Warfarin Toxicity and Individual Variability—Clinical Case
Abstract
:1. Introduction
1.1. Warfarin Toxicity
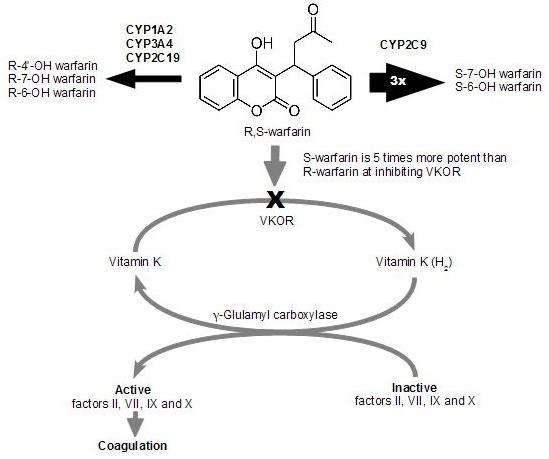
1.2. Warfarin Metabolism

1.3. Drug-drug Interaction
2. Patient Case
3. Discussion
- Most laboratories do not perform blood levels of warfarin and its metabolites.
- There are uncertainties in the interpretation of the toxicity level.
- Daily repeated measurement of the prothrombin time (PT) and the international normalized ratio (INR) are the best to describe the anticoagulant effect. However, these tests may not be elevated until 1–2 days postingestion.
- Depressed levels of vitamin K-dependent clotting factors (II, VII, IX, and X) may provide supporting evidence for suspected warfarin toxicity, but these tests are rarely available in a timely fashion and usually do not aid in clinical management.
- Other laboratory tests, such as a blood count for baseline hemoglobin and/or hematocrit or to assess for anemia, only register changes when severe warfarin toxicity has occurred.
- Genotype assessment of the cytochrome P450 variant alleles may help the clinician to individualize treatment and minimize bleeding complications in patients who are poor metabolizers and need lower warfarin doses or complete drug substitution.
4. Conclusions
Acknowledgements
References
- Makris, M.; Watson, H.G. The management of coumarin-induced over-anticoagulation Annotation. Br. J. Haematol. 2001, 114, 271–280. [Google Scholar]
- King, B.P.; Khan, T.I.; Aithal, G.P.; Kamali, F.; Daly, A.K. Upstream and coding region CYP2C9 polymorphisms: Correlation with warfarin dose and metabolism. Pharmacogenetics 2004, 14, 813–822. [Google Scholar]
- Gage, B.F.; Lesko, L.J. Pharmacogenetics of warfarin: Regulatory, scientific, and clinical issues. J. Thromb. Thrombolysis 2008, 25, 45–51. [Google Scholar]
- Palareti, G.; Leali, N.; Coccheri, S.; Poggi, M.; Manotti, C.; D’Angelo, A.; Pengo, V.; Erba, N.; Moia, M.; Ciavarella, N.; Devoto, G.; Berretini, M.; Musolesi, S. Hemorrhagic complications of oral anticoagulant therapy: Results of a prospective multicenter study ISCOAT (Italian Study on Complications of Oral Anticoagulant Therapy). G. Ital. Cardiol. 1997, 27, 231–243. [Google Scholar]
- Hirsh, J.; Bates, S.M. Clinical trials that have influenced the treatment of venous thromboembolism: A historical perspective. Ann. Intern. Med. 2001, 134, 409–417. [Google Scholar]
- Hirsh, J.; Dalen, J.; Anderson, D.R.; Poller, L.; Bussey, H.; Ansell, J.; Deykin, D. Oral anticoagulants: Mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest 2001, 119, 8S–21S. [Google Scholar]
- Jones, D.R.; Kim, S.Y.; Guderyon, M.; Yun, C.H.; Moran, J.H.; Miller, G.P. Hydroxywarfarin metabolites potently inhibit CYP2C9 metabolism of S-warfarin. Chem. Res. Toxicol. 2010, 23, 939–945. [Google Scholar]
- Kamali, F.; Wynne, H. Pharmacogenetics of warfarin. Annu. Rev. Med. 2010, 61, 63–75. [Google Scholar]
- Kaminsky, L.S.; Zhang, Z.Y. Human P450 metabolism of warfarin. Pharmacol. Ther. 1997, 73, 67–74. [Google Scholar]
- Adcock, D.M.; Koftan, C.; Crisan, D.; Kiechle, F.L. Effect of polymorphisms in the cytochrome P450 CYP2C9 gene on warfarin anticoagulation. Arch. Pathol. Lab. Med. 2004, 128, 1360–1363. [Google Scholar]
- Takahashi, H.; Echizen, H. Pharmacogenetics of warfarin elimination and its clinical implications. Clin. Pharmacokinet. 2001, 40, 587–603. [Google Scholar]
- Rettie, A.E.; Wienkers, L.C.; Gonzalez, F.J.; Trager, W.F.; Korzekwa, K.R. Impaired (S)-warfarin metabolism catalysed by the R144C allelic variant of CYP2C9. Pharmacogenetics 1994, 4, 39–42. [Google Scholar]
- Haining, R.L.; Hunter, A.P.; Veronese, M.E.; Trager, W.F.; Rettie, A.E. Allelic variants of human cytochrome P450 2C9: Baculovirus-mediated expression, purification, structural characterization, substrate stereoselectivity, and prochiral selectivity of the wild-type and I359L mutant forms. Arch. Biochem. Biophys. 1996, 333, 447–458. [Google Scholar]
- Steward, D.J.; Haining, R.L.; Henne, K.R.; Davis, G.; Rushmore, T.H.; Trager, W.F.; Rettie, A.E. Genetic association between sensitivity to warfarin and expression of CYP2C9*3. Pharmacogenetics 1997, 7, 361–367. [Google Scholar]
- Margaglione, M.; Colaizzo, D.; D’Andrea, G.; Brancaccio, V.; Ciampa, A.; Grandone, E.; Di Minno, G. Genetic modulation of oral anticoagulation with warfarin. Thromb. Haemost. 2000, 84, 775–778. [Google Scholar]
- Scordo, M.G.; Pengo, V.; Spina, E.; Dahl, M.L.; Gusella, M.; Padrini, R. Influence of CYP2C9 and CYP2C19 genetic polymorphisms on warfarin maintenance dose and metabolic clearance. Clin. Pharmacol. Ther. 2002, 72, 702–710. [Google Scholar]
- Visser, L.E.; van Vliet, M.; van Schaik, R.H.; Kasbergen, A.A.; De Smet, P.A.; Vulto, A.G.; Hofman, A.; van Duijn, C.M.; Stricker, B.H. The risk of overanticoagulation in patients with cytochrome P450 CYP2C9*2 or CYP2C9*3 alleles on acenocoumarol or phenprocoumon. Pharmacogenetics 2004, 14, 27–33. [Google Scholar]
- Wang, B.; Wang, J.; Huang, S.Q.; Su, H.H.; Zhou, S.F. Genetic Polymorphism of the Human Cytochrome P450 2C9 Gene and Its Clinical Significance. Curr. Drug. Metab. 2009, 10, 781–834. [Google Scholar]
- Furuya, H.; Fernandez-Salguero, P.; Gregory, W.; Taber, H.; Steward, A.; Gonzalez, F.J.; Idle, J.R. Genetic polymorphism of CYP2C9 and its effect on warfarin maintenance dose requirement in patients undergoing anticoagulation therapy. Pharmacogenetics 1995, 5, 389–392. [Google Scholar]
- Aithal, G.P.; Day, C.P.; Kesteven, P.J.; Daly, A.K. Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet 1999, 353, 717–719. [Google Scholar]
- Higashi, M.K.; Veenstra, D.L.; Kondo, L.M.; Wittkowsky, A.K.; Srinouanprachanh, S.L.; Farin, F.M.; Rettie, A.E. Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA 2002, 287, 1690–1698. [Google Scholar]
- Tabrizi, A.R.; Zehnbauer, B.A.; Borecki, I.B.; McGrath, S.D.; Buchman, T.G.; Freeman, B.D. The frequency and effects of cytochrome P450 (CYP) 2C9 polymorphisms in patients receiving warfarin. J. Am. Coll. Surg. 2002, 194, 267–273. [Google Scholar]
- Taube, J.; Halsall, D.; Baglin, T. Influence of cytochrome P-450 CYP2C9 polymorphisms on warfarin sensitivity and risk of over-anticoagulation in patients on long-term treatment. Blood 2000, 96, 1816–1819. [Google Scholar]
- Wadelius, M.; Sorlin, K.; Wallerman, O.; Karlsson, J.; Yue, Q.Y.; Magnusson, P.K.; Wadelius, C.; Melhus, H. Warfarin sensitivity related to CYP2C9, CYP3A5, ABCB1 (MDR1) and other factors. Pharmacogenomics J. 2004, 4, 40–48. [Google Scholar]
- Freeman, B.D.; Zehnbauer, B.A.; McGrath, S.; Borecki, I.; Buchman, T.G. Cytochrome P450 polymorphisms are associated with reduced warfarin dose. Surgery 2000, 128, 281–285. [Google Scholar]
- Takahashi, H.; Echizen, H. Pharmacogenetics of CYP2C9 and interindividual variability in anticoagulant response to warfarin. Pharmacogenomics J. 2003, 3, 202–214. [Google Scholar]
- London, S.J.; Daly, A.K.; Leathart, J.B.; Navidi, W.C.; Idle, J.R. Lung cancer risk in relation to the CYP2C9*1/CYP2C9*2 genetic polymorphism among African-Americans and Caucasians in Los Angeles County, California. Pharmacogenetics 1996, 6, 527–533. [Google Scholar]
- Nasu, K.; Kubota, T.; Ishizaki, T. Genetic analysis of CYP2C9 polymorphism in a Japanese population. Pharmacogenetics 1997, 7, 405–409. [Google Scholar]
- Bhasker, C.R.; Miners, J.O.; Coulter, S.; Birkett, D.J. Allelic and functional variability of cytochrome P4502C9. Pharmacogenetics 1997, 7, 51–58. [Google Scholar]
- Schalekamp, T.; Klungel, O.H.; Souverein, P.C.; de Boer, A. Increased bleeding risk with concurrent use of selective serotonin reuptake inhibitors and coumarins. Arch. Intern. Med. 2008, 168, 180–185. [Google Scholar]
- Dalton, S.O.; Sorensen, H.T.; Johansen, C. SSRIs and upper gastrointestinal bleeding: What is known and how should it influence prescribing? CNS Drugs 2006, 20, 143–151. [Google Scholar]
- Meijer, W.E.; Heerdink, E.R.; Leufkens, H.G.; Herings, R.M.; Egberts, A.C.; Nolen, W.A. Incidence and determinants of long-term use of antidepressants. Eur. J. Clin. Pharmacol. 2004, 60, 57–61. [Google Scholar]
- Greenblatt, D.J.; von Moltke, L.L.; Harmatz, J.S.; Shader, R.I. Drug interactions with newer antidepressants: Role of human cytochromes P450. J. Clin. Psychiatry 1998, 59 (Suppl. 15), 19–27. [Google Scholar]
- DeVane, C.L. Differential pharmacology of newer antidepressants. J. Clin. Psychiatry 1998, 59 (Suppl. 20), 85–93. [Google Scholar]
- Hauta-Aho, M.; Tirkkonen, T.; Vahlberg, T.; Laine, K. The effect of drug interactions on bleeding risk associated with warfarin therapy in hospitalized patients. Ann. Med. 2009, 41, 619–628. [Google Scholar]
- Wallerstedt, S.M.; Gleerup, H.; Sundstrom, A.; Stigendal, L.; Ny, L. Risk of clinically relevant bleeding in warfarin-treated patients—influence of SSRI treatment. Pharmacoepidemiol. Drug Saf. 2009, 18, 412–416. [Google Scholar]
- Wessinger, S.; Kaplan, M.; Choi, L.; Williams, M.; Lau, C.; Sharp, L.; Crowell, M.D.; Keshavarzian, A.; Jones, M.P. Increased use of selective serotonin reuptake inhibitors in patients admitted with gastrointestinal haemorrhage: A multicentre retrospective analysis. Aliment. Pharmacol. Ther. 2006, 23, 937–944. [Google Scholar]
- Duncan, D.; Sayal, K.; McConnell, H.; Taylor, D. Antidepressant interactions with warfarin. Int. Clin. Psychopharmacol. 1998, 13, 87–94. [Google Scholar]
- Sayal, K.S.; Duncan-McConnell, D.A.; McConnell, H.W.; Taylor, D.M. Psychotropic interactions with warfarin. Acta Psychiatr. Scand. 2000, 102, 250–255. [Google Scholar]
- Apseloff, G.; Wilner, K.D.; Gerber, N.; Tremaine, L.M. Effect of sertraline on protein binding of warfarin. Clin. Pharmacokinet. 1997, 32 (Suppl. 1), 37–42. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Piatkov, I.; Rochester, C.; Jones, T.; Boyages, S. Warfarin Toxicity and Individual Variability—Clinical Case. Toxins 2010, 2, 2584-2592. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins2112584
Piatkov I, Rochester C, Jones T, Boyages S. Warfarin Toxicity and Individual Variability—Clinical Case. Toxins. 2010; 2(11):2584-2592. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins2112584
Chicago/Turabian StylePiatkov, Irina, Colin Rochester, Trudi Jones, and Steven Boyages. 2010. "Warfarin Toxicity and Individual Variability—Clinical Case" Toxins 2, no. 11: 2584-2592. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins2112584