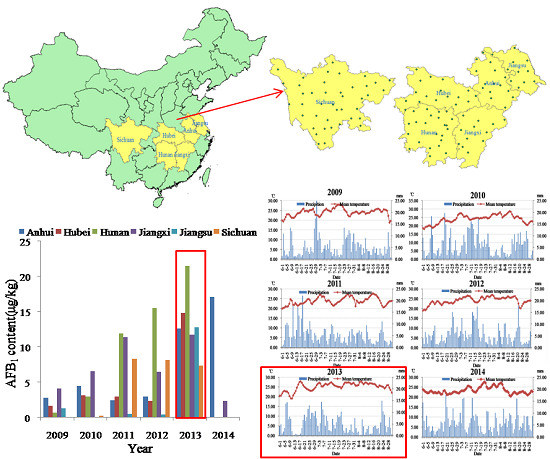
2.5.1. AFB1 Dietary Exposure Assessment
Based on the AFB
1 contamination data of the peanut samples combined with the peanut consumption data and demographic data (
Table 4), AFB
1 intake via peanuts of different population groups was simulated and calculated. The distributions of the estimated intake percentiles (90% confidence interval) are shown in
Table 5. Evenwithin the same region, the AFT-induced liver cancer risk varied significantly among different populations. The mean AFB
1 intake through peanuts for 2- to 6-year-old children was higher than adults. Simulated AFB
1 intake at the mean peanut consumption level ranges from 0.777 ng/(kg·d) (LB estimate) to 0.790 ng/(kg·d) (UB estimate), and at the high peanut consumption level it ranges from 11.660 ng/(kg·d) (LB estimate) to 11.853 ng/(kg·d) (UB estimate). The high percentile (P97.5) at the mean peanut consumption level ranges from 10.007 ng/(kg·d) (LB estimate) to 10.022 ng/(kg·d) (UB estimate), and at the high peanut consumption level it ranges from 150.104 ng/(kg·d) (LB estimate) to 150.330 ng/(kg·d) (UB estimate). The high AFT exposure for children and the effects of this exposure on children’s growth have been demonstrated in West Africa [
25]. Because the AFB
1 dietary exposure level in children is high and its influence on the high immunity level and different aspects of children’s health is significant [
26], China should establish more strict regulations on the control of the processing conditions of peanuts, sorting techniques and so on to limit AFT at the consumer level.
Table 4.
Peanut consumption groups and consumption amount.
Table 4.
Peanut consumption groups and consumption amount.
| Group | Weight/kg | Amount of Peanut Consumption/g |
|---|
| Mean-Level Consumption | High-Level Consumption |
|---|
| 2- to 6- year-old children | 15.18 | 1.66 | 24.9 |
| Standard adult a | 62.57 | 3.02 | 35.7 |
2.5.2. AFB1 Risk Characterization
Based on AFB
1 intake in the above two population groups through peanuts and risk assessment method established by Joint FAO/WHO Expert Committee on Food Additives (JECFA) [
27], liver cancer risk related to AFB
1 dietary exposure through peanuts and an average potency figure obtained from the individual potencies of hepatitis B surface antigen-positive and -negative groups were evaluated by JECFA. In China, the hepatitis B prevalence rate is assumed to be 7.18%. The cancer risk characterization results are shown in
Table 6. In light of the national average HBsAg+ prevalence rate, even at the high risk level of P97.5, the liver cancer risk (90% confidence interval) resulting from peanut AFB
1 exposure in adults with high peanut consumption ranged from 1.619 (1.524–1.692) cases/(100,000 persons·year) (LB estimate) to 1.621 (1.524–1.693) cases/(100,000 persons·year) (UB estimate), less than China’s current liver cancer incidence of 24.6 cases/(100,000 persons·year) [
28]. Research showed that liver cancer is a disease prevalent in some developing parts of the world, such as China, South East Asia and sub-Saharan Africa [
27]. Harris
et al. (1996) reviewed the evidence that “a dose-dependent relationship between dietary AFB
1 intake and p53 mutations (codon 249 ser) is observed in hepatocellular carcinoma from Asia, Africa and North America” [
29]. Therefore, the heavily-contaminated regions of AFB
1 are in good agreement with liver cancer prevalence regions. Due to high AFB
1 intake by 2- to 6-year-old children with high peanut consumption, their liver cancer risk was relatively high and noteworthy, and essential surveillance measures should be taken to protect their health.
Generally, based on the raw peanut samples from the post-harvest fields in the Yangtze River ecological region, dietary exposure assessment results indicate that the occurrence of AFB1 in raw peanuts at harvest does not appear to be a serious problem and that the risk concerning public health is low in China.
Different countries have varied AFB
1 exposure levels. Data assembled in the researches indicated that the exposure to AFTs through frequently contaminated foods was 3.5–14.8 ng/(kg·d) in Kenya, 11.4–158.6 ng/(kg·d) in Swaziland, 38.6–183.7 ng/(kg·d) in Mozambique, 16.5 ng/(kg·d) in Transkei (now South Africa), and 4–115 ng/(kg·d) in Gambia. The AFTs exposure in Ghana, as measured from peanut consumption alone, is estimated to be 9.9–99.2 ng/(kg·d). The estimated mean AFB
1 exposure for urban Lebanese adults was 0.63–0.66ng/(kg·d) and P95 was 1.40–1.46ng/(kg·d). Based on the mean dietary exposure level of AFB
1, the cancer risk was estimated to be0.0527–0.0545 cases/(100,000 persons·year) [
30]. As for Asia, the estimated AFTs intake was 11.7–2027ng/(kg·d) in southern Guangxi province of China and 6.5–53 ng/(kg·d) in Thailand.Sugita-Konishi
et al. (2010) assessed AFTs dietary exposure by food intake in different age groups, and the results suggested that the dietary intake of AFB
1 ranged from 0.003 to 0.004 ng/(kg·d) (from lower to upper limits), and the potential cancer risk was 0.00004–0.00005 cases/(100,000 persons·year) persons in the high level (95.0th percentile) of the consumer population. The mean dietary intake of AFB
1 through peanuts was 0.49ng/(kg·d) [
31]. However, the European Union estimated that the dietary exposure to AFTs ranged from 0.93 to 2.45 ng/(kg·d) for lower bound to upper bound [
27,
32]. In the United States, the exposure was estimated at 2.7 ng/(kg·d) [
8]. The AFB
1 intake estimates in our study were relatively high because peanuts were one of the products most likely affected by AFTs. Therefore, it is very important to regulate and monitor AFT contamination to protect peanut consumers, especially children and vegans. The results agreed with Wilda
et al.’s findings (2000) that the most heavily AFT-afflicted parts of the world were sub-Saharan Africa, Southeast Asia, and China [
33]. On the one hand, the reason may lie in the particularly high risk areas in tropical and subtropical regions and exposure to climatic conditions favorable for
A. flavus and
A. parasiticus proliferation. On the other hand, limited AFT control strategies were implemented in these countries. Prediction before harvest, good agricultural practice (GAP), good manufacturing practice (GMP), hazard analysis and critical control point (HACCP) are all effective measures for AFT control in peanuts.
Ding et al. (2012) indicated that the risk from peanut oil was about ten times than from raw peanuts in China. Consequently, AFB1 control for post-harvest products including storage conditions, processing methods and so on is critical to aggravate AFB1 contamination in the Yangtze River ecological region, besides growing and harvest. Further studies need to be focused on the process of peanut circulation and storage for AFB1 contamination risk assessment.
Table 5.
Simulated AFB1 intake through peanuts in different population groups in the Yangtze River ecological region.
Table 5.
Simulated AFB1 intake through peanuts in different population groups in the Yangtze River ecological region.
| Population | Consumption Level | Methods | Mean (90% Confidence Interval)/ng/(kg·d) | Percentiles of AFB1 Intake (90% Confidence Interval)/ng/(kg·d) |
|---|
| P50 | P75 | P90 | P95 | P97.5 |
|---|
| 2- to 6-year-old child | Mean | LB a | 0.777 (0.729–0.825) | 0 | 0.031 (0.028–0.034) | 1.377 (1.230–1.501) | 6.131 (5.796–6.383) | 10.007 (9.423–10.462) |
| UB a | 0.790 (0.745–0.837) | 0.022 | 0.031 (0.028–0.035) | 1.384 (1.231–1.503) | 6.144 (5.796–6.383) | 10.022 (9.423–10.465) |
| High | LB | 11.660 (10.934–12.370) | 0 | 0.462 (0.427–0.509) | 20.655 (18.454–22.509) | 91.972 (86.937–95.751) | 150.104 (141.338–156.929) |
| UB | 11.853 (11.174–12.556) | 0.328 | 0.463 (0.427–0.509) | 20.753 (18.470–22.538) | 92.162 (86.937–95.751) | 150.330 (141.338–156.972) |
| Standard adult | Mean | LB | 0.343 (0.322–0.364) | 0 | 0.014 (0.013–0.015) | 0.608 (0.544–0.662) | 2.706 (2.558–2.818) | 4.417 (4.159–4.618) |
| UB | 0.349 (0.329–0.370) | 0.010 | 0.014 (0.013–0.015) | 0.611 (0.543–0.663) | 2.712 (2.558–2.818) | 4.423 (4.159–4.619) |
| High | LB | 4.056 (3.803–4.303) | 0 | 0.161 (0.148–0.177) | 7.185 (6.419–7.829) | 31.991 (30.240–33.306) | 52.212 (49.162–54.586) |
| UB | 4.123 (3.887–4.367) | 0.114 | 0.161 (0.148–0.177) | 7.219 (6.425–7.840) | 32.057 (30.240–33.306) | 52.290 (49.162–54.601) |
Table 6.
Estimated liver cancer risk caused by AFB1 intake through peanuts in different population groups in the Yangtze River ecological region.
Table 6.
Estimated liver cancer risk caused by AFB1 intake through peanuts in different population groups in the Yangtze River ecological region.
| Population | Consumption Level | Methods | Mean (90% Confidence Interval)/cases/(105·persons·year) | Percentiles of AFB1-Induced Liver Cancer Risk (90% Confidence Interval)/cases/(100,000·persons·year) |
|---|
| P50 | P75 | P90 | P95 | P97.5 |
|---|
| 2- to 6-year-old child | Mean | LB a | 0.024 (0.023–0.026) | 0 | 0.001 | 0.043 (0.038–0.047) | 0.190 (0.180–0.198) | 0.310 (0.292–0.324) |
| UB a | 0.024 (0.023–0.026) | 0.001 | 0.001 | 0.043 (0.038–0.047) | 0.190 (0.180–0.198) | 0.311 (0.292–0.324) |
| High | LB | 0.361 (0.339–0.383) | 0 | 0.014 (0.013–0.016) | 0.640 (0.572–0.698) | 2.851 (2.695–2.968) | 4.653 (4.381–4.865) |
| UB | 0.367 (0.346–0.389) | 0.010 | 0.014 (0.013–0.016) | 0.643 (0.573–0.699) | 2.857 (2.695–2.968) | 4.660 (4.381–4.866) |
| Standard adult | Mean | LB | 0.011 (0.010–0.011) | 0 | 0 | 0.019 (0.017–0.021) | 0.084 (0.079–0.087) | 0.137 (0.129–0.143) |
| UB | 0.011 (0.010–0.012) | 0 | 0 | 0.019 (0.017–0.021) | 0.084 (0.079–0.087) | 0.137 (0.129–0.143) |
| High | LB | 0.126 (0.118–0.133) | 0 | 0.005 (0.004–0.006) | 0.223 (0.199–0.243) | 0.992 (0.937–1.032) | 1.619 (1.524–1.692) |
| UB | 0.128 (0.120–0.135) | 0.004 | 0.005 | 0.224 (0.199–0.243) | 0.994 (0.937–1.032) | 1.621 (1.524–1.693) |