Using Azadirachtin to Transform Spodoptera frugiperda from Pest to Natural Enemy
Abstract
:1. Introduction
2. Results
2.1. Toxicity of Azadirachtin to S. frugiperda
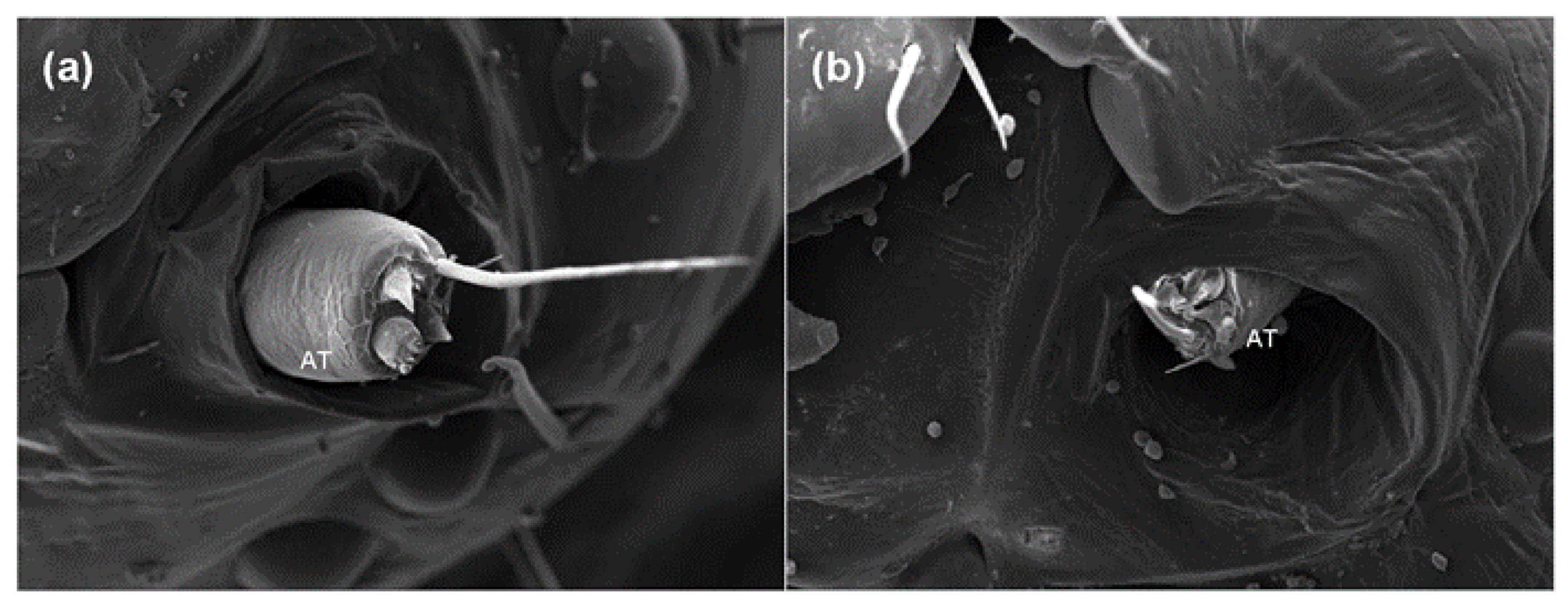
2.2. Effects of Azadirachtin on the Antenna and Sensilla of S. frugiperda
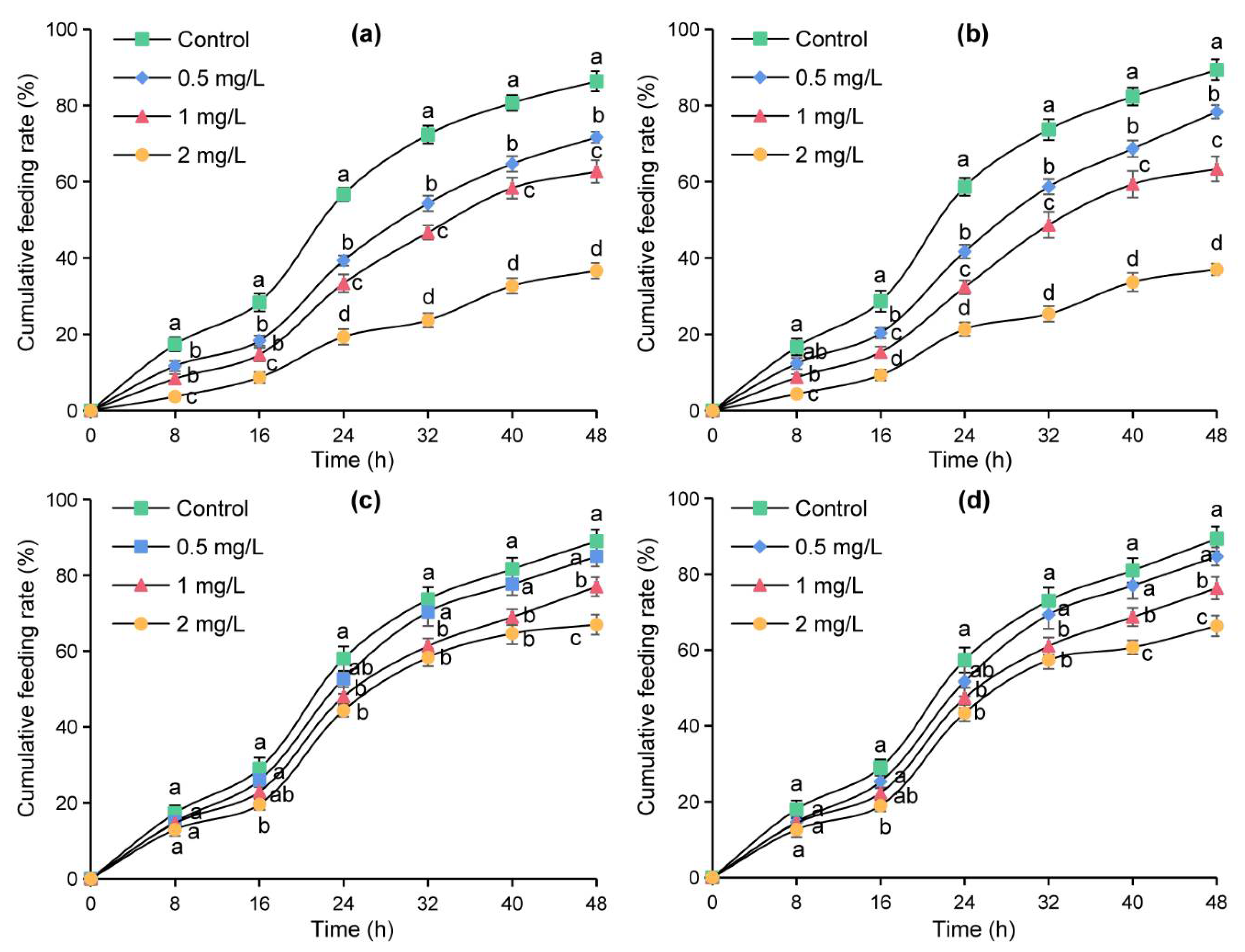
2.3. Effects of Azadirachtin on Sensilla in the Feeding of S. frugiperda
2.4. Effects of Azadirachtin on Predatory Behavior of S. frugiperda
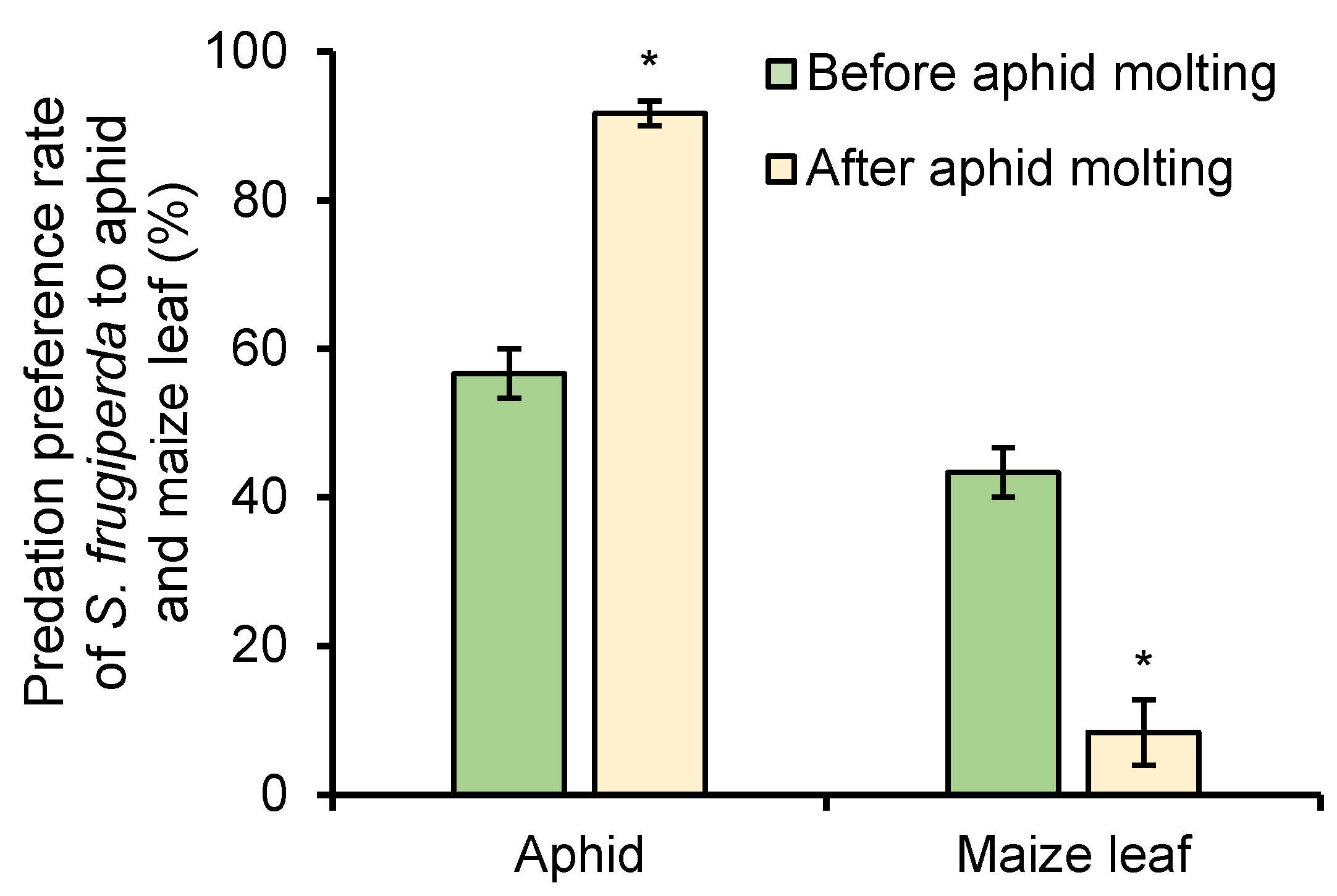
2.5. Effects of Aphid Molting on Predatory Behavior of S. frugiperda under Azadirachtin Treatment
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant, Insects, and Chemicals
5.2. Toxicity of Azadirachtin to S. frugiperda
5.3. Effects of Azadirachtin on the Antennae and Sensilla of S. frugiperda
5.4. Treatment of Four Sensilla by Azadirachtin Solution
5.5. Effects of Azadirachtin on Predatory Behavior of S. frugiperda
5.6. Effects of Aphid Molting on Predatory Behavior of S. frugiperda under Azadirachtin Treatment
5.7. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tao, F.; Zhang, S.; Zhang, Z.; Rötter, R.P. Temporal and spatial changes of maize yield potentials and yield gaps in the past three decades in China. Agric. Ecosyst. Environ. 2015, 208, 12–20. [Google Scholar] [CrossRef]
- He, H.; Hu, Q.; Li, R.; Pan, X.; Huang, B.; He, Q. Regional gap in maize production, climate and resource utilization in China. Field Crop. Res. 2020, 254, 107830. [Google Scholar] [CrossRef]
- Meng, Q.; Hou, P.; Wu, L.; Chen, X.; Cui, Z.; Zhang, F. Understanding production potentials and yield gaps in intensive maize production in China. Field Crop. Res. 2013, 143, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Lima, A.P.S.; Santana, E.D.R.; Santos, A.C.C.; Silva, J.E.; Ribeiro, G.T.; Pinheiro, A.M.; Santos, T.B.F.; Blank, A.F.; Araújo, A.P.A.; Bacci, L. Insecticide activity of botanical compounds against Spodoptera frugiperda and selectivity to the predatory bug Podisus nigrispinus. Crop. Prot. 2020, 136, 105230. [Google Scholar] [CrossRef]
- Luis, A.; Xavier, P. Effect of high temperature on the growth and reproduction of corn aphids (Homoptera: Aphididae) and implications for their population dynamics on the northeastern iberian peninsula. Environ. Entomol. 2001, 30, 1127–1134. [Google Scholar] [CrossRef]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; de Paula-Moraes, S.V.; Peterson, J.A. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef] [Green Version]
- Babendreier, D.; Koku, A.L.; Beseh, P.; Osae, M.; Nboyine, J.; Ofori, S.E.K.; Frimpong, J.O.; Attuquaye, C.V.; Kenis, M. The efficacy of alternative, environmentally friendly plant protection measures for control of fall armyworm, spodoptera frugiperda, in maize. Insects 2020, 11, 240. [Google Scholar] [CrossRef] [PubMed]
- Boaventura, D.; Bolzan, A.; Padovez, F.E.; Okuma, D.M.; Omoto, C.; Nauen, R. Detection of a ryanodine receptor target-site mutation in diamide insecticide resistant fall armyworm, Spodoptera frugiperda. Pest Manag. Sci. 2020, 76, 47–54. [Google Scholar] [CrossRef]
- Cui, L.; Bing, C.; Li, Y.; Wang, J.; Yang, D.; Yan, X.; Guo, Y.; Yuan, H. Research and application of chemical control technology against Spodoptera frugiperda (Lepidoptera:Noctuidae) in foreign countries. Plant Prot. 2019, 45, 7–13. [Google Scholar] [CrossRef]
- Early, R.; Gonzalez-Moreno, P.; Murphy, S.T.; Day, R. Forecasting the global extent of invasion of the cereal pest Spodoptera frugiperda, the fall armyworm. Neobiota 2018, 40, 25–50. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Xu, M.; Quan, Y.; Liao, L.; Gao, L. Potential geographic distribution of Spodoptera frugiperda in China based on MaxEnt model. Plant Quar. 2019, 33, 69–73. [Google Scholar] [CrossRef]
- Kansiime, M.K.; Mugambi, I.; Rwomushana, I.; Nunda, W.; Day, R. Farmer perception of fall armyworm (Spodoptera frugiderda J.E. Smith) and farm-level management practices in Zambia. Pest Manag. Sci. 2019, 75, 2840–2850. [Google Scholar] [CrossRef] [Green Version]
- Tambo, J.A.; Day, R.K.; Lamontagne-Godwin, J.; Silvestri, S.; Beseh, P.K.; Oppong-Mensah, B.; Phiri, N.A.; Matimelo, M. Tackling fall armyworm (Spodoptera frugiperda) outbreak in Africa: An analysis of farmers’ control actions. Int. J. Pest Manag. 2020, 6, 298–310. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Blanco, C.A.; Portilla, M.; Adamczyk, J.; Luttrell, R.; Huang, F. Evidence of multiple/cross resistance to Bt and organophosphate insecticides in Puerto Rico population of the fall armyworm, Spodoptera frugiperda. Pestic. Biochem. Physiol. 2015, 122, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Robert, L.M.J.; Gregg, S.N.; Rodney, N.N.; Mirian, M.H. Parasitoids attacking fall armyworm (Lepidoptera: Noctuidae) in sweet corn habitats. Biol. Control 2016, 95, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Wang, M.; Chen, H.; Wang, Y.; Zhang, H.; Chen, F.; Zhao, X.; Zhang, L. Predatory capacity and behavior of Picromerus lewisi Scott against Spodoptera frugiperda higher instar larve. Chin. J. Biol. Control 2019, 35, 698–703. [Google Scholar] [CrossRef]
- Kuo, M.H.; Chiu, M.C.; Perng, J.J. Temperature effects on life history traits of the corn leaf aphid, Rhopalosiphum maidis (Homoptera: Aphididae) on corn in Taiwan. Appl. Entomol. Zool. 2006, 41, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Foott, W.H. Biology of the corn leaf aphid, Rhopalosiphum Maidis (Homoptera: Aphididae), in Southwestern Ontario. Can. Entomol. 1977, 109, 1129–1135. [Google Scholar] [CrossRef]
- So, Y.S.; Ji, H.C.; Brewbaker, J.L. Resistance to corn leaf aphid (Rhopalosiphum maidis Fitch) in tropical corn (Zea mays L.). Euphytica 2010, 172, 373–381. [Google Scholar] [CrossRef]
- Shi, Z. Key points of integrated pest control in Maize. Rural Sci. Technol. 2019, 103–104. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Tomé, H.V.V.; Martins, J.C.; Corrêa, A.S.; Galdino, T.V.S.; Pican, O.M.C.; Guedes, R.N.C. Azadirachtin avoidance by larvae and adult females of the tomato leafminer Tuta absoluta. Crop Prot. 2013, 46, 63–69. [Google Scholar] [CrossRef]
- Liu, T.; Liu, S.S. Experience-altered oviposition responses to a neem-based product, Neemix, by the diamondback moth, Plutella xylostella. Pest Manag. Sci. 2010, 62, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Marrone, P.G. Pesticidal natural products-status and future potential. Pest Manag. Sci. 2019, 75, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.B.; Melo, J.W.S.; Guedes, N.M.P.; Gontijo, L.M.; Guedes, R.N.C.; Gondim, M.G.C.; Giancarlo, L.M. Bioinsecticide-Predator interactions: Azadirachtin behavioral and reproductive impairment of the coconut mite predator neoseiulus baraki. PLoS ONE 2015, 10, e118343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, D.P.; Guo, J.F.; Jiang, Y.Y.; Zhao, J.Z.; Sethi, A.; He, K.L.; Wang, Z.Y. Initial detections and spread of invasive Spodoptera frugiperda in China and comparisons with other noctuid larvae in cornfields using molecular techniques. Insect Sci. 2020, 27, 780–790. [Google Scholar] [CrossRef]
- Boaventura, D.; Ulrich, J.; Lueke, B.; Bolzan, A.; Okuma, D.; Gutbrod, O.; Geibel, S.; Zeng, Q.; Dourado, P.M.; Martinelli, S.; et al. Molecular characterization of Cry1F resistance in fall armyworm, Spodoptera frugiperda from Brazil. Insect Biochem. Mol. 2020, 116, 103280. [Google Scholar] [CrossRef]
- Huang, F.N.; Qureshi, J.A.; Meagher, R.L.; Reisig, D.D.; Head, G.P.; Andow, D.A.; Ni, X.Z.; Kerns, D.; Buntin, G.D.; Niu, Y.; et al. Cry1F resistance in fall armyworm Spodoptera frugiperda: Single gene versus pyramided Bt maize. PLoS ONE 2014, 9, e112958. [Google Scholar] [CrossRef] [Green Version]
- Bezzar-Bendjazia, R.; Kilani-Morakchi, S.; Maroua, F.; Aribi, N. Azadirachtin induced larval avoidance and antifeeding by disruption of food intake and digestive enzymes in Drosophila melanogaster (Diptera: Drosophilidae). Pestic. Biochem. Physiol. 2017, 143, 135–140. [Google Scholar] [CrossRef]
- Radhika, S.; Sahayaraj, K.; Senthil-Nathan, S.; Hunter, W.B. Individual and synergist activities of monocrotophos with neem based pesticide, Vijayneem against Spodoptera litura Fab. Physiol. Mol. Plant Pathol. 2017, 101, 54–68. [Google Scholar] [CrossRef]
- Mordue, A.J.; Morgan, E.D.; Nisbet, A.J. Azadirachtin, a natural product in insect control. Compr. Mol. Insect Sci. 2005, 6, 117–135. [Google Scholar] [CrossRef]
- Barbosa, W.F.; Meyer, L.D.; Guedes, R.N.C.; Smagghe, G. Lethal and sublethal effects of azadirachtin on the bumblebee Bombus terrestris (Hymenoptera: Apidae). Ecotoxicology 2015, 24, 130–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, M.; Liu, M.T.; Nguyen, M.Q.; Ryan, K. Single-Neuron comparison of the olfactory receptor response to deuterated and nondeuterated odorants. ACS Chem. Neurosci. 2019, 10, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Bleeker, M.A.K.; Smid, H.M.; Van Aelst, A.C.; Van Loon, J.J.A.; Vet, L.E.M. Antennal sensilla of two parasitoid wasps: A comparative scanning electron microscopy study. Microsc. Res. Tech. 2004, 63, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Loon, V.J.J.A. Chemosensory basis of feeding and oviposition behaviour in herbivorous insects: A glance at the periphery. Entomol. Exp. Appl. 1996, 80, 7–13. [Google Scholar] [CrossRef]
- Qin, D.; Zhang, P.; Zhou, Y.; Liu, B.; Zhang, Z. Antifeeding effects of azadirachtin on the fifth instar Spodoptera litura larvae and the analysis of azadirachtin on target sensilla around mouthparts. Arch. Insect Biochem. 2020, 103, 5318. [Google Scholar] [CrossRef]
- Mutuel, D.; Ravallec, M.; Chabi, B.; Multeau, C.; Salmon, J.M.; Fournier, P.; Ogliastro, M. Pathogenesis of Junonia coenia densovirus in Spodoptera frugiperda: A route of infection that leads to hypoxia. Virology 2010, 403, 137–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, N.C.; Kieckhefer, R.W.; Walgenbach, D.D. Effects of constant and fluctuating temperatures on developmental rates and demographic statistics for the corn leaf aphid (Homoptera: Aphididae). J. Econ. Entomol. 1988, 81, 1383–1389. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.; Li, S.; Liu, Z.; Zhang, L.; Wu, H.; Cheng, D.; Zhang, Z. Using Azadirachtin to Transform Spodoptera frugiperda from Pest to Natural Enemy. Toxins 2021, 13, 541. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13080541
Lin S, Li S, Liu Z, Zhang L, Wu H, Cheng D, Zhang Z. Using Azadirachtin to Transform Spodoptera frugiperda from Pest to Natural Enemy. Toxins. 2021; 13(8):541. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13080541
Chicago/Turabian StyleLin, Sukun, Shengnan Li, Zhenghui Liu, Li Zhang, Hao Wu, Dongmei Cheng, and Zhixiang Zhang. 2021. "Using Azadirachtin to Transform Spodoptera frugiperda from Pest to Natural Enemy" Toxins 13, no. 8: 541. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13080541




