Variations in the Microcystin Content of Different Fish Species Collected from a Eutrophic Lake
Abstract
:1. Introduction
| Fish species | Range of microcystin detected (µg/kg) | FW or DW | Extraction protocol | Analytical method | Author(s) | Year |
|---|---|---|---|---|---|---|
| Channel catfish (Ictalurus punctatus) | 123.1–250.0 | FW | Water:methanol:butanol (15:4:1) extraction, C18 cleanup | ELISA | [29] | 2001 |
| Tilapia rendalli | 3.0–337.0 | DW | 100% methanol extraction | ELISA | [30] | 2001 |
| Goldfish (Carassius auratus L.) | 50–300 (estimated from their Figure 1) | FW | 100% methanol extraction | PPIA | [31] | 2003 |
| 500–1960 (estimated from their Figure 2) | DW | Water:methanol:butanol (15:4:1) extraction, C18 and Si cleanup | LC-PDA | [22] | 2005 | |
| Yellow perch (Perca flavescens) | 0.12–4.0 | FW | 75% methanol and acetic acid extraction | ELISA | [32] | 2008 |
| 0.5–7.0 | DW | 100% methanol extraction | ELISA | [33] | 2011 | |
| Largemouth bass (Micropterus salmoides) | 210.0–320.0 | FW | Water:methanol:butanol (15:4:1) extraction, C18 cleanup | ELISA | [34] | 2011 |
| Nile tilapia (Oreochromis niloticus) | 45-225 (estimated from their Figure 1b) | FW | Homogenization in methanol, hexane | LC-MS | [35] | 2011 |
| 0.8–63.4 | DW | methanol extraction | ELISA | [33] | 2011 | |
| Common carp (Cyprinus carpio) | 46.3 | DW | Water:methanol:butanol (15:4:1) extraction, C18 and Si cleanup | LC-PDA | [22] | 2005 |
| 3.3–19.0 | FW | 50% methanol, hexane | ELISA | [36] | 2007 | |
| 2.85–138.7 | FW | 75% methanol, acetic acid | ELISA, LC-MS | [37] | 2011 | |
| 50–470 (estimated from their Figure 4) | FW | 100% methanol extraction | ELISA | [23] | 2012 | |
| 3.5 | FW | 5% acetic acid, 0.01M EDTA extraction, charcoal | LC-MS/MS | Present study | 2012 | |
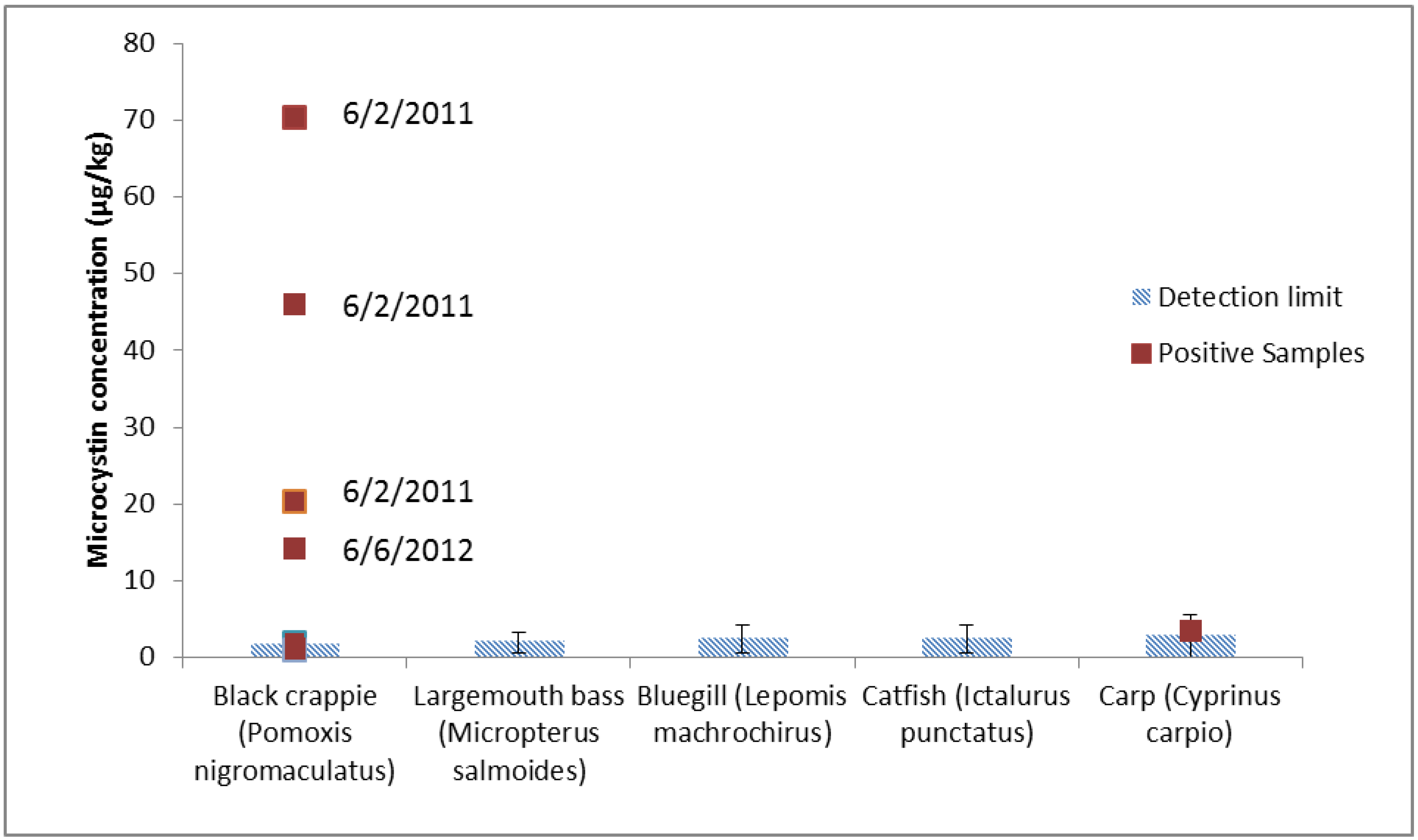
| Black crappie (Pomoxis nigromaculatus) | 399.0 | FW | 100% methanol and acidified water, cleanup with C18 cleanup | LC-MS/MS | [38] | 2009 |
| 1.5–1.9 | DW | 50% methanol extraction | ELISA | [33] | 2011 | |
| 1.04–70.43 | FW | 5% acetic acid, 0.01M EDTA extraction, charcoal cleanup | LC-MS/MS | Present study | 2012 | |
| White crappie (Pomoxis annularis) | 270.0–320.0 | FW | Water:methanol:butanol (15:4:1) extraction, C18 cleanup | ELISA | [34] | 2011 |
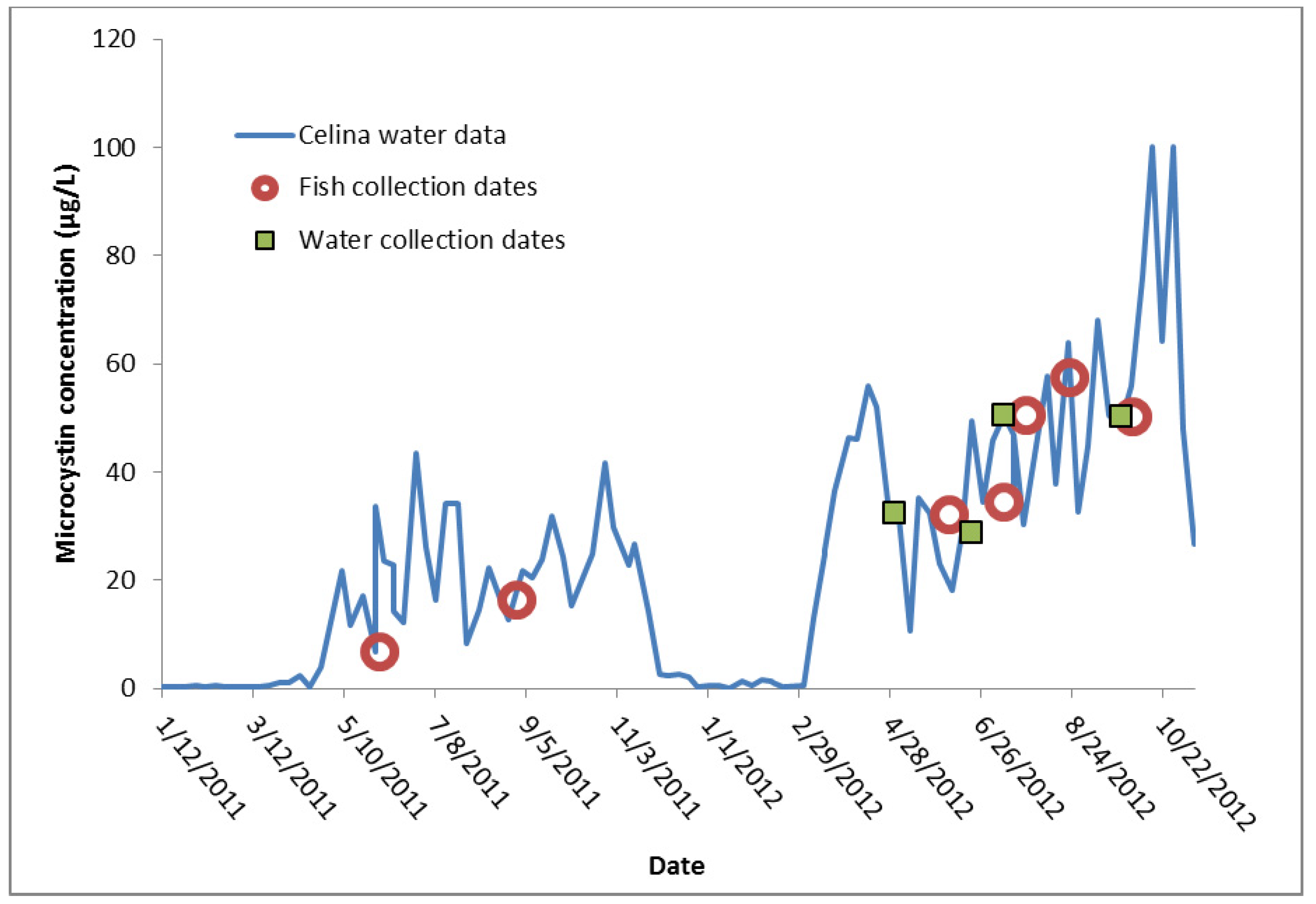
2. Results and Discussion
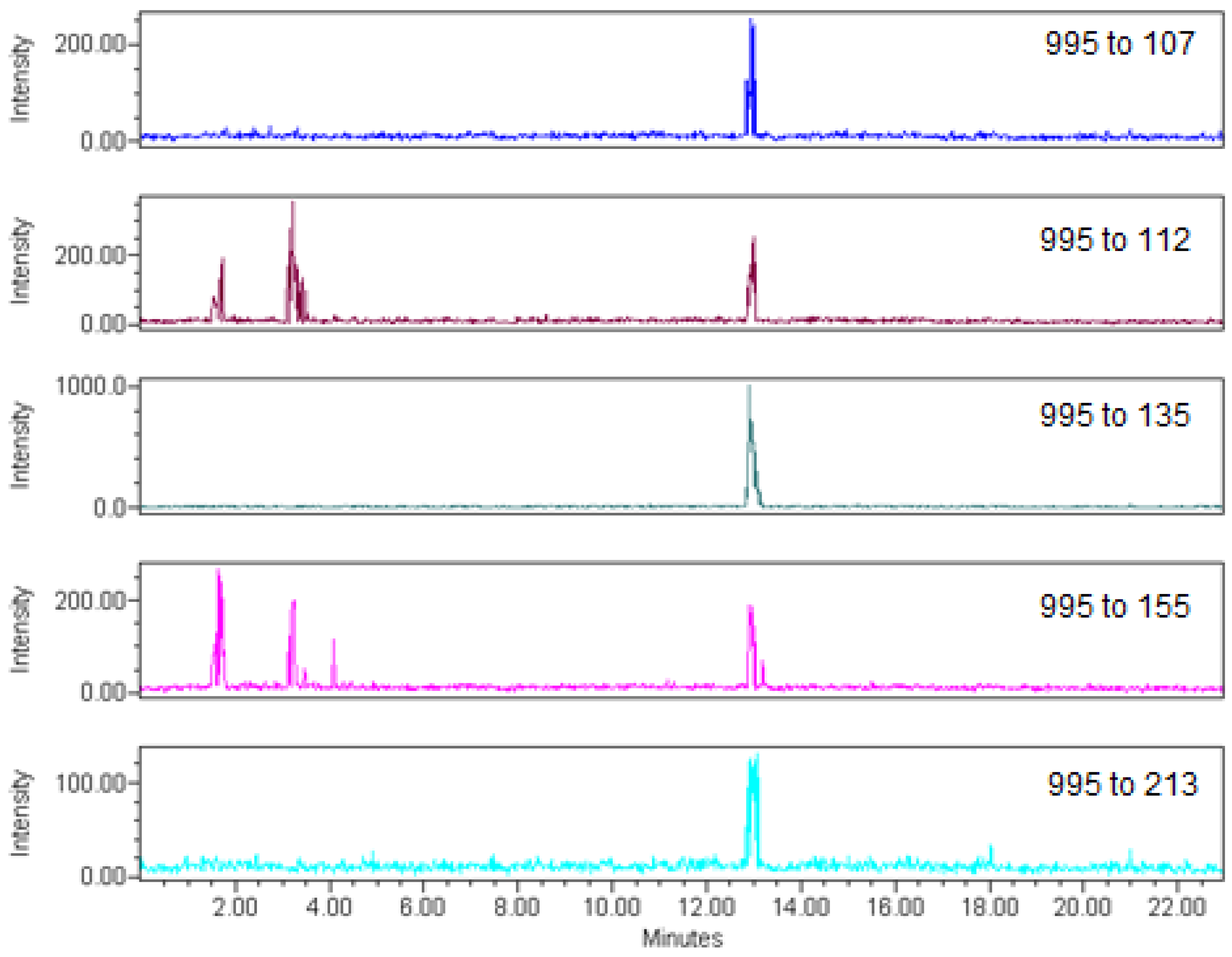
| Transition (m/z) | Instrument detection limit (µg on column) | Method detection limit (µg/kg) | Cone Voltage (V) | Collision Energy (V) |
|---|---|---|---|---|
| 995→107 | 0.03 | 0.09 | 85 | 90 |
| 995→112 | 0.05 | 0.15 | 85 | 95 |
| 995→135 | 0.02 | 0.05 | 85 | 90 |
| 995→155 | 0.06 | 0.18 | 85 | 85 |
| 995→213 | 0.08 | 0.24 | 85 | 87 |
| Date of collection | Microcystin concentration (µg/g dry weight) | Congeners |
|---|---|---|
| 6/17/2010 | 132 | 100% microcystin-LR |
| 7/19/2010 | 537 | 55% microcystin-LR |
| 28% microcystin-LW | ||
| 9% microcystin-YR | ||
| 8% microcystin-RR | ||
| 8/11/2010 | 46 | 100% microcystin-LR |
| 8/24/2010 | 20 | 100% microcystin-LR |
3. Experimental Section
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Smarda, J.; Marsalek, B. Microcystis aeruginosa (Cyanobacteria): Ultrastructure in a pelagic and in a benthic ecosystem. Int. J. Phycol. Res. 2008, 126, 73–86. [Google Scholar]
- Paerl, H.W.; Hall, N.S.; Calandrino, E.S. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climate-induced change. Sci. Total Environ. 2011, 409, 1739–1745. [Google Scholar]
- Carmichael, W. Cyanobacteria secondary metabolites—the cyanotoxins. J. Appl. Bacteriol. 1992, 72, 445–459. [Google Scholar] [CrossRef]
- Carmichael, W. Health effects of toxin-producing cyanobacteria: “the cyanoHABs”. Hum. Ecol. Risk Assess. 2001, 7, 1393–1407. [Google Scholar] [CrossRef]
- Dittman, E.; Wiegand, C. Cyanobacterial toxins—occurrence, biosynthesis and impact on human affairs. Mol. Nutr. Food Res. 2006, 50, 7–17. [Google Scholar] [CrossRef]
- Graham, J.L.; Loftin, K.A.; Meyer, M.T.; Ziegler, A.C. Cyanotoxin mixtures and taste-and-odor compound in cyanobacterial blooms from the Midwestern United States. Environ. Sci. Technol. 2010, 44, 7361–7368. [Google Scholar] [CrossRef]
- De Figueiredo, D.R.; Azeiteiro, U.M.; Esteves, S.M.; Gonçalves, F.J.M.; Pereira, M.J. Microcystin-producing blooms—a serious global public health issue. Ecotox. Environ. Safe. 2004, 59, 151–163. [Google Scholar] [CrossRef]
- Zurawell, R.W.; Chen, H.; Burke, J.M.; Prepas, E.E. Hepatotoxic cyanobacteria: A review of the biological importance of microcystins in freshwater environments. J. Toxicol. Environ. Health 2005, 8, 1–37. [Google Scholar] [CrossRef]
- Falconer, I.R. Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Springer Press: New York, NY, USA, 1998; pp. 607–612. [Google Scholar]
- Smith, J.L.; Boyer, G.L.; Zimba, P.V. A review of cyanobacterial odorous and bioactive metabolites: Impacts and management alternatives in aquaculture. Aquaculture 2008, 280, 5–20. [Google Scholar]
- MacKintosh, R.W.; Dalby, K.N.; Campbell, D.G.; Cohen, P.T.W.; Cohen, P.; MacKintosh, C. The cyanobacterial toxin microcystin binds covalently to cysteine-273 on protein phosphatase 1. FEBS Lett. 1995, 371, 236–240. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Wiegand, C.; Oberemm, A.; Beattie, K.A.; Krause, E.; Codd, G.A.; Steinberg, C.E.W. Identification of an enzymatically formed glutathione conjugate of the cyanobacterial hepatotoxin microcystin-LR: The first step of detoxication. Biochim. Biophys. Acta 1998, 1425, 527–533. [Google Scholar] [CrossRef]
- Pflugmacher, S. Promotion of oxidative stress in the aquatic macrophyte Ceratophyllum demersum during biotransformation of the cyanobacterial toxin microcystin-LR. Aquat. Toxicol. 2004, 70, 169–178. [Google Scholar] [CrossRef]
- Robinson, N.A.; Pace, J.G.; Matson, C.F.; Miura, G.A.; Lawrence, W.B. Tissue distribution, excretion, and hepatic biotransformation of microcystin-LR in mice. J. Pharmacol. Exp. Ther. 1991, 256, 176–182. [Google Scholar]
- Amorim, A.; Vasconcelos, V. Dynamics of microcystins in the mussel Mytilus galloprovincialis. Toxicon 1999, 37, 1041–1052. [Google Scholar] [CrossRef]
- Vanderploeg, H.A.; Liebig, J.R.; Carmichael, W.W.; Agy, M.A.; Johengen, T.H.; Fahnenstiel, G.L.; Nalepa, T.F. Zebra mussel (Dreissena polymorpha) selective filtration promoted toxic Microcystis blooms in Saginaw Bay (Lake Huron) and Lake Erie. Can. J. Fish. Aquat. Sci. 2001, 58, 1208–1221. [Google Scholar] [CrossRef]
- Hooser, S.B.; Kuhlenschmidt, M.S.; Dahlem, A.M.; Beasley, V.R.; Carmichael, W.W.; Haschek, W.M. Uptake and subcellular localization of tritiated dihydro-microcystin-LR in rat liver. Toxicon 1991, 29, 589–601. [Google Scholar] [CrossRef]
- Runnegar, M.T.C.; Gerdes, R.G.; Falconer, I.R. The uptake of the cyanobacterial hepatotoxin microcystin by isolated rat hepatocytes. Toxicon 1991, 29, 43–51. [Google Scholar] [CrossRef]
- Sahin, A.; Tencalla, F.G.; Dietrich, D.R.; Naegeli, H. Biliary excretion of biochemically active cyanobacteria (blue-green algae) hepatotoxins in fish. Toxicology 1996, 106, 123–130. [Google Scholar] [CrossRef]
- Prepas, E.E.; Kotak, B.G.; Campbell, L.M.; Evans, J.C.; Hrudey, S.E.; Holmes, C.F.B. Accumulation and elimination of cyanobacterial hepatotoxins by the freshwater clam Anodonta grandis simpsoniana. Can. J. Fish. Aquat. Sci. 1997, 54, 41–46. [Google Scholar]
- Williams, D.E.; Dawe, S.C.; Kent, M.; Andersen, R.J.; Craig, M.; Holmes, C.F.B. Bioaccumulation and clearance of microcystins from salt water mussels, Mytilus edulis, and in vivo evidence for covalently bound microcystins in mussel tissues. Toxicon 1997, 35, 1617–1625. [Google Scholar] [CrossRef]
- Xie, L.; Xie, P.; Guo, L.; Miyabara, Y.; Park, H. Organ distribution and bioaccumulation of microcystins in freshwater fish and different trophic levels from the eutrophic Lake Chaohu, China. Environ. Toxicol. 2005, 20, 293–300. [Google Scholar] [CrossRef]
- Papadimitriou, T.; Kagalou, I.; Stalikas, C.; Pilidis, G.; Leonardos, I.D. Assessment of microcystin distribution and biomagnification in tissues of aquatic food web compartments from a shallow lake and evaluation of potential risks to public health. Ecotoxicology 2012, 21, 1155–1166. [Google Scholar] [CrossRef]
- Zhang, D.; Den, X.; Xie, P.; Yang, Q.; Chen, J.; Dai, M. Determination of microcystin-LR and its metabolites in snail (Bellamya aeruginosa), shrimp (Macrobrachium nipponensis) and silver carp (Hypophthalmichthys molitrix) from Lake Taihu, China. Chemosphere 2009, 76, 974–981. [Google Scholar] [CrossRef]
- Li, X.Y.; Chung, I.K.; Kim, J.I.; Lee, J.E. Subchronic oral toxicity of microcystins in common carp (Cyprius carpio L.) exposed to Microcystis under laboratory conditions. Toxicon 2004, 44, 821–827. [Google Scholar] [CrossRef]
- Xie, L.; Xie, P.; Ozawa, K.; Honma, T.; Yokoyama, A.; Park, H.D. Dynamics of microcystins-LR and –RR in the planktivorous silver carp in a sub-chronic toxicity experiment. Environ. Poll. 2004, 127, 431–439. [Google Scholar] [CrossRef]
- Smith, J.L.; Haney, J.F. Foodweb transfer, accumulation, and depuration of microcystins, a cyanobacterial toxin, in pumpkinseed sunfish (Lepomis gibbosus). Toxicon 2006, 48, 580–589. [Google Scholar] [CrossRef]
- Qiu, T.; Xie, P.; Guo, L.; Zhang, D. Plasma biochemical responses of the planktivorous filter-feeding silver carp (Hypophthalmichthys molitrix) and bighead carp (Aristichthys nobilis) to prolonged toxic cyanobacterial blooms in natural waters. Environ. Toxicol. Pharm. 2009, 27, 350–356. [Google Scholar] [CrossRef]
- Zimba, P.V.; Khoo, L.; Gaunt, P.S.; Brittain, S.; Carmichael, W.W. Confirmation of catfish, Ictalurus punctatus (Rafinesque), mortality from Microcystis toxins. J. Fish Dis. 2001, 24, 41–47. [Google Scholar] [CrossRef]
- Freitas de Magalhães, V.F.; Soares, R.M.; Azevedo, S.M.F.O. Microcystin contamination in fish from the Jacarepagua Lagoon (Rio de Janeiro, Brazil): Ecological implication and human health risk. Toxicon 2001, 39, 1077–1085. [Google Scholar] [CrossRef]
- Malbrouck, C.; Trausch, G.; Devos, P.; Kestemont, P. Hepatic accumulation and effects of microcystin-LR on juvenile goldfish Carassius auratus L. Comp. Biochem. Phys. C 2003, 135, 39–48. [Google Scholar] [CrossRef]
- Wilson, A.E.; Gossiaux, D.C.; Höök, T.O.; Berry, J.P.; Landrum, P.F.; Dyble, J.; Guildford, S.J. Evaluation of the human health threat associated with the hepatotoxin microcystin in the muscle and liver tissues of yellow perch (Perca flavescens). Can. J. Fish. Aquat. Sci. 2008, 65, 1487–1497. [Google Scholar] [CrossRef]
- Poste, A.; Hecky, R.E.; Guildford, S.J. Evaluating microcystin exposure risk through fish consumption. Environ. Sci. Technol. 2011, 45, 5806–5811. [Google Scholar] [CrossRef]
- Microcystin Toxin Migration, Bioaccumulation, and Treatment; Nebraska Department of Environmental Quality: Fremont Lake #20, Dodge County, NE, USA, 2011.
- Palíková, M.; Mares, J.; Kopp, R.; Hlavkova, J.; Navratil, S.; Adamovský, O.; Chmelar, L.; Bláha, L. Accumulation of microcystins in Nile tilapia, Oreochromis niloticus L., and effects of a complex cyanobacterial bloom on the dietetic quality of muscles. In Bull. Environ. Contam. Toxicol.; 2011; Volume 87, pp. 26–30. [Google Scholar]
- Adamovský, O.; Kopp, R.; Hilscherová, K.; Babica, P.; Palíková, M.; Pašková, V.; Navrátil, S.; Maršálek, B.; Bláha, L. Microcystin kinetics (bioaccumulation and elimination) and biochemical responses in common carp (Cyprinus carpio) and silver carp (Hypophthalmichthys molitrix) exposed to toxic cyanobacterial blooms. Environ. Toxicol. Chem. 2007, 26, 2687–2693. [Google Scholar] [CrossRef]
- Berry, J.P.; Lee, E.; Walton, K.; Wilson, A.E.; Bernal-Brooks, F. Bioaccumulation of microcystins by fish associated with a persistent cyanobacterial bloom in Lago de Patzcuaro (Michoacan, Mexico). Environ. Toxicol. Chem. 2011, 30, 1621–1628. [Google Scholar]
- Mekebri, A.; Blondina, G.J.; Crane, D.B. Method validation of microcystins in water and tissue by enhanced liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 3147–3155. [Google Scholar] [CrossRef]
- Kozlowsky-Suzuki, B.; Wilson, A.E.; Ferrão-Filho, A. Biomagnification or biodilution of microcystins in aquatic foodwebs? Meta-analyses of laboratory and field studies. Harmful Algae 2012, 18, 47–55. [Google Scholar] [CrossRef]
- Falconer, I.R.; Jackson, A.R.B.; Langley, J.; Runnegar, M.T. Liver pathology in mice in poisoning by the blue-green alga Microcystis aeruginosa. Aust. J. Biol. Sci. 1981, 34, 179–187. [Google Scholar]
- Chorus, I.; Bartram, J. Toxic Cyanobacteria in Water: A Guide to Public Health Consequence, Monitoring and Management; E. and F.N. Spon: London, UK, 1999. [Google Scholar]
- Kotak, B.G.; Semalulu, S.; Fritz, D.L.; Prepas, E.E.; Hrudey, S.E.E.; Coppock, R.W. Hepatic and renal pathology of intraperitoneally administered microcystin-LR in rainbow trout (Oncorhynchus mykiss). Toxicon 1996, 34, 517–525. [Google Scholar] [CrossRef]
- Hairston, N.G.; Lampert, W.; Cáceres, C.E.; Holtmeier, C.L.; Weider, L.J.; Gaedke, U.; Fischer, J.M.; Fox, J.A.; Post, D.M. Rapid evolution revealed by dormant eggs. Nature 1999, 401, 446. [Google Scholar]
- Gustafsson, S.; Hanson, L. Development of tolerance against toxic cyanobacteria in Daphnia. Aquat. Ecol. 2004, 38, 37–44. [Google Scholar] [CrossRef]
- Guo, N.; Xie, P. Development of tolerance against toxic Microcystis aeruginosa in three cladocerans and the ecological implications. Environ. Pollut. 2006, 143, 513–518. [Google Scholar] [CrossRef]
- Wood, S.A.; Briggs, L.R.; Sprosen, J.; Ruck, J.G.; Wear, R.G.; Holland, P.T.; Bloxham, M. Changes in concentrations of microcystins in rainbow trout, freshwater mussels, and cyanobacteria in lakes Rotoiti and Rotoehu. Environ. Toxicol. 2006, 21, 205–221. [Google Scholar] [CrossRef]
- Lawrence, J.R.; Menard, C. Determination of microcystins in blue-green algae, fish and water using liquid chromatography with ultraviolet detection after sample clean-up employing immunoaffinity chromatography. J. Chromatogr. A 2001, 922, 111–117. [Google Scholar] [CrossRef]
- Moreno, I.A.; Herrador, M.A.; Atencio, L.; Puerto, M.; González, A.G.; Cameán, A.M. Differentiation between microcystin contaminated and uncontaminated fish by determination of unconjugated MCs using an ELISA anti-ADDA test based on receiver-operating characteristic curves threshold values: Application to Tinca tinca from natural ponds. Environ. Toxicol. 2009, 26, 45–56. [Google Scholar]
- Geis-Asteggiante, L.; Lehotay, S.J.; Fortis, L.L.; Paoli, G.; Wijey, C.; Heinzen, H. Development and validation of a rapid method for microcystins in fish and comparing LC-MS/MS results with ELISA. Anal. Bioanal. Chem. 2011, 401, 2617–2630. [Google Scholar] [CrossRef]
- Amé, M.V.; Galanti, L.N.; Menone, M.L.; Gerpe, M.S.; Moreno, V.J.; Wunderlin, D.A. Microcystin-LR, -RR, -YR, and –LA in water samples and fishes from a shallow lake in Argentina. Harmful Algae 2010, 9, 66–73. [Google Scholar] [CrossRef]
- Norris, R.L.G.; Eaglesham, G.K.; Shaw, G.R.; Senogles, P.; Chiswell, R.K.; Smith, M.J.; Davis, B.C.; Seawright, A.A.; Moore, M.R. Extraction and purification of the zwitterions cylindrospermopsin and deoxycylindrospermopsin from Cylindrospermopsis raciborskii. Environ. Toxicol. 2001, 16, 391–396. [Google Scholar] [CrossRef]
- Donati, C.; Drikas, M.; Hayes, R.; Newcombe, G. Microcystin-LR adsorption by powdered activated carbon. Wat. Res. 1994, 28, 1735–1742. [Google Scholar] [CrossRef]
- Huang, W.; Cheng, B.; Cheng, Y. Adsorption of microcystin-LR by three types of activated carbon. J. Hazard. Mater. 2007, 141, 115–122. [Google Scholar] [CrossRef]
- Ho, L.; Lambling, P.; Bustamante, H.; Duker, P.; Newcombe, G. Application of powdered activated carbon for the adsorption of cylindrospermopsin and microcystin toxins from drinking water supplies. Wat. Res. 2011, 45, 2954–2964. [Google Scholar] [CrossRef]
- Ohio EPA. Algae Info Webpage. 2012. Available online: ohioalgaeinfo.com (accessed on 5 March 2013).
- Hoorman, J.J.; Prochaska, S.C.; Rausch, J.N.; Fritz, M. Ohio swine farm manure application survey. Ohio J. Sci. 2005a, 104, A-34. [Google Scholar]
- Hoorman, J.J.; Prochaska, S.C.; Rausch, J.N.; Fritz, M. Ohio dairy farm manure application survey. Ohio J. Sci. 2005b, 104, A-34. [Google Scholar]
- Hoorman, J.J.; Hone, T.; Sudman, T., Jr.; Dirksen, T.; Iles, J.; Islam, K.R. Agricultural impacts on lake and stream water quality in Grand Lake St. Marys, Western Ohio. Water Air Soil Pollut. 2008, 193, 309–322. [Google Scholar] [CrossRef]
- Steffan, M.M.; Li, Z.; Effler, C.; Hauser, L.J.; Boyer, G.L.; Wilhelm, S.W. Comparative metagenomics of toxic freshwater cyanobacterial bloom communities on two continents. PLoS One 2012, 7, 1–9. [Google Scholar]
- Seaburg, K.G.; Moyle, J.B. Feeding habits, digestive rates, and growth of some Minnesota warmwater fishes. Trans. Am. Fish. Soc. 2011, 93, 269–285. [Google Scholar] [CrossRef]
- Catherine, A.; Quiblier, C.; Yéprémiam, C.; Got, P.; Groleau, A.; Vincon-Leite, B.; Bernard, C.; Trousselier, M. Collapse of Planktothrix agardhii perennial bloom and microcystin dynamics in response to reduce phosphate concentrations in a temperate late. FEMS Microbiol. Ecol. 2008, 65, 61–73. [Google Scholar] [CrossRef]
- Rinta-Kanto, J.M.; Wilhelm, S.W. Diversity of microcystin-producing cyanobacteria in spatially isolated regions of Lake Erie. Appl. Environ. Microbiol. 2006, 72, 5083–5085. [Google Scholar] [CrossRef]
- Anderson, D.M. Bloom dynamics of toxic Alexandrium species in the north-eastern United States. Limnol. Oceanogr. 1997, 42, 1009–1022. [Google Scholar] [CrossRef]
- Anderson, D.M. Prevention, Control, and Mitigation of Harmful Algal Blooms: Multiple Approaches to HAB Management. In Harmful Algae Management and Mitigation; Hudnell, H.K., Etheridge, S., Anderson, D., Kleindinst, J., Zhu, M., Zou, Y., Eds.; Asia Pacific Economic Cooperation: Singapore, 2004; pp. 123–130. [Google Scholar]
- Kohoutek, J.; Adamovský, O.; Oravec, M.; Simek, Z.; Palíková, M.; Kopp, R.; Bláha, L. LC-MS analyses of microcystins in fish tissues overestimate toxin levels-critical comparison with LC-MS/MS. Anal. Bioanal. Chem. 2010, 398, 1231–1237. [Google Scholar]
- Wendelken, S.C.; Munch, D.J.; Pepich, B.V.; Later, D.W.; Pohl, C.A. Method 331.0: Determination of Perchlorate in Drinking Water by Liquid Chromatography Electrospray Ionization Mass Spectrometry, revision 1.0; US Environmental Protection Agency: Cincinnati, OH, USA, 2005; EPA Document #: 815-R-05-007. [Google Scholar]
- Tsvetanova, B.C.; Price, N.P.J. Liquid chromatography–electrospray mass spectrometry of Tunicamycin-type antibiotics. Anal. Biochem. 2001, 289, 147–156. [Google Scholar]
- Fawell, J.K.; James, C.P.; James, H.A. Toxins from Blue-Green Algae: Toxicological Assessment of micRocystin-LR and a Method for Its Determination in Water; Foundation for Water Research: Medmenham, Marlow, Bucks, UK, 1994; pp. 1–46. [Google Scholar]
- World Health Organization, Cyanobacterial Toxins: Microcystin-LR in Drinking Water. In Guidelines for Drinking Water Quality; WHO: Geneva, Italy, 2008.
- Ibelings, B.W.; Chorus, I. Accumulation of cyanobacterial toxins in freshwater “seafood” and its consequence for public health: A review. Environ. Pollut. 2007, 150, 177–192. [Google Scholar] [CrossRef]
- Mulvenna, V.; Dale, K.; Priestly, B.; Mueller, U.; Humpage, A.; Shaw, G.; Allinson, G.; Falconer, I. Health risk assessment for cyanobacterial toxins in seafood. Int. J. Environ. Res. Public Health 2012, 9, 807–820. [Google Scholar]
- Smith, J.L.; Schulz, K.L.; Zimba, P.V.; Boyer, G.L. Possible mechanism for the foodweb transfer of covalently bound microcystins. Ecotoxicol. Environ. Saf. 2010, 73, 757–761. [Google Scholar]
- Williams, D.E.; Craig, M.; Dawe, S.C.; Kent, M.L.; Holmes, C.F.B. 14C-labeled microcystin-LR administered to Atlantic salmon via intraperitoneal injection provides in vivo evidence for covalent binding of microcystin-LR in salmon livers. Toxicon 1997a, 35, 985–989. [Google Scholar]
- Williams, D.E.; Craig, M.; Dawe, S.C.; Kent, M.L.; Holmes, C.F.B.; Andersen, R.J. Evidence for a covalently bound form of microcystin-LR in salmon liver and dungeness crab larvae. Chem. Res. Toxicol. 1997b, 10, 463–469. [Google Scholar]
- Ibelings, B.W.; Bruning, K.; de Jonge, J.; Wolfstein, K.; Pires, L.M.; Postma, J.; Burger, T. Distribution of microcystins in a lake foodweb: No evidence for biomagnification. Microb. Ecol. 2005, 49, 487–500. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Beattie, K.A.; Pflugmacher, S.; Codd, G.A. Immuno-crossreactivity and toxicity assessment of conjugation products of the cyanobacterial toxin, microcystin-LR. FEMS Microbiol. Lett. 2000, 189, 155–158. [Google Scholar] [CrossRef]
- Ito, E.; Takai, A.; Kondo, F.; Masui, H.; Imanishi, S.; Harada, K. Comparison of protein phosphatase inhibitory activity and apparent toxicity of microcystins and related compounds. Toxicon 2002, 40, 1017–1025. [Google Scholar]
- Falconer, I.R.; Bartram, J.; Chorus, I.; Kuiper-Goodman, T.; Utkilen, H.; Burch, M.; Codd, G.A. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; E&FN Spon: London, UK, 1999; pp. 155–578. [Google Scholar]
- Campos, A.; Vasconcelos, V. Molecular mechanisms of microcystin toxicity in animal cells. Int. J. Mol. Sci. 2010, 11, 268–287. [Google Scholar] [CrossRef]
- Ohio EPA, Preparation of Tissue Samples for Extraction of Organic and Metals Determination; Ohio EPA method 581.10; Ohio EPA: Cincinnati, OH, USA, 2007.
- Boyer, G.L. The occurrence of Cyanobacterial toxins in New York lakes: Lessons for the MERHAB-Lower Great lakes program. Lake Reserv. Manag. 2007, 23, 153–160. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Schmidt, J.R.; Shaskus, M.; Estenik, J.F.; Oesch, C.; Khidekel, R.; Boyer, G.L. Variations in the Microcystin Content of Different Fish Species Collected from a Eutrophic Lake. Toxins 2013, 5, 992-1009. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins5050992
Schmidt JR, Shaskus M, Estenik JF, Oesch C, Khidekel R, Boyer GL. Variations in the Microcystin Content of Different Fish Species Collected from a Eutrophic Lake. Toxins. 2013; 5(5):992-1009. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins5050992
Chicago/Turabian StyleSchmidt, Justine R., Mylynda Shaskus, John F. Estenik, Carl Oesch, Roman Khidekel, and Gregory L. Boyer. 2013. "Variations in the Microcystin Content of Different Fish Species Collected from a Eutrophic Lake" Toxins 5, no. 5: 992-1009. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins5050992




