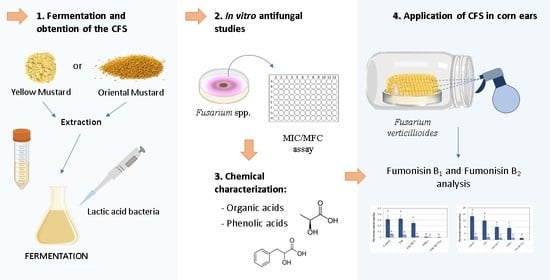
Use of Mustard Extracts Fermented by Lactic Acid Bacteria to Mitigate the Production of Fumonisin B1 and B2 by Fusarium verticillioides in Corn Ears
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antifungal Activity of the Fermented Mustard Extracts
2.2. Phenolic Acids and Organic Acids Profile of the CFS
2.3. Application of the CFS on Corn Ears as an Antimycotoxigenic Agent
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Microorganisms and Culture Conditions
4.3. Fermentation Conditions and Preparation of CFS
4.4. Qualitative Antifungal Test on PDA Plates
4.5. Determination of the MIC and MFC Values of the CFS
4.6. Organic Acids and Phenolic Acids Determination in the CFS
4.7. Application of the CFS in Corn Ears
4.8. Extraction and Determination of Mycotoxins by Q-TOF
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 3rd ed.; Springer Science & Business Media: New York, NY, USA, 2009; pp. 1–2. ISBN 978-1-4615-6391-4. [Google Scholar]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2019, 60, 2773–2789. [Google Scholar] [CrossRef]
- Moretti, A.; Logrieco, A.F.; Susca, A. Mycotoxins: An Underhand Food Problem BT. In Mycotoxigenic Fungi: Methods and Protocols; Moretti, A., Susca, A., Eds.; Springer: New York, NY, USA, 2017; pp. 3–12. ISBN 978-1-4939-6707-0. [Google Scholar]
- Giorni, P.; Bertuzzi, T.; Battilani, P. Impact of fungi co-occurrence on mycotoxin contamination in maize during the growing season. Front. Microbiol. 2019, 10, 1265. [Google Scholar] [CrossRef]
- Palumbo, R.; Gonçalves, A.; Gkrillas, A.; Logrieco, A.; Dorne, J.; Dall, C.; Venâncio, A.; Battilani, P.; Palumbo, R.; Gonçalves, A.; et al. Phytopathologia Mediterranea Mycotoxins in maize: Mitigation actions, with a chain management approach. Phytopathol. Mediterr. 2020, 59, 5–28. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [Green Version]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in Occurrence, Importance, and Mycotoxin Control Strategies: Prevention and Detoxification in Foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.M.; Ameye, M.; Phan, L.T.-K.; Devlieghere, F.; De Saeger, S.; Eeckhout, M.; Audenaert, K. Post-harvest contamination of maize by Fusarium verticillioides and fumonisins linked to traditional harvest and post-harvest practices: A case study of small-holder farms in Vietnam. Int. J. Food Microbiol. 2021, 339, 109022. [Google Scholar] [CrossRef]
- Hassan, Z.U.; Al Thani, R.; Alsafran, M.; Migheli, Q.; Jaoua, S. Selection of Bacillus spp. with decontamination potential on multiple Fusarium mycotoxins. Food Control 2021, 127, 108119. [Google Scholar] [CrossRef]
- Esposito, F.; Fasano, E.; Scognamiclio, G.; Nardone, A.; Triassi, M.; Cirillo, T. Exposure assessment to fumonisin B1, B2 and B3 through consumption of gluten-free foodstuffs intended for people affected by celiac disease. Food Chem. Toxicol. 2016, 97, 395–401. [Google Scholar] [CrossRef]
- Yang, X.; Gao, J.; Liu, Q.; Yang, D. Co-occurrence of mycotoxins in maize and maize-derived food in China and estimation of dietary intake. Food Addit. Contam. Part B 2019, 12, 124–134. [Google Scholar] [CrossRef]
- Andretta, I.; Kipper, M.; Hauschild, L.; Lehnen, C.R.; Remus, A.; Melchior, R. Meta-analysis of individual and combined effects of mycotoxins on growing pigs. Sci. Agric. 2016, 73, 328–331. [Google Scholar] [CrossRef] [Green Version]
- Kifer, D.; Jakšić, D.; Šegvić Klarić, M. Assessing the Effect of Mycotoxin Combinations: Which Mathematical Model Is (the Most) Appropriate? Toxins 2020, 12, 153. [Google Scholar] [CrossRef] [Green Version]
- Gajbhiye, M.H.; Kapadnis, B.P. Antifungal-activity-producing lactic acid bacteria as biocontrol agents in plants. Biocontrol Sci. Technol. 2016, 26, 1451–1470. [Google Scholar] [CrossRef] [Green Version]
- Želonková, K.; Havadej, S.; Verebová, V.; Holečková, B.; Uličný, J.; Staničová, J. Fungicide Tebuconazole Influences the Structure of Human Serum Albumin Molecule. Molecules 2019, 24, 3190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Lay, C.; Mounier, J.; Vasseur, V.; Weill, A.; Le Blay, G.; Barbier, G.; Coton, E. In vitro and in situ screening of lactic acid bacteria and propionibacteria antifungal activities against bakery product spoilage molds. Food Control 2016, 60, 247–255. [Google Scholar] [CrossRef]
- van der Ven, L.T.M.; Rorije, E.; Sprong, R.C.; Zink, D.; Derr, R.; Hendriks, G.; Loo, L.-H.; Luijten, M. A Case Study with Triazole Fungicides to Explore Practical Application of Next-Generation Hazard Assessment Methods for Human Health. Chem. Res. Toxicol. 2020, 33, 834–848. [Google Scholar] [CrossRef]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. 2020, 201, 110812. [Google Scholar] [CrossRef] [PubMed]
- Bahmid, N.A.; Dekker, M.; Fogliano, V.; Heising, J. Development of a moisture-activated antimicrobial film containing ground mustard seeds and its application on meat in active packaging system. Food Packag. Shelf Life 2021, 30, 100753. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Holley, R.A. Inhibition of Listeria monocytogenes on cooked cured chicken breasts by acidified coating containing allyl isothiocyanate or deodorized Oriental mustard extract. Food Microbiol. 2016, 57, 90–95. [Google Scholar] [CrossRef]
- Avato, P.; Argentieri, P.M. Brassicaceae: A rich source of health improving phytochemicals. Phytochem. Rev. 2015, 14, 1019–1033. [Google Scholar] [CrossRef]
- Saladino, F.; Luz, C.; Manyes, L.; Fernández-Franzón, M.; Meca, G. In vitro antifungal activity of lactic acid bacteria against mycotoxigenic fungi and their application in loaf bread shelf life improvement. Food Control 2016, 67, 273–277. [Google Scholar] [CrossRef]
- Lv, X.; Ma, H.; Lin, Y.; Bai, F.; Ge, Y.; Zhang, D.; Li, J. Antifungal activity of Lactobacillus plantarum C10 against Trichothecium roseum and its application in promotion of defense responses in muskmelon (Cucumis melo L.) fruit. J. Food Sci. Technol. 2018, 55, 3703. [Google Scholar] [CrossRef] [PubMed]
- Mokoena, M.P.; Omatola, C.A.; Olaniran, A.O. Applications of Lactic Acid Bacteria and Their Bacteriocins against Food Spoilage Microorganisms and Foodborne Pathogens. Molecules 2021, 26, 7055. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.M.; Torrijos, R.; Luciano, F.B.; Mañes, J.; Meca, G. Aflatoxins and A. flavus Reduction in Loaf Bread through the Use of Natural Ingredients. Molecules 2018, 23, 1638. [Google Scholar] [CrossRef] [Green Version]
- Torrijos, R.; Nazareth, T.d.M.; Quiles, J.M.; Mañes, J.; Meca, G. Application of White Mustard Bran and Flour on Bread as Natural Preservative Agents. Foods 2021, 10, 431. [Google Scholar] [CrossRef]
- Luz, C.; Izzo, L.; Graziani, G.; Gaspari, A.; Ritieni, A.; Mañes, J.; Meca, G. Evaluation of biological and antimicrobial properties of freeze-dried whey fermented by different strains of Lactobacillus plantarum. Food Funct. 2018, 9, 3688–3697. [Google Scholar] [CrossRef]
- Izzo, L.; Luz, C.; Ritieni, A.; Beses, J.Q.; Mañes, J.; Meca, G. Inhibitory effect of sweet whey fermented by Lactobacillus plantarum strains against fungal growth: A potential application as an antifungal agent. J. Food Sci. 2020, 85, 3920–3926. [Google Scholar] [CrossRef]
- Nicácio, A.E.; Rodrigues, C.A.; Visentainer, J.V.; Maldaner, L. Evaluation of the QuEChERS method for the determination of phenolic compounds in yellow (Brassica alba), brown (Brassica juncea), and black (Brassica nigra) mustard seeds. Food Chem. 2021, 340, 128162. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Ebrahimi, M.; Mortazavi, S.A.; Abedfar, A. Application of the selected antifungal LAB isolate as a protective starter culture in pan whole-wheat sourdough bread. Food Control 2019, 95, 298–307. [Google Scholar] [CrossRef]
- Schmidt, M.; Zannini, E.; Lynch, K.M.; Arendt, E.K. Novel approaches for chemical and microbiological shelf life extension of cereal crops. Crit. Rev. Food Sci. Nutr. 2018, 59, 3395–3419. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ju, H.; Wang, Y.; Du, G.; Yan, X.; Cui, Y.; Yuan, Y.; Yue, T. Antifungal activity and mode of action of lactic acid bacteria isolated from kefir against Penicillium expansum. Food Control 2021, 130, 108274. [Google Scholar] [CrossRef]
- Rajanikar, R.V.; Nataraj, B.H.; Naithani, H.; Ali, S.A.; Panjagari, N.R.; Behare, P.V. Phenyllactic acid: A green compound for food biopreservation. Food Control 2021, 128, 108184. [Google Scholar] [CrossRef]
- Cortés-Zavaleta, O.; López-Malo, A.; Hernández-Mendoza, A.; García, H.S. Antifungal activity of lactobacilli and its relationship with 3-phenyllactic acid production. Int. J. Food Microbiol. 2014, 173, 30–35. [Google Scholar] [CrossRef]
- Daou, R.; Joubrane, K.; Maroun, R.G.; Khabbaz, L.R.; Ismail, A.; El Khoury, A.; Daou, R.; Joubrane, K.; Maroun, R.G.; Khabbaz, L.R.; et al. Mycotoxins: Factors influencing production and control strategies. AIMS Agric. Food 2021, 6, 416–447. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 401/2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union. 2006, 70, 12–34. [Google Scholar]
- de Melo Nazareth, T.; Luz, C.; Torrijos, R.; Quiles, J.M.; Luciano, F.B.; Mañes, J.; Meca, G. Potential application of lactic acid bacteria to reduce aflatoxin B1 and fumonisin B1 occurrence on corn kernels and corn ears. Toxins 2019, 12, 21. [Google Scholar] [CrossRef] [Green Version]
- Dopazo, V.; Luz, C.; Mañes, J.; Quiles, J.M.; Carbonell, R.; Calpe, J.; Meca, G. Bio-preservative potential of microorganisms isolated from red grape against food contaminant fungi. Toxins 2021, 13, 412. [Google Scholar] [CrossRef]
- Ben Taheur, F.; Mansour, C.; Kouidhi, B.; Chaieb, K. Use of lactic acid bacteria for the inhibition of Aspergillus flavus and Aspergillus carbonarius growth and mycotoxin production. Toxicon 2019, 166, 15–23. [Google Scholar] [CrossRef]
- Luz, C.; D’Opazo, V.; Quiles, J.M.; Romano, R.; Mañes, J.; Meca, G. Biopreservation of tomatoes using fermented media by lactic acid bacteria. LWT 2020, 130, 109618. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard CLSI Document M38-A2; Clinical and Laboratory Standards Institute, WayneCLS: Wayne, PA, USA, 2008. [Google Scholar]
- Khosravi, F.; Rastakhiz, N.; Iranmanesh, B.; Jafari Olia, S.S.S. Determination of Organic Acids in Fruit juices by UPLC. Int. J. Life Sci. 2015, 9, 41–44. [Google Scholar] [CrossRef] [Green Version]
- Brosnan, B.; Coffey, A.; Arendt, E.K.; Furey, A. The QuEChERS approach in a novel application for the identification of antifungal compounds produced by lactic acid bacteria cultures. Talanta 2014, 129, 364–373. [Google Scholar] [CrossRef]
- Denardi-Souza, T.; Luz, C.; Mañes, J.; Badiale-Furlong, E.; Meca, G. Antifungal effect of phenolic extract of fermented rice bran with Rhizopus oryzae and its potential use in loaf bread shelf life extension. J. Sci. Food Agric. 2018, 98, 5011–5018. [Google Scholar] [CrossRef] [PubMed]


| Fungal Strain | Control | IRK751 | IRK82 | SMF76 | POM | TR7 | TR71 | TR14 | TR2 | CECT 8962 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| YM | OM | YM | OM | YM | OM | YM | OM | YM | OM | YM | OM | YM | OM | YM | OM | YM | OM | YM | OM | |
| F. graminearum ITEM 126 | − | − | − | − | − | − | − | − | − | − | + | − | + | − | + | + | − | − | + | − |
| F. graminearum ITEM 6352 | − | − | − | − | − | − | − | − | − | − | − | − | + | − | + | + | − | − | + | + |
| F. graminearum ITEM 6415 | − | − | − | − | − | − | − | − | − | − | + | − | + | − | + | − | − | − | + | − |
| F. proliferatum ITEM 12072 | − | − | − | − | − | − | − | − | − | − | + | + | + | + | + | + | − | − | + | + |
| F. proliferatum ITEM 12103 | − | − | − | − | − | − | − | − | − | − | − | − | + | − | + | − | − | − | − | − |
| F. proliferatum ITEM 16031 | − | − | − | − | − | − | − | − | − | − | + | + | + | + | + | + | − | − | + | + |
| F. verticillioides ITEM 12052 | − | − | − | − | − | − | − | − | − | − | + | + | + | + | + | + | − | − | + | + |
| F. verticillioides ITEM 12043 | − | − | − | − | − | − | − | − | − | − | + | + | + | + | + | + | − | − | + | + |
| F. verticillioides ITEM 12044 | − | − | − | − | − | − | − | − | − | − | + | + | + | + | + | + | − | − | + | + |
| F. sporotrichioides ITEM 121 | − | − | − | − | − | − | − | − | − | − | + | − | + | − | + | + | − | − | − | − |
| F. langsethiae ITEM 11031 | − | − | − | − | − | − | − | − | − | − | + | − | + | + | + | + | − | − | + | + |
| F. poae ITEM 9131 | − | − | − | − | − | − | − | − | − | − | − | − | + | − | + | − | − | − | + | − |
| F. poae ITEM 9151 | − | − | − | − | − | − | − | − | − | − | + | − | + | − | + | − | − | − | + | − |
| F. poae ITEM 9211 | − | − | − | − | − | − | − | − | − | − | + | − | + | + | + | + | − | − | + | + |
| (a) | ||||||||
| Fungal Strain | TR7 | TR71 | TR14 | CECT 8962 | ||||
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |
| F. graminearum ITEM 126 | 15.6 | 31.3 | 7.8 | 15.6 | 7.8 | 15.6 | 31.3 | 62.5 |
| F. graminearum ITEM 6352 | 15.6 | 31.3 | 7.8 | 15.6 | 15.6 | 31.3 | 15.6 | 31.3 |
| F. gramienarum ITEM 6415 | 7.8 | 15.6 | 7.8 | 15.6 | 7.8 | 15.6 | 7.8 | 15.6 |
| F. proliferatum ITEM 12072 | 31.3 | 62.5 | 7.8 | 15.6 | 15.6 | 31.3 | 15.6 | 31.3 |
| F. proliferatum ITEM 12103 | 15.6 | 31.3 | 15.6 | 31.3 | 15.6 | 31.3 | 15.6 | 31.3 |
| F. proliferatum ITEM 16031 | 15.6 | 62.5 | 15.6 | 31.3 | 31.3 | 62.5 | 15.6 | 31.3 |
| F. verticillioides ITEM 12052 | 15.6 | 31.3 | 15.6 | 31.3 | 31.3 | 62.5 | 15.6 | 31.3 |
| F. verticillioides ITEM 12043 | 7.8 | 15.6 | 7.8 | 15.6 | 15.6 | 31.3 | 15.6 | 31.3 |
| F. verticillioides ITEM 12044 | 15.6 | 31.3 | 15.6 | 31.3 | 31.3 | 62.5 | 15.6 | 31.3 |
| F. sporotrichioides ITEM 121 | 31.3 | 62.5 | 7.8 | 15.6 | 7.8 | 15.6 | 15.6 | 31.3 |
| F. langsethiae ITEM 11031 | 15.6 | 31.3 | 7.8 | 15.6 | 7.8 | 15.6 | 7.8 | 15.6 |
| F. poae ITEM 9131 | 15.6 | 31.3 | 15.6 | 31.3 | 15.6 | 31.3 | 15.6 | 31.3 |
| F. poae ITEM 9151 | 7.8 | 15.6 | 7.8 | 15.6 | 15.6 | 31.3 | 31.3 | 62.5 |
| F. poae ITEM 9211 | 15.6 | 31.3 | 15.6 | 31.3 | 15.6 | 31.3 | 31.3 | 62.5 |
| (b) | ||||||||
| Fungal Strain | TR7 | TR71 | TR14 | CECT 8962 | ||||
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |
| F. graminearum ITEM 126 | 31.3 | 62.5 | 31.3 | 62.5 | 15.6 | 62.5 | 31.3 | 62.5 |
| F. graminearum ITEM 6352 | 31.3 | 62.5 | 31.3 | 62.5 | 31.3 | 62.5 | 15.6 | 31.3 |
| F. gramienarum ITEM 6415 | 15.6 | 31.3 | 15.6 | 31.3 | 15.6 | 62.5 | 15.6 | 31.3 |
| F. proliferatum ITEM 12072 | 31.3 | 62.5 | 31.3 | 62.5 | 31.3 | 62.5 | 31.3 | 62.5 |
| F. proliferatum ITEM 12103 | 31.3 | 62.5 | 31.3 | 62.5 | 31.3 | 62.5 | 31.3 | 62.5 |
| F. proliferatum ITEM 16031 | 62.5 | 125.0 | 31.3 | 62.5 | 31.3 | 62.5 | 62.5 | 125.0 |
| F. verticillioides ITEM 12052 | 31.3 | 62.5 | 31.3 | 62.5 | 31.3 | 62.5 | 31.3 | 62.5 |
| F. verticillioides ITEM 12043 | 31.3 | 62.5 | 31.3 | 62.5 | 31.3 | 62.5 | 31.3 | 62.5 |
| F. verticillioides ITEM 12044 | 31.3 | 62.5 | 31.3 | 62.5 | 62.5 | 125.0 | 31.3 | 62.5 |
| F. sporotrichioides ITEM 121 | 31.3 | 62.5 | 31.3 | 62.5 | 31.3 | 62.5 | 31.3 | 62.5 |
| F. langsethiae ITEM 11031 | 15.6 | 31.3 | 15.6 | 31.3 | 7.8 | 15.6 | 15.6 | 31.3 |
| F. poae ITEM 9131 | 31.3 | 125.0 | 62.5 | 125.0 | 62.5 | 125.0 | 31.3 | 62.5 |
| F. poae ITEM 9151 | 31.3 | 62.5 | 31.3 | 62.5 | 31.3 | 62.5 | 62.5 | 125.0 |
| F. poae ITEM 9211 | 31.3 | 62.5 | 31.3 | 62.5 | 62.5 | 125.0 | 62.5 | 125.0 |
| (a) Yellow mustard extract | |||||
| Phenolic Acid | Control | TR7 | TR71 | TR14 | CECT 8962 |
| 1,2-Dihydroxybenzene | 62.88 ± 10.86 a | 259.53 ± 11.12 b | 292.85 ± 62.97 b | 194.99 ± 64.37 c | 250.17 ± 5.76 b |
| 3,4-Dihydroxicinnamic acid | 11.45 ± 3.67 a | 32.73 ± 4.13 b | 44.95 ± 9.76 c | 26.01 ± 5.26 b | 28.76 ± 8.24 b |
| Benzoic acid | 14.74 ± 2.36 a | 134.42 ± 5.09 b | 220.12 ± 27.12 c | 128.19 ± 11.15 b | 127.29 ± 10.51 b |
| 3-Phenyllactic acid | 13.67 ± 1.68 a | 45.66 ± 6.87 ab | 559.15 ± 78.51 c | 57.48 ± 12.07b | 45.62 ± 8.16 ab |
| Hydroxycinnamic acid | 6.84 ± 0.93 | n.d | n.d | n.d | n.d |
| P-Coumaric acid | 16.13 ± 6.07 a | 42.66 ± 5.37 b | 76.07 ± 15.81 c | 65.25 ± 7.68 cd | 62.13 ± 11.97 d |
| Protocatechuic | 31.13 ± 7.60 a | 158.68 ± 12.96 b | 17.25 ± 2.11 c | 8.96 ± 3.69 c | 39.08 ± 17.86 a |
| Sinapic acid | 61.72 ± 4.86 a | 8.29 ± 2.39b | 16.79 ± 0.12 c | 28.40 ± 7.78 d | 40.50 ± 8.51 e |
| Vanillin | 4.17 ± 1.56 a | n.d | 30.28 ± 7.92 b | 17.75 ± 6.06 c | 20.71 ± 7.62 c |
| Syringic acid | 4.30 ± 1.34 | n.d | n.d | n.d | n.d |
| Ferulic acid | 11.69 ± 4.69 | n.d | n.d | n.d | n.d |
| Organic acid | |||||
| Lactic acid | n.d | 728.00 ± 20.97 a | 799.88 ± 27.08 b | 591.56 ± 25.50 c | 570.26 ± 29.64 c |
| (b) Oriental mustard extract | |||||
| Phenolic Acid | Control | TR7 | TR71 | TR14 | CECT 8962 |
| 1,2-Dihydroxybenzene. | 7.23 ± 2.64 a | n.d | 39.27 ± 6.70 b | 34.62 ± 5.39 b | n.d |
| 3,4-Dihydroxicinnamic acid | 2.83 ± 0.98 a | 146.01 ± 16.30 b | 217.67 ± 35.00 c | 190.56 ± 52.87 cd | 157.06 ± 18.40 bd |
| Benzoic acid | 76.51 ± 8.85 a | 140.53 ± 30.09 b | 159.10 ± 8.99 b | 228.43 ± 16.90 c | 143.08 ± 36.13 b |
| 3-Phenyllactic acid | 6.65 ± 2.87 a | 34.68 ± 3.37 bc | 37.16 ± 4.57 bc | 42.43 ± 14.18 b | 31.20 ± 7.27 c |
| Hydroxycinnamic acid | 10.14 ± 4.04 | n.d | n.d | n.d | n.d |
| P-Coumaric acid | 58.09 ± 15.02 ab | 68.29 ± 20.46 ac | 76.50 ± 8.62 d | 30.70 ± 13.59 ce | 42.44 ± 10.27 be |
| Protocatechuic | 20.30 ± 1.33 a | 44.24 ± 13.22 b | 32.23 ± 9.96 c | 31.33 ± 2.52 c | 33.39 ± 4.15 c |
| Sinapic acid | 62.37 ± 27.75 a | 25.09 ± 2.96 b | 22.59 ± 6.09 b | 48.21 ± 11.49 a | 19.18 ± 4.12 b |
| Vanillin | 9.47 ± 1.24 a | 18.64 ± 2.57 a | 60.14 ± 19.03 b | 58.86 ± 10.49 b | 59.47 ± 11.65 b |
| Syringic acid | 6.81 ± 1.45 | n.d | n.d | n.d | n.d |
| Ferulic acid | 19.74 ± 7.55 | n.d | n.d | n.d | n.d |
| Organic acid | |||||
| Lactic acid | n.d | 209.04 ± 66.16 a | 203.39 ± 6.53 a | 89.24 ± 20.20 b | 174.55 ± 7.26 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torrijos, R.; de Melo Nazareth, T.; Vila-Donat, P.; Mañes, J.; Meca, G. Use of Mustard Extracts Fermented by Lactic Acid Bacteria to Mitigate the Production of Fumonisin B1 and B2 by Fusarium verticillioides in Corn Ears. Toxins 2022, 14, 80. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins14020080
Torrijos R, de Melo Nazareth T, Vila-Donat P, Mañes J, Meca G. Use of Mustard Extracts Fermented by Lactic Acid Bacteria to Mitigate the Production of Fumonisin B1 and B2 by Fusarium verticillioides in Corn Ears. Toxins. 2022; 14(2):80. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins14020080
Chicago/Turabian StyleTorrijos, Raquel, Tiago de Melo Nazareth, Pilar Vila-Donat, Jordi Mañes, and Giuseppe Meca. 2022. "Use of Mustard Extracts Fermented by Lactic Acid Bacteria to Mitigate the Production of Fumonisin B1 and B2 by Fusarium verticillioides in Corn Ears" Toxins 14, no. 2: 80. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins14020080