Effects of Botulinum Toxin Type A on Pain among Trigeminal Neuralgia, Myofascial Temporomandibular Disorders, and Oromandibular Dystonia
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Treatment Outcomes
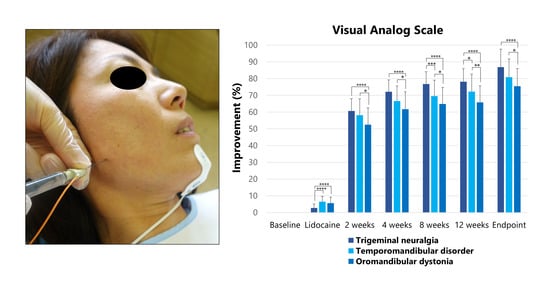
2.2.1. Visual Analog Scale
2.2.2. Pain Frequency
2.2.3. Pain Scale of the Oromandibular Dystonia Rating Scale (OMDRS)
2.3. Trigeminal Neuralgia (TN)
2.4. Myofascial Temporomandibular Disorders (TMD)
2.5. Oromandibular Dystonia (OMD)
3. Discussion
3.1. Mechanism of BoNT/A on Muscle Relaxation and Pain Relief
3.2. Limitations of This Study
3.3. Effects of BoNT/A on TN
3.4. Effects of BoNT/A on Myofascial TMD
3.5. Effects of BoNT/A on OMD
3.6. Difference of Effects of BoNT/A among the Three Groups
3.7. Adverse Effects of BoNT/A
3.8. Future Study on the Clinical Use of BoNT/A
4. Conclusions
5. Materials and Methods
5.1. Patients
5.1.1. TN
5.1.2. Myofascial TMD
5.1.3. OMD
5.2. Botulinum Toxin Therapy
5.2.1. TN
5.2.2. Myofascial TMD and OMD
5.3. Analysis
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simpson, L.L. The origin, structure, and pharmacologic activity of botulinum toxin. Pharmacol. Rev. 1981, 33, 155–188. [Google Scholar]
- Jankovic, J.; Brin, M.F. Therapeutic uses of botulinum toxin. N. Engl. J. Med. 1991, 324, 1186–1194. [Google Scholar] [PubMed]
- Truong, D.D.; Stenner, A.; Reichel, G. Current clinical applications of botulinum toxin. Curr. Pharm. Des. 2009, 15, 3671–3680. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M.; Albanese, A.; Dressler, D.; Segal, K.R.; Simpson, D.M.; Truong, D.; Jankovic, J. Evidence-based review and assessment of botulinum neurotoxin for the treatment of movement disorders. Toxicon 2013, 67, 94–114. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. An update on new and unique uses of botulinum toxin in movement disorders. Toxicon 2017, 147, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Blitzer, A.; Brin, M.; Greene, P.E.; Fahn, S. Botulinum toxin injection for the treatment of oromandibular dystonia. Ann. Otol. Rhinol. Laryngol. 1989, 98, 93–97. [Google Scholar] [CrossRef]
- Tan, E.K.; Jankovic, J. Botulinum toxin A in patients with oromandibular dystonia Long-term follow-up. Neurology 1999, 53, 2102–2107. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Iizuka, T. Botulinum toxin treatment for upper airway collapse resulting from temporomandibular joint dislocation due to jaw-opening dystonia. Cranio 2006, 24, 217–222. [Google Scholar] [CrossRef]
- Sinclair, C.F.; Gurey, L.E.; Blitzer, A. Oromandibular dystonia: Long-term management with botulinum toxin. Laryngoscope 2013, 123, 3078–3083. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Computer-aided design/computer-assisted manufacture-derived needle guide for injection of botulinum toxin into the lateral pterygoid muscle in patients with oromandibular dystonia. J. Oral Facial Pain Headache 2018, 32, e13–e21. [Google Scholar] [CrossRef]
- Yoshida, K. How do I inject botulinum toxin into the lateral and medial pterygoid muscles? Mov. Disord. Clin. Pract. 2016, 4, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, K. Botulinum neurotoxin therapy for lingual dystonia using an individualized injection method based on clinical features. Toxins 2019, 11, 51. [Google Scholar] [CrossRef] [Green Version]
- Borodic, G.E.; Acquadro, M.A. The use of botulinum toxin for the treatment of chronic facial pain. J. Pain 2002, 3, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Piovesan, E.J.; Teive, H.G.; Kowacs, P.A.; Della Coletta, M.V.; Werneck, L.C.; Silberstein, S.D. An open study of botulinum-A toxin treatment of trigeminal neuralgia. Neurology 2005, 65, 1306–1308. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga, C.; Díaz, S.; Piedimonte, F.; Micheli, F. Beneficial effects of botulinum toxin type A in trigeminal neuralgia. Arq. Neuropsiquiatr. 2008, 66, 500–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohluli, B.; Motamedi, M.H.; Bagheri, S.C.; Bayat, M.; Lassemi, E.; Navi, F.; Moharamnejad, N. Use of botulinum toxin A for drug-refractory trigeminal neuralgia: Preliminary report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 111, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.J.; Lian, Y.J.; Zheng, Y.K.; Zhang, H.F.; Chen, Y.; Xie, N.C.; Wang, L.J. Botulinum toxin type A for the treatment of trigeminal neuralgia: Results from a randomized, double-blind, placebo-controlled trial. Cephalalgia 2012, 32, 443–450. [Google Scholar] [CrossRef]
- Zúñiga, C.; Piedimonte, F.; Díaz, S.; Micheli, F. Acute treatment of TN with onabotulinum toxin A. Clin. Neuropharmacol. 2013, 36, 146–150. [Google Scholar] [CrossRef]
- Shehata, H.S.; El-Tamawy, M.S.; Shalaby, N.M.; Ramzy, G. Botulinum toxin type A: Could it be an effective treatment option in intractable trigeminal neuralgia? J. Headache Pain 2013, 14, 92. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Lian, Y.; Ma, Y.; Chen, Y.; He, C.; Xie, N.; Wu, C. Two doses of botulinum toxin type A for the treatment of trigeminal neuralgia: Observation of therapeutic effect from a randomized, double-blind, placebo-controlled trial. J. Headache Pain 2014, 15, 65. [Google Scholar] [CrossRef] [Green Version]
- Freund, B.; Schwartz, M. The use of botulinum toxin for the treatment of temporomandibular disorder. Oral Health 1998, 88, 32–37. [Google Scholar]
- von Lindern, J.J. Type A botulinum toxin in the treatment of chronic facial pain associated with temporo-mandibular dysfunction. Acta Neurol. Belg. 2001, 101, 39–41. [Google Scholar]
- Nixdorf, D.R.; Heo, G.; Major, P.W. Randomized controlled trial of botulinum toxin for chronic myogenous orofacial pain. Pain 2002, 99, 465–473. [Google Scholar] [CrossRef]
- Guarda-Nardini, L.; Manfredini, D.; Salamone, M.; Salmaso, S.; Tonello, S.; Ferronato, G. Efficacy of botulinum toxin in treating myofascial pain in bruxers: A controlled placebo pilot study. Cranio 2008, 26, 126–135. [Google Scholar] [CrossRef]
- Kurtoglu, C.; Gur, O.H.; Kurkcu, M.; Sertdemir, Y.; Guler-Uysal, F.; Uysal, H. Effect of botulinum toxin-A in myofascial pain patients with or without functional disc displacement. J. Oral Maxillofac. Surg. 2008, 66, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Ernberg, M.; Hedenberg-Magnusson, B.; List, T.; Svensson, P. Efficacy of botulinum toxin type A for treatment of persistent myofascial TMD pain: A randomized, controlled, double-blind multicenter study. Pain 2011, 152, 1988–1996. [Google Scholar] [CrossRef]
- Guarda-Nardini, L.; Stecco, A.; Stecco, C.; Masiero, S.; Manfredini, D. Myofascial pain of the jaw muscles: Comparison of short-term effectiveness of botulinum toxin injections and Fascial Manipulation technique. Cranio 2012, 30, 95–102. [Google Scholar] [CrossRef]
- Patel, A.A.; Lerner, M.Z.; Blitzer, A. IncobotulinumtoxinA injection for temporomandibular joint disorder. Ann. Otol. Rhinol. Laryngol. 2017, 126, 328–333. [Google Scholar] [CrossRef] [PubMed]
- De la Torre Canales, G.; Alvarez-Pinzon, N.; Victor Ricardo Manuel Muñoz-Lora, V.R.M.; Peroni, L.V.; Amanda Farias Gomes, A.F.; Sánchez-Ayala, A.; Haiter-Neto, F.; Manfredini, D.; Rizzatti-Barbosa, C.M. Efficacy and safety of botulinum toxin type A on persistent myofascial pain: A randomized clinical trial. Toxins 2020, 12, 395. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse-Smith, D.; Begley, A.; Dodd, M. Clinical evaluation of botulinum toxin A in the management of temporomandibular myofascial pain. Br. J. Oral Maxillofac. Surg. 2020, 58, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Montes-Carmona, J.; Gonzalez-Perez, L.; Infante-Cossio, P. Treatment of localized and referred masticatory myofascial pain with botulinum toxin injection. Toxins 2021, 13, 6. [Google Scholar] [CrossRef]
- Pellett, S.; Yaksh, T.L.; Ramachandran, R. Current status and future directions of botulinum neurotoxins for targeting pain processing. Toxins 2015, 7, 4519–4563. [Google Scholar] [CrossRef]
- Matak, I.; Bölcskei, K.; Bach-rojecky, L.; Helyes, Z. Mechanisms of botulinum toxin type A action on pain. Toxins 2019, 11, 459. [Google Scholar] [CrossRef] [Green Version]
- Burstein, R.; Blumenfeld, A.M.; Silberstein, S.D.; Adams, A.M.; Brin, M.F. Mechanism of action of onabotulinumtoxinA in chronic migraine: A narrative review. Headache 2020, 60, 1259–1272. [Google Scholar] [CrossRef]
- Headache classification committee of the international headache society (IHS). The international classification of headache disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Bhatia, K.; Bressman, S.B.; Delong, M.R.; Fahn, S.; Fung, V.S.; Hallett, M.; Jankovic, J.; Jinnah, H.A.; Klein, C.; et al. Phenomenology and classification of dystonia: A consensus up date. Mov. Disord. 2013, 28, 863–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, K. Oromandibular dystonia screening questionnaire for differential diagnosis. Clin. Oral Investig. 2019, 23, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Development and validation of a disease-specific oromandibular dystonia rating scale (OMDRS). Front. Neurol. 2020, 11, 583177. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Behandlungsstrategien bei oromandibulärer Dystonie. Fortschr. Neurol. Psychiatr. 2021, 89, 1–11. [Google Scholar] [CrossRef]
- Yoshida, K. Botulinum neurotoxin injection for the treatment of recurrent temporomandibular joint dislocation with and without neurogenic muscular hyperactivity. Toxins 2018, 10, 174. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K. Sphenopalatine ganglion block with botulinum neurotoxin for treating trigeminal neuralgia using CAD/CAM-derived injection guide. J. Oral Facial Pain Headache 2020, 34, 135–140. [Google Scholar] [CrossRef]
- Bach-Rojecky, L.; Lackovi’c, Z. Central origin of the antinociceptive action of botulinum toxin type A. Pharmacol. Biochem. Behav. 2009, 94, 234–238. [Google Scholar] [CrossRef] [Green Version]
- Matak, I.; Bach-Rojecky, L.; Filipovi´c, B.; Lackovi´c, Z. Behavioral and immunohistochemical evidence for central antinociceptive activity of botulinum toxin A. Neuroscience 2011, 186, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Purkiss, J.; Welch, M.; Doward, S.; Foster, K. Capsaicin-stimulated release of substance P from cultured dorsal root ganglion neurons: Involvement of two distinct mechanisms. Biochem. Pharmacol. 2000, 59, 1403–1406. [Google Scholar] [CrossRef]
- Aoki, K. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. NeuroToxicology 2005, 26, 785–793. [Google Scholar] [CrossRef]
- Yoshida, K. Involuntary Movements of the Stomatognathic Region. 2021. Available online: https://sites.google.com/site/oromandibulardystoniaenglish (accessed on 11 April 2021).
- Yoshida, K. Multilingual website and cyberconsultations for oromandibular dystonia. Neurol. Int. 2018, 10, 7536. [Google Scholar] [CrossRef]
- Isselée, H.; De Laat, A.; Bogaerts, K.; Lysens, R. Short-term reproducibility of pressure pain thresholds in masticatory muscles measured with a new algometer. J. Orofac. Pain 1998, 12, 203–209. [Google Scholar]
- Ferreira-Valente, M.A.; Pais-Ribeiro, J.L.; Jensen, M.P. Validity of four pain intensity rating scales. Pain 2011, 152, 2399–2404. [Google Scholar] [CrossRef]
- Yoshida, K.; Kaji, R.; Kubori, T.; Kohara, N.; Iizuka, T.; Kimura, J. Muscle afferent block for the treatment of oromandibular dystonia. Mov. Disord. 1998, 13, 699–705. [Google Scholar] [CrossRef]
- Yoshida, K.; Kaji, R.; Shibasaki, H.; Iizuka, T. Factors influencing the therapeutic effect of muscle afferent block for oromandibular dystonia and dyskinesia: Implications for their distinct pathophysiology. Int. J. Oral Maxillofac. Surg. 2002, 31, 499–505. [Google Scholar] [CrossRef]
- Yoshida, K.; Kaji, R.; Takagi, A.; Iizuka, T. Customized EMG needle insertion guide for the muscle afferent block of jaw-deviation and jaw-opening dystonias. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1999, 88, 664–669. [Google Scholar] [CrossRef]
- Al-Moraissi, E.A.; Alradom, J.; Aladashi, O.; Goddard, G.; Christidis, N. Needling therapies in the management of myofascial pain of the masticatory muscles: A network meta-analysis of randomised clinical trials. J. Oral Rehabil. 2020, 47, 910–922. [Google Scholar] [CrossRef]
- McMillan, A.S.; Nolan, A.; Kelly, P.J. The efficacy of dry needling and procaine in the treatment of myofascial pain in the jaw muscles. J. Orofac. Pain 1997, 11, 307–314. [Google Scholar] [PubMed]
- Cummings, T.M.; White, A.R. Needling therapies in the management of myofascial trigger point pain: A systematic review. Arch. Phys. Med. Rehabil. 2001, 82, 986–992. [Google Scholar] [CrossRef] [Green Version]
- Türk, Ü.; Ilhan, S.; Alp, R.; Sur, H. Botulinum toxin and intractable TN. Clin. Neuropharmacol. 2005, 28, 161–162. [Google Scholar] [CrossRef]
- Türk Börü, Ü.; Duman, A.; Bölük, C.; Coşkun Duman, S.; Taşdemir, M. Botulinum toxin in the treatment of trigeminal neuralgia: 6-Month follow-up. Medicine 2017, 96, e8133. [Google Scholar] [CrossRef]
- Bratbak, D.F.; Nordgård, S.; Stovner, L.J.; Linde, M.; Dodick, D.W.; Aschehoug, I.; Folvik, M.; Tronvik, E. Pilot study of sphenopalatine injection of onabotulinum toxin A for the treatment of intractable chronic migraine. Cephalalgia 2017, 37, 356–364. [Google Scholar] [CrossRef] [Green Version]
- Ohrbach, R.; Dworkin, S.F. The Evolution of TMD Diagnosis: Past, Present, Future. J. Dent. Res. 2016, 95, 1093–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qerama, E.; Fuglsang-Frederiksen, A.; Jensen, T.S. The role of botulinum toxin in management of pain: An evidence-based review. Curr. Opin. Anaesthesiol. 2010, 23, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Adatia, A.; Zarin, W.; Moitri, M.; Vijenthira, A.; Chu, R.; Thabane, L.; Kean, W. The efficacy of botulinum toxin type A in managing chronic musculoskeletal pain: A systematic review and meta analysis. Inflammopharmacology 2011, 19, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Prevalence and incidence of oromandibular dystonia: An oral and maxillofacial surgery service-based study. Clin. Oral Investig. 2021, 1–10. [Google Scholar] [CrossRef]
- Yoshida, K. Sensory trick splint as a multimodal therapy for oromandibular dystonia. J. Prosthodont. Res. 2018, 62, 239–244. [Google Scholar] [CrossRef]
- Yoshida, K. Coronoidotomy as treatment for trismus due to jaw-closing oromandibular dystonia. Mov. Disord. 2006, 21, 1028–1031. [Google Scholar] [CrossRef]
- Yoshida, K. Surgical intervention for oromandibular dystonia-related limited mouth opening: Long-term follow-up. J. Cranio Maxillofac. Surg. 2017, 45, 56–62. [Google Scholar] [CrossRef]
- Yoshida, K. Mouth opening retaining appliance after coronoidotomy for the treatment of trismus: Effects on pain during postoperative training and maximal extent of mouth opening. Clin. Surg. 2020, 5, 2737. [Google Scholar]
- Rafferty, K.L.; Liu, Z.J.; Ye, W.; Navarrete, A.L.; Nguyen, T.T.; Salamati, A.; Herring, S.W. Botulinum toxin in masticatory muscles: Short- and long-term effects on muscle, bone, and craniofacial function in adult rabbits. Bone 2012, 50, 651–662. [Google Scholar] [CrossRef] [Green Version]
- Matthys, T.; Ho Dang, H.A.; Rafferty, K.L.; Herring, S.W. Bone and cartilage changes in rabbit mandibular condyles after 1 injection of botulinum toxin. Am. J. Orthod. Dentofac. Orthop. 2015, 148, 999–1009. [Google Scholar] [CrossRef] [Green Version]
- Balanta-Melo, J.; Toro-Ibacache, V.; Torres-Quintana, M.A.; Kupczik, K.; Vega, C.; Morales, C.; Hernández-Moya, N.; Arias-Calderón, M.; Beato, C.; Buvinic, S. Early molecular response and microanatomical changes in the masseter muscle and mandibular head after botulinum toxin intervention in adult mice. Ann. Anat. 2018, 216, 112–119. [Google Scholar] [CrossRef]
- Raphael, K.G.; Tadinada, A.; Bradshaw, J.M.; Janal, M.N.; Sirois, D.A.; Chan, K.C.; Lurie, A.G. Osteopenic consequences of botulinum toxin injections in the masticatory muscles: A pilot study. J. Oral Rehabil. 2014, 41, 555–563. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, S.-J.; Lee, K.-J.; Yu, H.-S.; Baik, H.-S. Repeated injections of botulinum toxin into the masseter muscle induce b5ony changes in human adults: A longitudinal study. Korean J. Orthod. 2017, 47, 222–228. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.S.; Bergeron, L.; Yu, C.C.; Chen, P.K.; Chen, Y.R. Mandible changes evaluated by computed tomography following Botulinum Toxin A injections in square-faced patients. Aesthetic Plast. Surg. 2011, 35, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Yokoe, Y.; Yasuda, S.; Tsuboi, Y.; Iizuka, T. Prolonged mandibular hypomobility patient with a “square mandible” configuration with coronoid process and angle hyperplasia. Cranio 2000, 18, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.P. Diagnostic Criteria for TMD (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J. Orofac. Pain 2014, 28, 6–27. [Google Scholar] [CrossRef]
- Yoshida, K. Clinical characteristics of functional movement disorders in the stomatognathic system. Front. Neurol. 2020, 11, 23. [Google Scholar] [CrossRef] [Green Version]



| Disease Groups | N | Age (Years) [Mean (SD)] | Sex (Female, Male) [N (%)] | Duration (Years) [Mean (SD)] | Visual Analog Scale at Baseline [Mean (SD)] | Pain Frequency at Baseline (Times/Day) [Mean (SD)] | Pain Scale of Oromandibular Dystonia Rating Scale (OMDRS) (Points) [Mean (SD)] | Psychiatric Disease [N (%)] | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Severity | Duration | Degree | Total | ||||||||
| TN | 28 | 68.2 (13.6) | 23 (82.1), 5 (17.9) | 6.3 (5.6) | 89.3 7.5) | 19.1 (7.7) | 18.9 (5.1) | 4.8 (1.5) | 4.9 (1.4) | 28.6 (3.6) | 4 (14.3) |
| TMD | 53 | 46.1 (17.6) | 39 (73.6), 14 (26.4) | 10.1 (6.5) | 71.3 (19.3) | 13.8 (6.8) | 10.9 (5.9) | 3.6 (1.2) | 3.5 (1.1) | 21.6 (7.5) | 17 (32.1) |
| OMD | 89 | 56.4 (16.2) | 60 (67.4), 29 (32.6) | 3.7 (4.5) | 70.3 (19.1) | 12.5 (5.7) | 10.4 (5.2) | 3.5 (1.3) | 3.1 (1.0) | 20.3 (7.5) | 40 (44.9) |
| Total | 170 | 55.2 (17.9) | 122 (71.8), 48 (28.2) | 4.9 (5.5) | 73.4 (19.1) | 14.0 (6.8) | 11.6 (5.6) | 3.7 (1.2) | 3.4 (1.1) | 22.1 (7.6) | 61 (35.9) |
| Groups | Visual Analog Scale [Mean (SD)] | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | After Lidocaine Injection | After BoNT/A Injection | |||||
| 2 Weeks | 4 Weeks | 8 Weeks | 12 Weeks | Endpoint | |||
| TN | 89.3 (7.5) | 86.8 (7.3) | 35.1 (6.6) | 25.0 (6.8) | 20.8 (7.0) | 19.5 (7.3) | 11.9 (9.8) |
| TMD | 71.2 (19.3) | 65.6 (18.1) | 28.8 (9.4) | 23.2 (8.9) | 21.2 (9.2) | 19.8 (9.8) | 14.2 (9.2) |
| OMD | 70.0 (19.1) | 64.5 (17.7) | 32.4 (8.2) | 26.0 (7.5) | 24.0 (7.8) | 23.5 (8.0) | 17.3 (8.5) |
| Total | 73.4 (19.1) | 66.3 (17.6) | 31.7 (8.6) | 25.0 (7.9) | 22.6 (8.2) | 21.7 (8.6) | 15.4 (9.1) |
| Groups | Pain Frequency (Times/Day) [Mean (SD)] | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | After Lidocaine Injection | After BoNT/A Injection | |||||
| 2 Weeks | 4 Weeks | 8 Weeks | 12 Weeks | Endpoint | |||
| TN | 19.1 (7.7) | 18.6 (7.5) | 9.8 (4.9) | 5.6 (3.5) | 4.2 (2.9) | 3.7 (2.6) | 3.1 (2.3) |
| TMD | 13.8 (6.8) | 13.2 (6.5) | 8.0 (4.7) | 5.3 (3.2) | 4.3 (2.6) | 4.0 (2.6) | 3.3 (2.3) |
| OMD | 12.5 (5.7) | 12.0 (5.7) | 7.4 (4.0) | 5.1 (2.9) | 4.0 (2.3) | 3.6 (2.0) | 3.0 (1.7) |
| Total | 14.0 (6.8) | 13.5 (6.7) | 8.0 (4.5) | 5.3 (3.1) | 4.2 (2.5) | 3.7 (2.3) | 3.1 (2.0) |
| Groups | Pain Scale of Oromandibular Dystonia Rating Scale (OMDRS) (Point) [Mean (SD)] | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | After Lidocaine Injection | After BoNT/A Injection | |||||
| 2 Weeks | 4 Weeks | 8 Weeks | 12 Weeks | Endpoint | |||
| TN | 28.6 (3.6) | 27.3 (3.5) | 13.1 (3.3) | 7.8 (1.5) | 5.3 (1.9) | 4.6 (2.3) | 3.1 (2.3) |
| TMD | 21.6 (7.5) | 20.4 (7.0) | 10.3 (4.1) | 6.5 (3.0) | 4.9 (2.2) | 4.6 (2.4) | 3.3 (2.2) |
| OMD | 20.3 (7.5) | 19.1 (7.1) | 9.8 (4.2) | 6.4 (3.2) | 5.3 (2.8) | 5.1 (2.8) | 3.9 (2.3) |
| Total | 22.1 (7.6) | 20.4 (7.3) | 10.5 (4.2) | 6.7 (2.9) | 5.2 (2.5) | 4.9 (2.6) | 3.6 (2.3) |
| 1. Rate the severity of pain during the last week on a scale of 0 to 10, where a score of 1 represents a minimal ache and 10 represents the most excruciating pain imaginable. | |
| Best | 0 to 10 |
| Worst | 0 to 10 |
| Usual | 0 to 10 |
| 2. Rate the duration of pain | |
| None | 0 |
| Present < 10% of the time | 1 |
| Present 10% to <25% of the time | 2 |
| Present 25% to <50% of the time | 3 |
| Present 50% to <75% of the time | 4 |
| Present ≥ 75% of the time | 5 |
| 3. Rate the degree to which pain contributes to disability | |
| No limitation or interference from pain | 0 |
| Pain is quite bothersome but not a source of disability | 1 |
| Pain definitely interferes with some tasks a major contributor to disability | 2 |
| Pain accounts for some (less than half) disability | 3 |
| Pain is a major source of difficulty with activities; separate from this, muscle contraction is also a source of some (less than half) disability | 4 |
| Pain is the major source of disability; without it, most impaired activities could be performed quite satisfactorily | 5 |
| 4. Total Pain Score | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, K. Effects of Botulinum Toxin Type A on Pain among Trigeminal Neuralgia, Myofascial Temporomandibular Disorders, and Oromandibular Dystonia. Toxins 2021, 13, 605. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13090605
Yoshida K. Effects of Botulinum Toxin Type A on Pain among Trigeminal Neuralgia, Myofascial Temporomandibular Disorders, and Oromandibular Dystonia. Toxins. 2021; 13(9):605. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13090605
Chicago/Turabian StyleYoshida, Kazuya. 2021. "Effects of Botulinum Toxin Type A on Pain among Trigeminal Neuralgia, Myofascial Temporomandibular Disorders, and Oromandibular Dystonia" Toxins 13, no. 9: 605. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13090605