functional redundancy in a high diversity system. Funct. Ecol. 2019, 33, 1144–1155. [Google Scholar] [CrossRef]
- Tebbett, S.B.; Chase, T.J.; Bellwood, D.R. Farming damselfishes shape algal turf sediment dynamics on coral reefs. Mar. Environ. Res. 2020, 160, 104988. [Google Scholar] [CrossRef]
- Wilson, S.; Bellwood, D.R. Cryptic dietary components of territorial damselfishes (Pomacentridae, Labroidei). Mar. Ecol. Prog. Ser. 1997, 153, 299–310. [Google Scholar] [CrossRef] [Green Version]
- Edwards, C.B.; Friedlander, A.M.; Green, A.G.; Hardt, M.J.; Sala, E.; Sweatman, H.P.; Williams, I.D.; Zglicynski, B.; Sandin, S.A.; Smith, J.E. Global assessment of the status of coral reef herbivorous fishes: Evidence for fishing effects. Proc. Royal Soc. B 2013, 281, 20131835. [Google Scholar] [CrossRef] [Green Version]
- Andersen, C.; Clarke, K.; Higgs, J.; Ryan, S. Ecological Assessment of the Queensland Coral Reef Fin Fish Fishery; Queensland Department of Primary Industries: Brisbane, Australia, 2005; p. 149.
- Marriott, R.J.; Mapstone, B.D.; Begg, G.A. Age-specific demographic parameters, and their implication for management of the red bass, Lutjanus bohar (Forsskál 1775): A large, long-lived fish. Fish. Res. 2007, 83, 204–215. [Google Scholar] [CrossRef]
- Choat, J.H.; Axe, L.M. Growth and longevity in acanthurid fishes; an analysis of otolith increments. Mar. Ecol. Prog. Ser. 1996, 134, 15–26. [Google Scholar] [CrossRef] [Green Version]
- Kraa, E.; Campbell, B. Ciguatera outbreak, NSW, New South Wales. Public Health Bull. 1994, 5, 69. Available online: http://www.health.nsw.gov.au/phb/Documents/1994-06.pdf (accessed on 20 July 2021).
- Lewis, R.J.; Ruff, T.A. Ciguatera: Ecological, clinical and socioeconomic perspectives. Crit. Rev. Environ. Sci. Technol. 1993, 23, 137–156. [Google Scholar] [CrossRef]
- Wong, C.-K.; Hung, P.; Lo, J.Y.C. Ciguatera fish poisoning in Hong Kong-A 10-year perspective on the class of ciguatoxins. Toxicon 2014, 86, 96–106. [Google Scholar] [CrossRef]
- Leigh, G.M.; Williams, A.J.; Begg, G.A.; Gribble, N.A.; Whybird, O.J. Stock Assessment of the Queensland East Coast Red Throat Emperor (Lethrinius miniatus) Fishery; Queensland Department of Primary Industries and Fisheries: Brisbane, Australia, 2006; p. 127.
- Hessel, D.W.; Halstead, B.W.; Peckham, N.H. Marine biotoxins. 1. Ciguatera poison: Some biological and chemical aspects. Ann. N. Y. Acad. Sci. 1960, 90, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Banner, A.H.; Helfrich, P.; Scheuer, P.J.; Yoshida, T. Research on ciguatera in the tropical Pacific. In Proceedings of the 16th Gulf and Caribbean Fisheries Insititute, Coral Gables, FL, USA, May 1964; pp. 84–98. [Google Scholar]
- Vernoux, J.-P. La ciguatera dans l’île de Saint-Barthélémy: Aspects épidémiologiques, toxicologiques et préventifs. Oceanol. Acta 1988, 11, 37–46. [Google Scholar]
- Bravo, J.; Cabrera Suárez Ramírez, A.S.; Acosta, F. Ciguatera, an emerging human poisoning in Europe. J. Aqua. Mar. Biol. 2015, 3, 53. [Google Scholar] [CrossRef]
- Tsumuraya, T.; Hirama, M. Rationally designed synthetic haptens to generate anti-ciguatoxin monoclonal antibodies, and development of a practical sandwich ELISA to detect ciguatoxins. Toxins 2019, 11, 533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonardo, S.; Gaiani, G.; Tsumuraya, T.; Hirama, M.; Turquet, J.; Sagristá, N.; Rambla-Alegre, M.; Flores, C.; Caixach, J.; Diogène, J.; et al. Addressing the analytical challenges for the detection of ciguatoxins using an electrochemical biosensor. Anal. Chem. 2020, 92, 4858–4865. [Google Scholar] [CrossRef] [PubMed]
- Bagnis, R.; Bennett, J.; Barsinas, M.; Drollet, J.H.; Jacquet, G.; Legrand, A.M.; Cruchet, P.H.; Pascal, H. Correlation between Ciguateric Fish and Damage to Reefs in the Gambier Islands (French Polynesia). In Proceedings of the 6th International Coral Reef Symposium Executive Committee, Townsville, Australia, 1 January 1988; Volume 2, pp. 195–200. [Google Scholar]
- Belliveau, S.A.; Paul, V.J. Effects of herbivory and nutrients on the early colonization of crustose coralline and fleshy algae. Mar. Ecol. Prog. Ser. 2002, 232, 105–114. [Google Scholar] [CrossRef]
- Stuart-Smith, R.D.; Brown, C.J.; Ceccarelli, D.M.; Edgar, G.J. Ecosystem restructuring along the Great Barrier Reef following mass coral bleaching. Nature 2018, 560, 92–96. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Pratchett, M.S.; Morrison, T.H.; Gurney, G.G.; Highes, T.P.; Álvarez-Romero, J.G.; Day, J.C.; Grantham, R.; Grech, A.; Hoey, A.S.; et al. Coral reef conservation in the Anthropocene: Confronting spatial mismatches and prioritizing functions. Biol. Cons. 2019, 236, 604–615. [Google Scholar] [CrossRef]
- Cheal, A.J.; Emslie, M.; MacNeil, A.; Miller, I.; Sweatman, H. Spatial variation in the functional characteristics of herbivorous fish communities and the resilience of coral reefs. Ecol. Appl. 2013, 23, 174–188. [Google Scholar] [CrossRef] [Green Version]
- Turner, M.G.; Calder, W.J.; Cumming, G.S.; Hughes, T.P.; Jentsch, A.; LaDeau, S.L.; Lenton, T.M.; Shuman, B.N.; Turetsky, M.R.; Ratajczak, Z.; et al. Climate change, ecosystems and abrupt change: Science priorities. Phil. Trans. R. Soc. B 2020, 375, 20190105. [Google Scholar] [CrossRef] [Green Version]
- Kaly, U.L.; Jones, G.P. Test of the effect of disturbance on ciguatera in Tuvalu. Mem. Qld Mus. 1994, 34, 523–532. [Google Scholar]
- Robinson, J.P.W.; McDevitt-Irwin, J.; Dajka, J.-C.; Hadj-Hammou, J.; Howlett, S.; Graba-Landry, A.; Hoey, A.S.; Nash, K.L.; Wilson, S.K.; Graham, N.A.J. Habitat and fishing control grazing potential on coral reefs. Funct. Ecol. 2019, 34, 240–251. [Google Scholar] [CrossRef]
- McClure, E.C.; Richardson, L.E.; Graba-Landry, A.; Loffler, Z.; Russ, G.R.; Hoey, A.S. Cross-shelf differences in the response of herbivorous fish assemblage to severe environmental disturbances. Diversity 2019, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Hales, S.; Weinstein, P.; Woodward, A. Ciguatera (fish poisoning), El Niño, and Pacific sea surface temperatures. Ecosyst. Health 1999, 5, 20–25. [Google Scholar] [CrossRef]
- Chateau-Degat, M.-L.; Chinain, M.; Cerf, N.; Gingras, S.; Hubert, B.; Dewailly, É. Seawater temperature, Gambierdiscus spp. variability and incidence of ciguatera in French Polynesia. Harmful Algae 2005, 4, 1053–1062. [Google Scholar] [CrossRef]
- Llewellyn, L.E. Revisiting the association between sea surface temperature and the epidemiology of fish poisoning in the South Pacific: Reassessing the link between ciguatera and climate change. Toxicon 2010, 56, 691–697. [Google Scholar] [CrossRef]
- Tester, P.A.; Feldman, R.L.; Nau, A.W.; Kibler, S.R.; Litaker, R.W. Ciguatera fish poisoning and sea surface temperatures in the Caribbean Sea and the West Indies. Toxicon 2010, 56, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Heimann, K.; Capper, A.; Sparrow, L. Ocean surface warming: Impact on toxic benthic dinoflagellates causing ciguatera. In The Encyclopaedia of Life Sciences A23373; Wiley Publishing: New York, NY, USA, 2011. [Google Scholar]
- Wells, M.L.; Karlson, B.; Wulff, A.; Kudela, R.; Trick, C.; Asnaghi, V.; Berdalet, E.; Cochlan, W.; Davidson, K.; De Rijcke, M.; et al. Future HAB science: Directions and challenges in a changing climate. Harmful Algae 2020, 91, 101632. [Google Scholar] [CrossRef]
- Hallegraeff, G.M.; Fraga, S. Bloom dynamics of the toxic dinoflagellate Gymnodinium catenatum, with emphasis on Tasmanian and Spanish coastal waters. In Physiological Ecology of Harmful Algal Blooms; Anderson, D.M., Cembella, A.D., Hallegraeff, G.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 59–80. [Google Scholar]
- Holmes, M.J.; Bolch, C.J.S.; Green, D.H.; Cembella, A.D.; Teo, S.L.M. Singapore isolates of the dinoflagellate Gymnodinium catenatum (Dinophyceae) produce a unique profile of paralytic shellfish poisoning toxins. J. Phycol. 2002, 38, 96–106. [Google Scholar] [CrossRef]
- Lewis, R.J. Socioeconomic impacts and management ciguatera in the Pacific. Bull. Path. Soc. Exot. 1992, 85, 427–434. [Google Scholar]
- Zheng, L.; Gatti, C.M.; Gamarro, E.G.; Suzuki, A.; Teah, H.Y. Modeling the time-lag effect of sea surface temperatures on ciguatera poisoning in the South Pacific: Implications for surveillance and response. Toxicon 2020, 182, 21–29. [Google Scholar] [CrossRef] [PubMed]

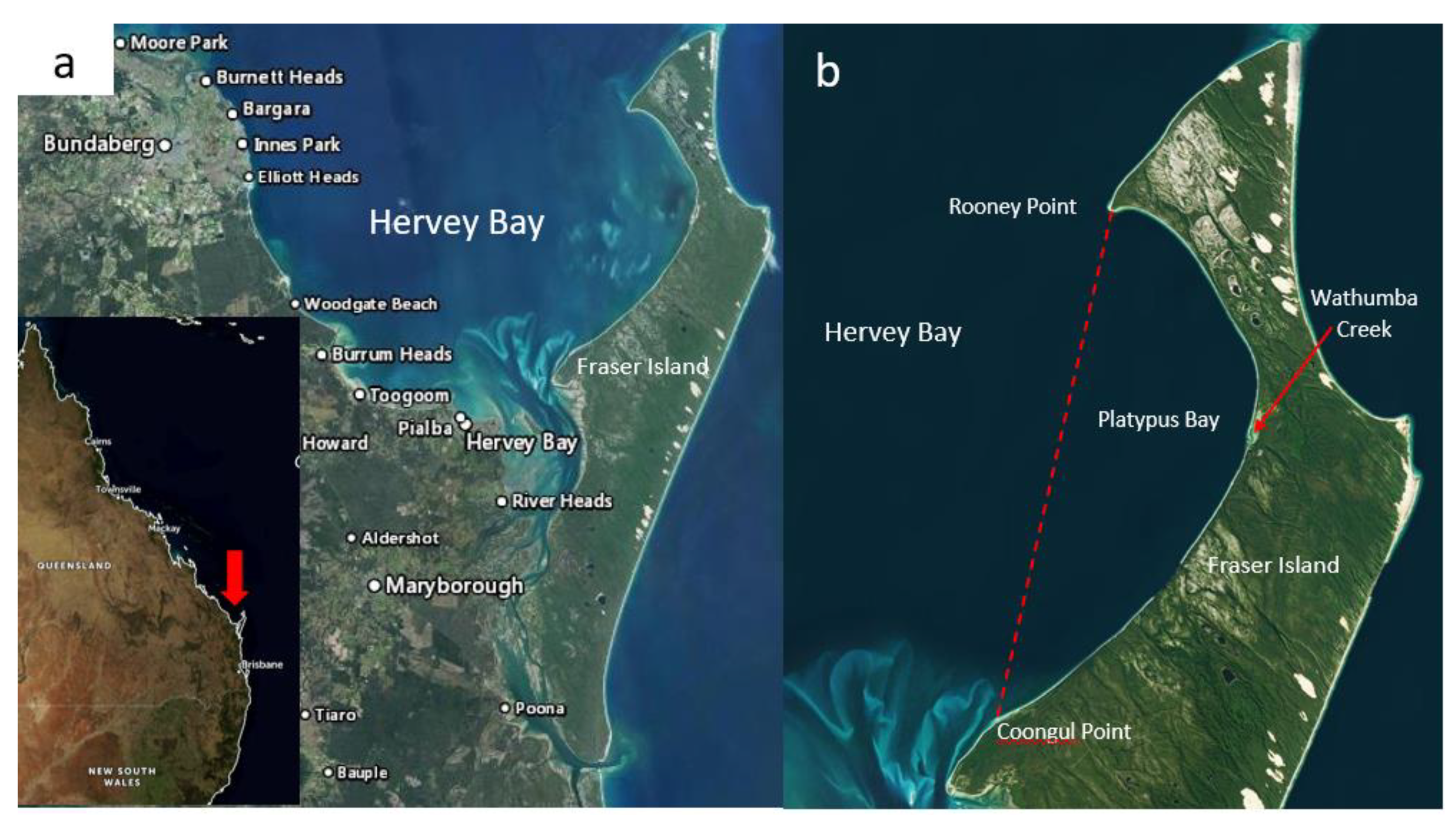
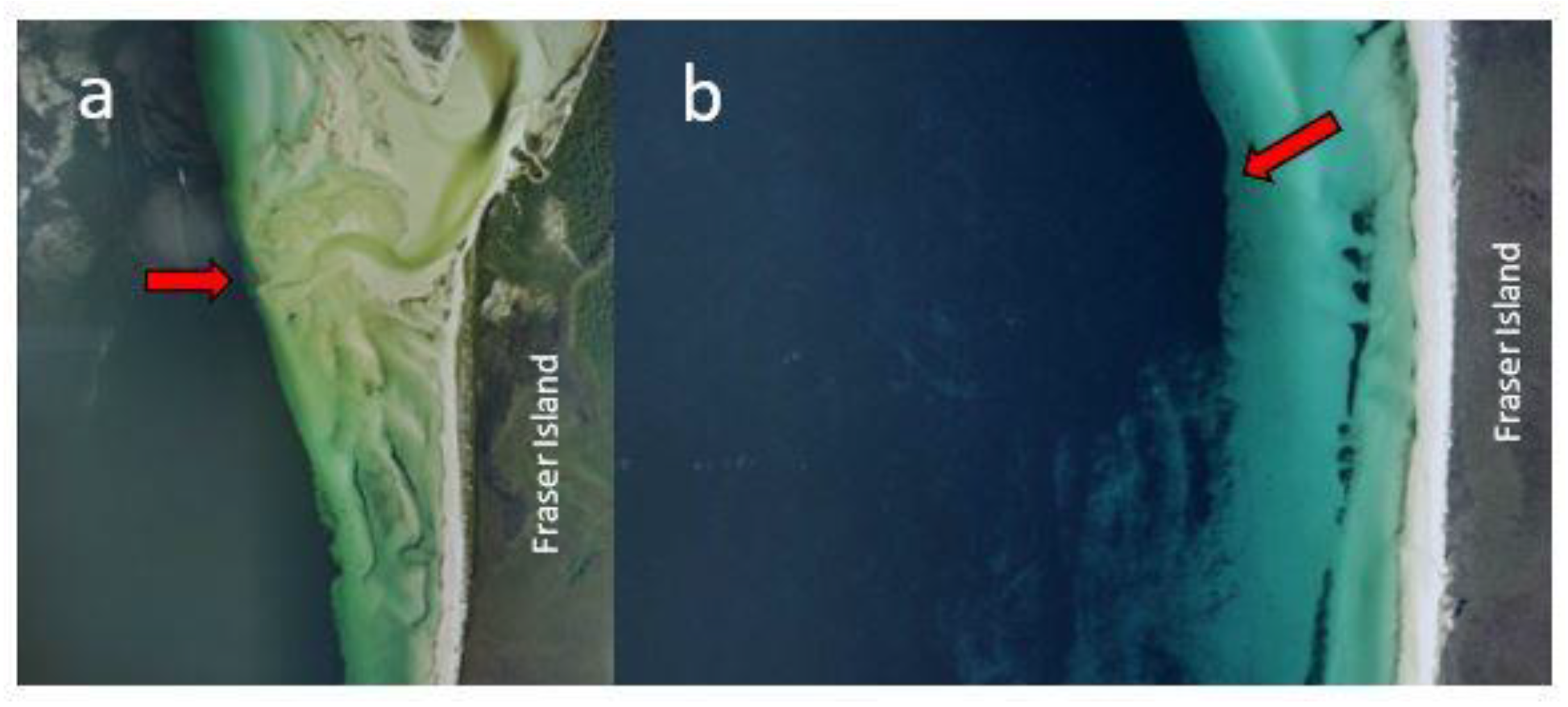


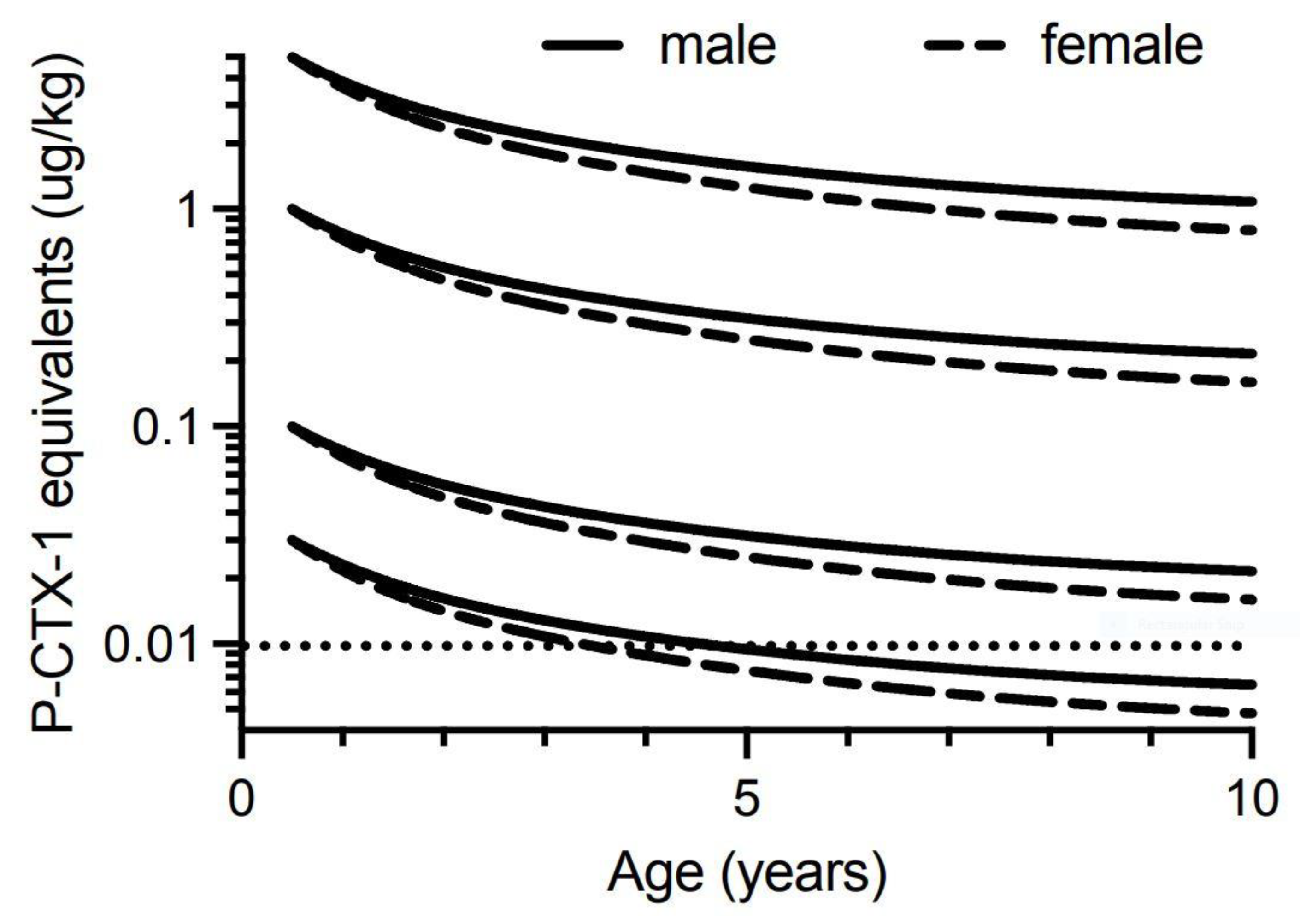
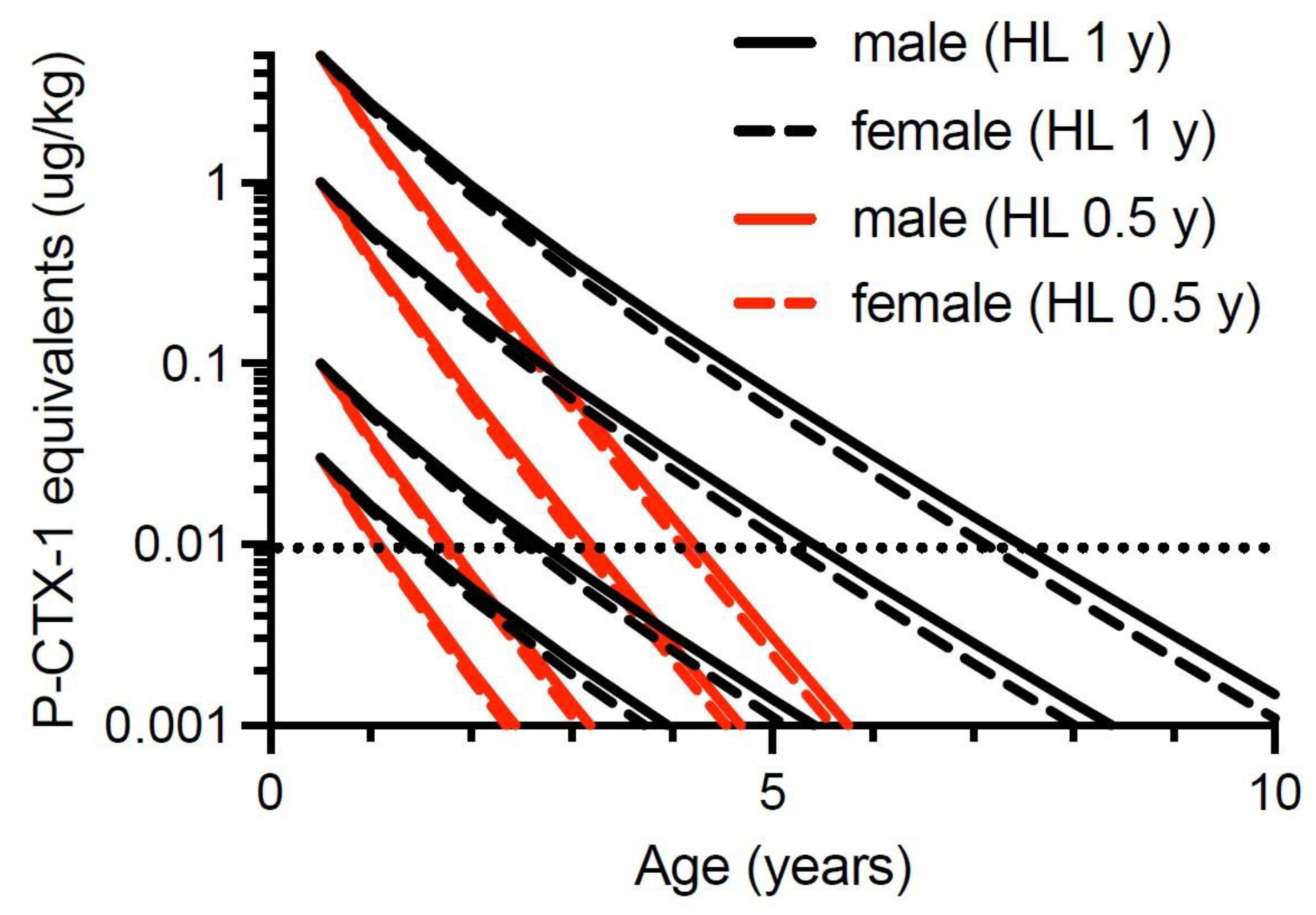

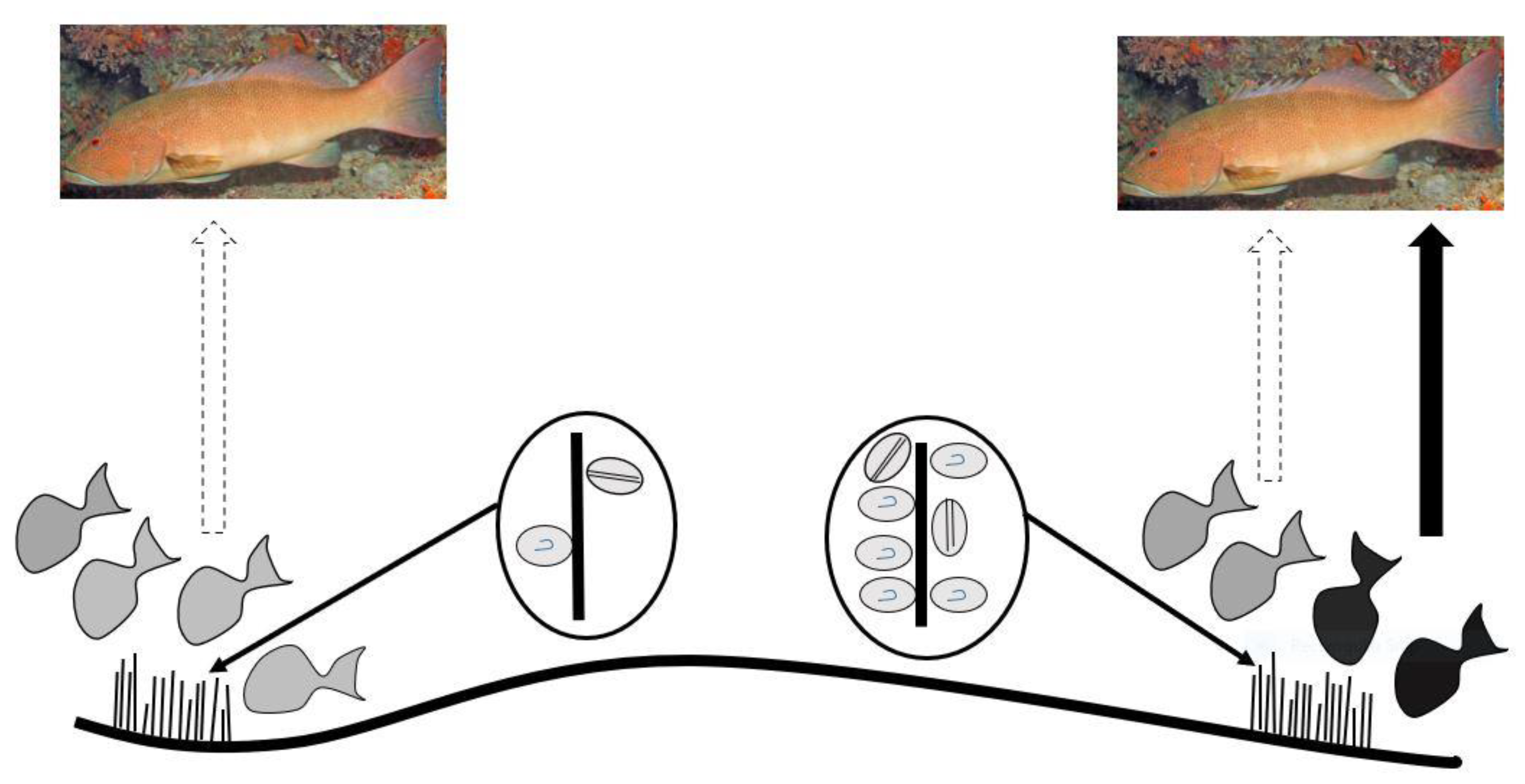
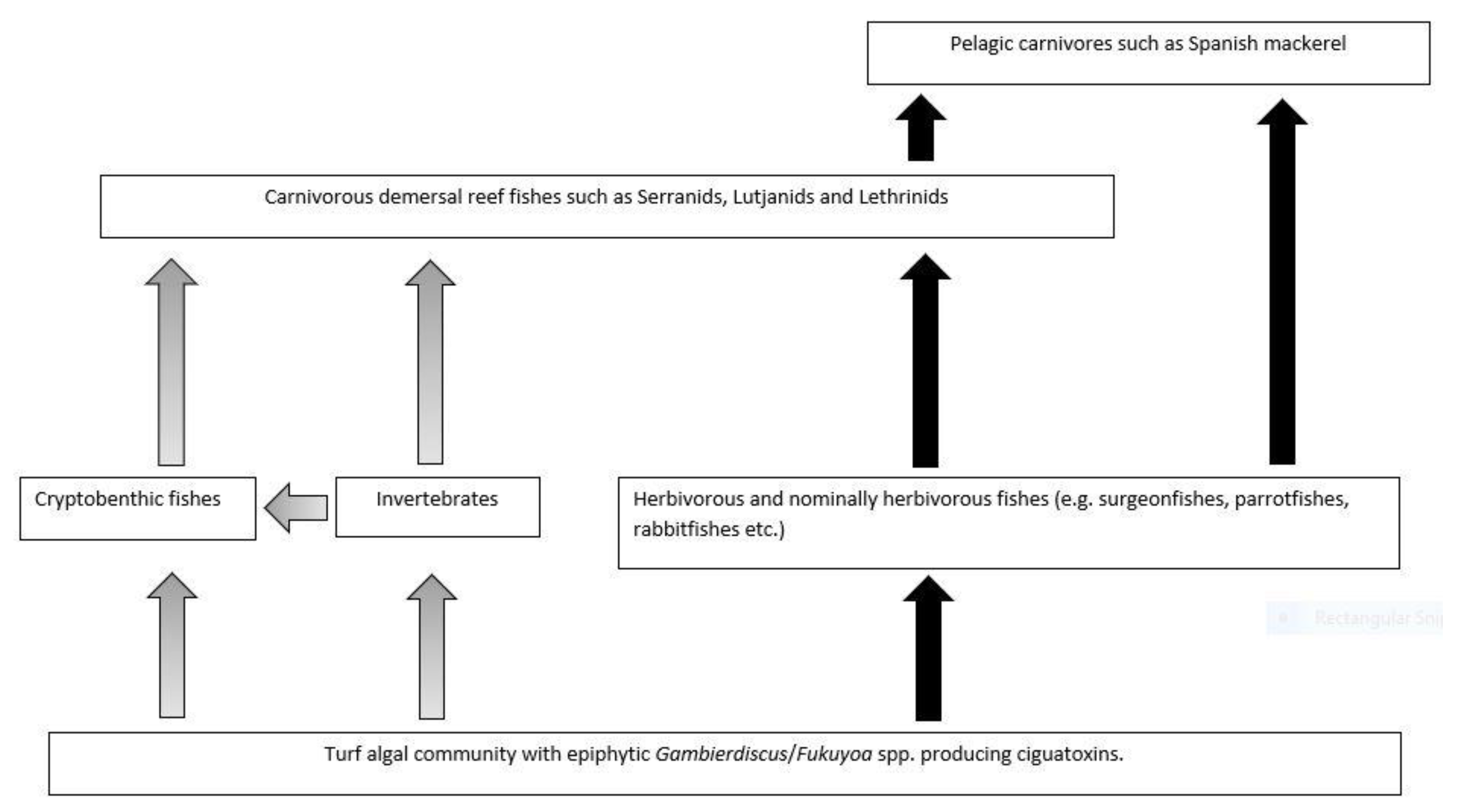
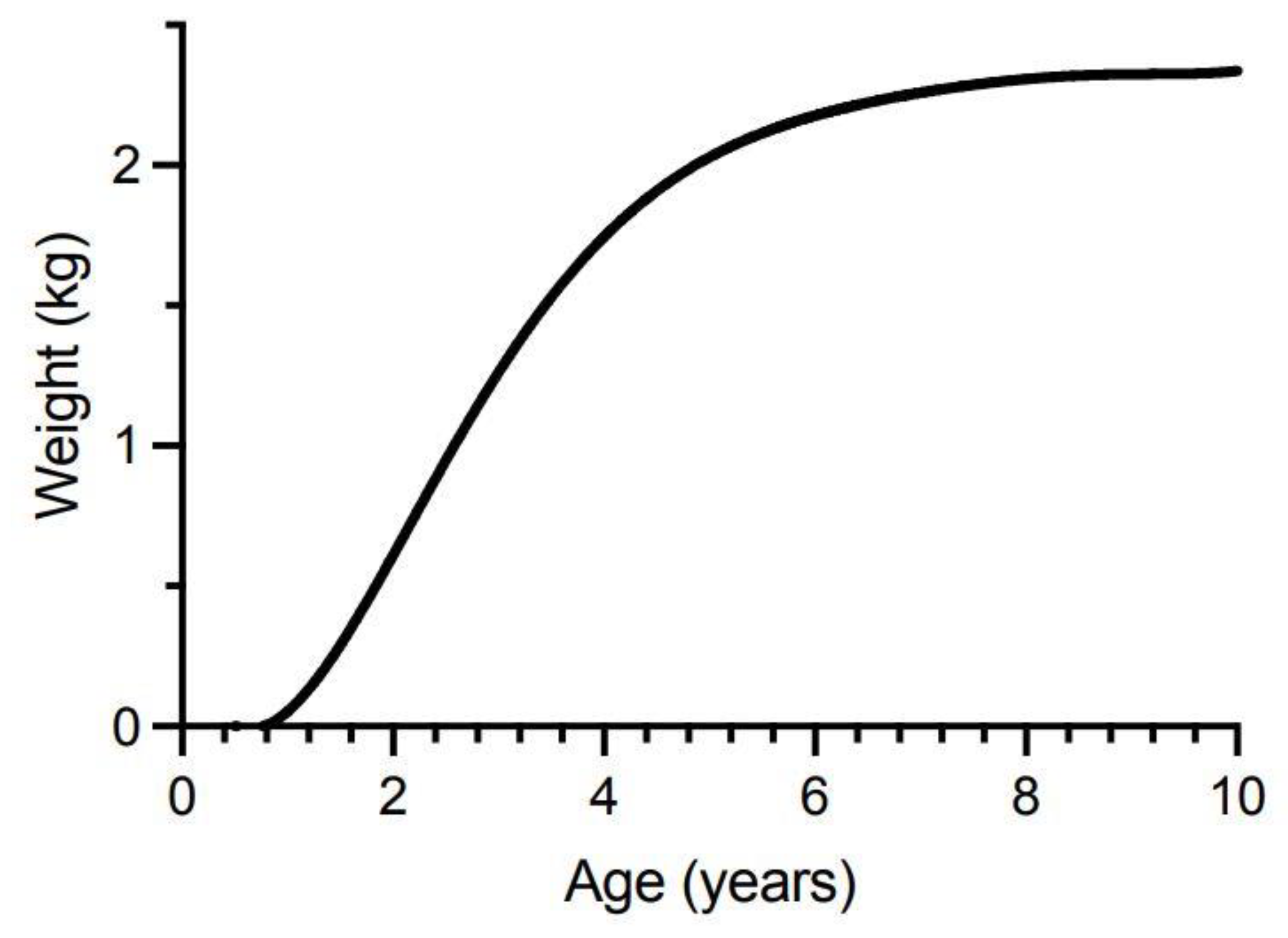
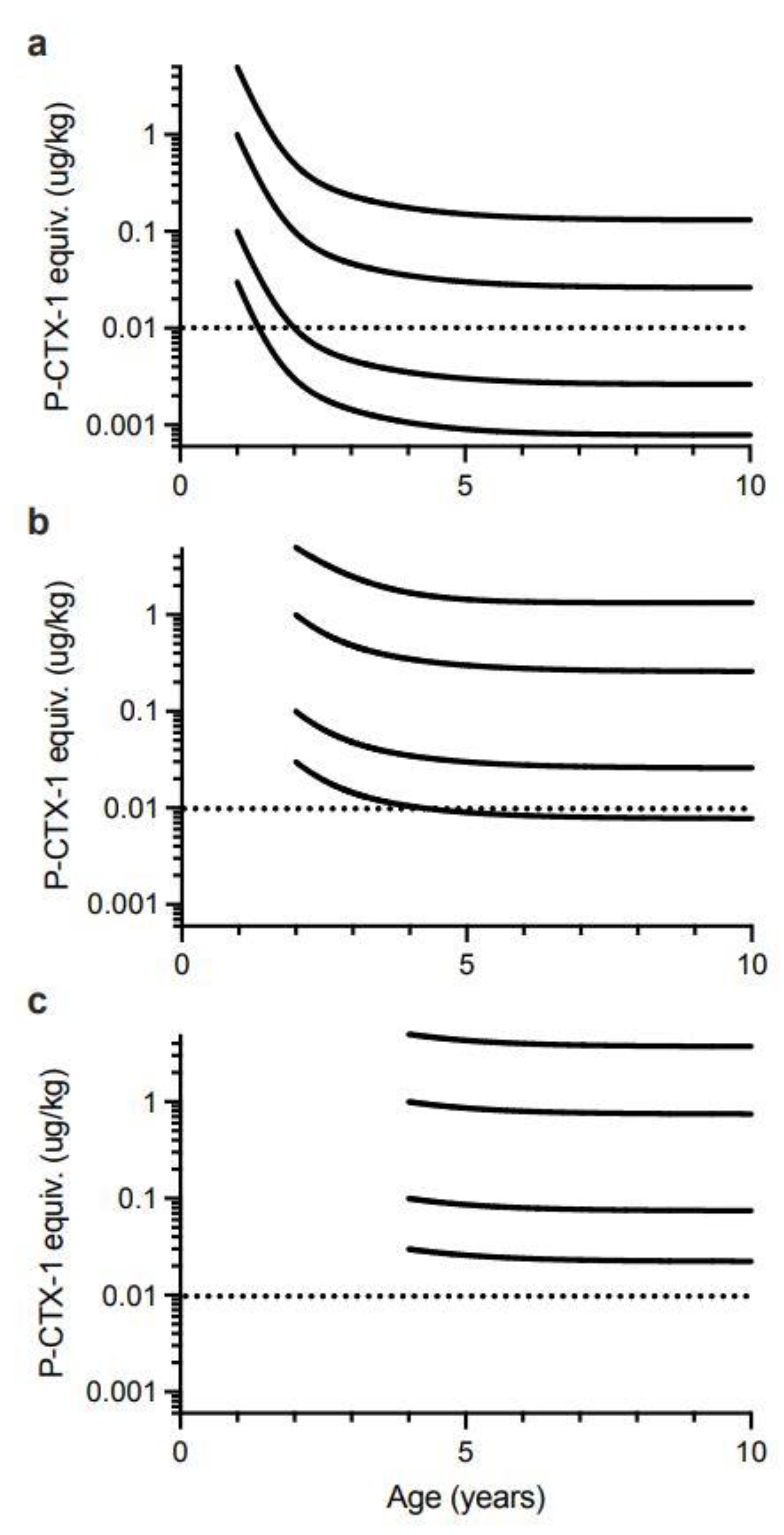
| Years | Rabbitfish Catch (Tonnes) |
|---|---|
| 1994–1998 | 22.12 |
| 1999–2003 | 6.35 |
| 2004–2008 | 13.59 |
| 2009–2013 | 3.19 |
| 2014–2018 | 0.16 |
| Total for all years | 45.41 |
| Gender and Approx. Weight (kg) of 2- or 3-Year-old Ciguatoxic Spanish Mackerel | Initial [P-CTX-1] µg/kg Equivalents | Modelled Fish Age (Years) at Which P-CTX-1 Concentrations of 1.0 or 0.1 µg/kg Reach the Threshold of 0.01 µg/kg Equivalents, from Somatic Growth and Depuration (for Depuration Half-Life’s of 0.5, 1.0, 2.0 or 4.0 Years) | |||
|---|---|---|---|---|---|
| Half-Life = 0.5 Year | Half-Life = 1.0 Year | Half-Life = 2.0 Year | Half-Life = 4.0 Year | ||
| Male 2-year-old, 5.5 kg | 1.0 | 4.9 | 7.5 | 12.4 | 21.6 |
| Male 3-year-old, 7.0 kg | 1.0 | 6.0 | 8.8 | 13.9 | 23.9 |
| Female 2-year-old, 6.6 kg | 1.0 | 4.9 | 7.3 | 11.8 | 20.1 |
| Female 3-year-old, 8.7 kg | 1.0 | 6.0 | 8.6 | 13.4 | 22.5 |
| Male 2-year-old, 5.5 kg | 0.1 | 3.4 | 4.6 | 6.6 | 10.0 |
| Male 3-year-old, 7.0 kg | 0.1 | 4.5 | 5.7 | 8.0 | 11.9 |
| Female 2-year-old, 6.6 kg | 0.1 | 3.4 | 4.5 | 6.3 | 9.2 |
| Female 3-year-old, 8.7 kg | 0.1 | 4.5 | 5.7 | 7.7 | 11.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holmes, M.J.; Venables, B.; Lewis, R.J. Critical Review and Conceptual and Quantitative Models for the Transfer and Depuration of Ciguatoxins in Fishes. Toxins 2021, 13, 515. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13080515
Holmes MJ, Venables B, Lewis RJ. Critical Review and Conceptual and Quantitative Models for the Transfer and Depuration of Ciguatoxins in Fishes. Toxins. 2021; 13(8):515. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13080515
Chicago/Turabian StyleHolmes, Michael J., Bill Venables, and Richard J. Lewis. 2021. "Critical Review and Conceptual and Quantitative Models for the Transfer and Depuration of Ciguatoxins in Fishes" Toxins 13, no. 8: 515. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13080515





