A Magnetic Nanoparticle Based Enzyme-Linked Immunosorbent Assay for Sensitive Quantification of Zearalenone in Cereal and Feed Samples
Abstract
:1. Introduction

2. Results and Discussion
2.1. Identification of ZEN-BSA Conjugate and ZEN-BSA-Biotin

2.2. Identification of Anti-ZEN Immunomagnetic Nanoparticles

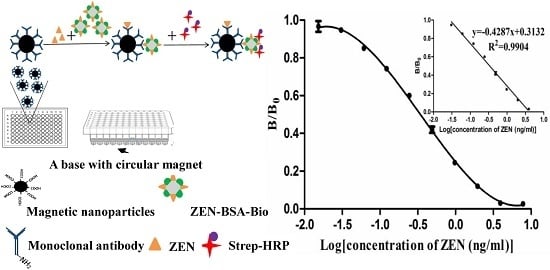
2.3. Optimization of Indirect Competitive MNP-bsELISA for Quantitation of ZEN

2.4. Specificity Study
2.5. Calibration Curve and Matrix Interference Analysis

| Methods | Detection Time | Limit of Detection | IC50 | Working Range | Ref. |
|---|---|---|---|---|---|
| DC-ELISA | 1h | 0.15 ng/mL | 1.13 ng/mL | 41.0–909.8 μg/kg | [40] |
| ic-ELISA | 2h | 0.8 ng/mL | - | 0.8–150 ng/mL | [41] |
| Immunosensor assay | - | 0.007 ng/mL | - | 0.019–0.422 ng/mL | [35] |
| Fluorescence assay | - | 137 μg/kg | - | 150–1000 μg/kg | [39] |
| Array Immunoassay | 1.5 | 0.51 ng/mL | 2.1 ng/mL | 0.73–6.8 ng/mL | [42] |
| mAb 2C9 based ic-ELISA | 2 h | 0.12 ng/mL | 1.24ng/mL | 0.21–9.76 ng/mL | [34] |
| mAb 2C9 based MNP-bsELISA | 1.5 | 0.04 ng/mL | 0.37 ng/mL | 0.07–2.41 ng/mL | This study |

2.6. Recovery Rates of ZEN in Spiked Corn Samples and Detection of ZEN in Natural Samples
| Samples | Spiked Level (μg/kg) | Inter-Assay a | |||
|---|---|---|---|---|---|
| n | Measured (μg/kg) | Recovery (%) | CV b (%) | ||
| 1 | 1.25 | 3 | 1.18 ± 0.04 | 94.5 ± 2.9 | 3.1 |
| 2 | 2.5 | 3 | 2.32 ± 0.18 | 92.8 ± 6.9 | 7.5 |
| 3 | 5 | 3 | 5.42 ± 0.26 | 108.4 ± 5.2 | 4.8 |
| 4 | 10 | 3 | 11.19 ± 0.67 | 111.9 ± 6.7 | 6.1 |
| 5 | 20 | 3 | 21.43 ± 1.13 | 107.2 ± 5.6 | 5.3 |
| Samples | MNP-bsELISA (μg/kg), Mean ± SD a | LC-MS/MS (μg/kg), Mean ± SD |
|---|---|---|
| Corn 1 | 7.96 ± 0.49 | 9.99 ± 0.08 |
| Corn 2 | 12.47 ± 0.66 | 14.01 ± 0.45 |
| Corn 3 | 14.61 ± 0.37 | 14.27 ± 0.15 |
| Corn 4 | 11.61 ± 0.46 | 10.91 ± 0.28 |
| Corn 5 | 3.02 ± 0.19 | - b |
| Wheat 1 | 15.34 ± 0.39 | 16.67 ± 0.05 |
| Wheat 2 | 3.76 ± 0.21 | - b |
| Wheat 3 | 12.31 ± 0.26 | 14.41 ± 0.35 |
| Feedstuff 1 | 19.73 ± 1.69 | 21.25 ± 0.11 |
| Feedstuff 2 | 16.19 ± 0.71 | 17.52 ± 0.29 |
| Feedstuff 3 | 14.13 ± 0.77 | 14.84 ± 0.28 |
| Feedstuff 4 | 18.77 ± 1.19 | 19.35 ± 0.16 |

3. Experimental Section
3.1. Chemicals and Reagents
3.2. Equipment
3.3. Synthesis of the ZEN-BSA Conjugate
3.4. Biotinylation of ZEN-BSA and Identification
3.5. Preparation of Immunomagnetic Nanoparticles
3.6. Optimization of the MNP-bsELISA
3.7. Development of Indirect Competitive MNP-bsELISA
3.8. Specificity
3.9. Elimination of Matrix Interference and Recovery of Spiked Samples
3.10. Detection of Natural Samples by MNP-bsELISA and LC-MS/MS
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, I.-H.; Son, H.-Y.; Cho, S.-W.; Ha, C.-S.; Kang, B.-H. Zearalenone induces male germ cell apoptosis in rats. Toxicol. Lett. 2003, 138, 185–192. [Google Scholar] [PubMed]
- Placinta, C.; Dʼmello, J.; Macdonald, A. A review of worldwide contamination of cereal grains and animal feed with fusarium mycotoxins. Anim. Feed Sci. Technol. 1999, 78, 21–37. [Google Scholar] [CrossRef]
- Miller, J.D. Mycotoxins in grain. Rev. Ins. Med. Trop. Sao Paulo 1994, 36, 326–326. [Google Scholar]
- Rodricks, J.V.; Hesseltine, C.W.; Mehlman, M.A. Mycotoxins in Human and Animal Health. In Proceedings of a Conference Convened at University of Maryland, College Park, MD, USA, 4–8 October 1976.
- Collins, T.F.; Sprando, R.L.; Black, T.N.; Olejnik, N.; Eppley, R.M.; Alam, H.Z.; Rorie, J.; Ruggles, D.I. Effects of zearalenone on in utero development in rats. Food Chem. Toxicol. 2006, 44, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Panel, E.C. Scientific opinion on the risks for public health related to the presence of zearalenone in food. EFSA J. 2011, 9, 2197. [Google Scholar]
- EFSA, J. Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to zearalenone as undesirable substance in animal feed. EFSA J. 2004, 89, 1–35. [Google Scholar]
- Šegvić Klarić, M.; Cvetnić, Z.; Pepeljnjak, S.; Kosalec, I. Co-occurrence of aflatoxins, ochratoxin A, fumonisins, and zearalenone in cereals and feed, determined by competitive direct enzyme-linked immunosorbent assay and thin-layer chromatography. Arh. Higijenu Rada Toksikol. 2009, 60, 427–433. [Google Scholar]
- Tsiplakou, E.; Anagnostopoulos, C.; Liapis, K.; Haroutounian, S.A.; Zervas, G. Determination of mycotoxins in feedstuffs and ruminantʼs milk using an easy and simple LC-MS/MS multiresidue method. Talanta 2014, 130, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wong, J.W.; Krynitsky, A.J.; Trucksess, M.W. Determining mycotoxins in baby foods and animal feeds using stable isotope dilution and liquid chromatography tandem mass spectrometry. J. Agric. Food Chem. 2014, 62, 8935–8943. [Google Scholar] [CrossRef] [PubMed]
- Lacina, O.; Zachariasova, M.; Urbanova, J.; Vaclavikova, M.; Cajka, T.; Hajslova, J. Critical assessment of extraction methods for the simultaneous determination of pesticide residues and mycotoxins in fruits, cereals, spices and oil seeds employing ultra-high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2012, 1262, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Giovannoli, C.; Passini, C.; Di Nardo, F.; Anfossi, L.; Baggiani, C. Determination of ochratoxin a in italian red wines by molecularly imprinted solid phase extraction and HPLC analysis. J. Agric. Food Chem. 2014, 62, 5220–5225. [Google Scholar] [CrossRef] [PubMed]
- Stanker, L.H.; Scotcher, M.C.; Cheng, L.; Ching, K.; McGarvey, J.; Hodge, D.; Hnasko, R. A monoclonal antibody based capture ELISA for botulinum neurotoxin serotype B: Toxin detection in food. Toxins 2013, 5, 2212–2226. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Pang, J.; Yu, J.; Wang, R.; Liu, L.; Ma, Y.; Zhang, Y.; Jin, N.; Wang, S. Preparation and identification of monoclonal antibody against fumonisin B1 and development of detection by Ic-ELISA. Toxicon 2014, 80, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, X.; Yang, S.; Cao, X.; Wang, Z.; Shi, W.; Zhang, S. High specific monoclonal antibody production and development of an ELISA method for monitoring T-2 toxin in rice. J. Agric. Food Chem. 2014, 62, 1492–1497. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.B.; Dzantiev, B.B.; Eremin, S.A.; Chung, D.H. One-step simultaneous immunochromatographic strip test for multianalysis of ochratoxin A and zearalenone. J. Microbiol. Biotechnol. 2009, 19, 83–92. [Google Scholar] [PubMed]
- Kolosova, A.Y.; de Saeger, S.; Sibanda, L.; Verheijen, R.; van Peteghem, C. Development of a colloidal gold-based lateral-flow immunoassay for the rapid simultaneous detection of zearalenone and deoxynivalenol. Anal. Bioanal. Chem. 2007, 389, 2103–2107. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-K.; Yan, Y.-X.; Ji, W.-H.; Wang, H.-A.; Li, S.-Q.; Zou, Q.; Sun, J.-H. Rapid simultaneous quantification of zearalenone and fumonisin B1 in corn and wheat by lateral flow dual immunoassay. J. Agric. Food Chem. 2013, 61, 5031–5036. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, S.H.; Palleschi, G.; Compagnone, D.; Pascale, M.; Visconti, A.; Barna-Vetró, I. Monoclonal antibody based electrochemical immunosensor for the determination of ochratoxin A in wheat. Talanta 2006, 69, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Du, L.; Zou, L.; Zou, Y.; Hu, N.; Wang, P. An ultrasensitive electrochemical immunosensor for carcinoembryonic antigen detection based on staphylococcal protein A—Au nanoparticle modified gold electrode. Sens. Actuators B: Chem. 2014, 197, 220–227. [Google Scholar] [CrossRef]
- Matsukuma, E.; Kato, Z.; Omoya, K.; Hashimoto, K.; Li, A.; Yamamoto, Y.; Ohnishi, H.; Hiranuma, H.; Komine, H.; Kondo, N. Development of fluorescence-linked immunosorbent assay for high throughput screening of interferon-γ. Allergology Int. 2006, 55, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Li, P.; Zhang, Q.; Zhang, W.; Hu, X.; Ding, X. Monoclonal antibody-quantum dots CdTe conjugate-based fluoroimmunoassay for the determination of aflatoxin B1 in peanuts. Food Chem. 2014, 146, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, H.; Li, C.; Yu, Q.; Shen, J.; De Saeger, S. Development and application of a quantitative fluorescence-based immunochromatographic assay for fumonisin B1 in maize. J. Agric. Food Chem. 2014, 62, 6294–6298. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-T.; Yeh, J.Z.; Jiang, C.-M.; Wu, M.-C. Magnetic particle-linked anti hCG β antibody for immunoassay of human chorionic gonadotropin (hCG), potential application to early pregnancy diagnosis. J. Immunol. Methods 2012, 381, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Pappert, G.; Rieger, M.; Niessner, R.; Seidel, M. Immunomagnetic nanoparticle-based sandwich chemiluminescence-ELISA for the enrichment and quantification of E. coli. Microchim. Acta 2010, 168, 1–8. [Google Scholar] [CrossRef]
- Urusov, A.E.; Petrakova, A.V.; Vozniak, M.V.; Zherdev, A.V.; Dzantiev, B.B. Rapid immunoenzyme assay of aflatoxin B1 using magnetic nanoparticles. Sensors 2014, 14, 21843–21857. [Google Scholar] [CrossRef] [PubMed]
- Ohne, K.; Kani, S.; Ohashi, M.; Shinkai, N.; Inoue, T.; Wakimoto, Y.; Tanaka, Y. clinical evaluation of a newly developed high-sensitive detection of hepatitis B virus surface antigen by a semi-automated immune complex transfer chemiluminescent enzyme immunoassay. Rinsho byori. Jpn. J. Clin. Pathol. 2013, 61, 787–794. [Google Scholar]
- Smith, J.E.; Sapsford, K.E.; Tan, W.; Ligler, F.S. Optimization of antibody-conjugated magnetic nanoparticles for target preconcentration and immunoassays. Anal. Biochem. 2011, 410, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Diler, E.; Obst, U.; Schmitz, K.; Schwartz, T. A lysozyme and magnetic bead based method of separating intact bacteria. Anal. Bioanal. Chem. 2011, 401, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Shen, G.; Zhu, H.; Jiang, G. A class-specific enzyme-linked immunosorbent assay based on magnetic particles for multiresidue organophosphorus pesticides. J. Agric. Food Chem. 2010, 58, 2801–2806. [Google Scholar] [CrossRef] [PubMed]
- Bu, D.; Zhuang, H.S.; Yang, G.X. A sensitive enzyme-linked immunosorbent assay amplified by biotin-streptavidin system for detecting non-steroidal anti-inflammatory drug ketoprofen. J. Environ. Sci. Health Part B 2014, 49, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wang, X.; Li, Z.-J.; Ren, S.-Q.; Chen, G.-N.; Ying, X.-T.; Lin, J.-M. Development of a sensitive, rapid, biotin–streptavidin based chemiluminescent enzyme immunoassay for human thyroid stimulating hormone. Talanta 2008, 75, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Chai, Y.; Zhuo, Y.; Yuan, R. Ultrasensitive simultaneous detection of four biomarkers based on hybridization chain reaction and biotin-streptavidin signal amplification strategy. Biosens. Bioelectron. 2015, 68, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Wang, Y.; Chen, Z.; Yan, Y.; Hao, Q.; Li, S.; Yu, C.; Yang, C.; Sun, J. Preparation of anti-zearalenone monoclonal antibodies and development of an indirect competitive elisa for zearalenone. Microbiol. China 2011, 38, 1793–1800. [Google Scholar]
- Urraca, J.L.; Benito-Peña, E.; Pérez-Conde, C.; Moreno-Bondi, M.C.; Pestka, J.J. Analysis of zearalenone in cereal and swine feed samples using an automated flow-through immunosensor. J. Agric. Food Chem. 2005, 53, 3338–3344. [Google Scholar] [CrossRef] [PubMed]
- Burmistrova, N.A.; Goryacheva, I.Y.; Basova, E.Y.; Franki, A.-S.; Elewaut, D.; Van Beneden, K.; Deforce, D.; van Peteghem, C.; de Saeger, S. Application of a new anti-zearalenone monoclonal antibody in different immunoassay formats. Anal. Bioanal. Chem. 2009, 395, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Burkin, A.; Kononenko, G.; Soboleva, N. Group-specific antibodies against zearalenone and its metabolites and synthetic analogs. Appl. Biochem. Microbiol. 2002, 38, 169–176. [Google Scholar] [CrossRef]
- Kido, K.; Edakuni, K.; Morinaga, O.; Tanaka, H.; Shoyama, Y. An enzyme-linked immunosorbent assay for aconitine-type alkaloids using an anti-aconitine monoclonal antibody. Anal. Chim. Acta 2008, 616, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.S.; Choi, E.H.; Chang, H.-J.; Choi, S.-W.; Eremin, S.A. A fluorescence polarization immunoassay for the detection of zearalenone in corn. Anal. Chim. Acta 2009, 639, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Thongrussamee, T.; Kuzmina, N.; Shim, W.-B.; Jiratpong, T.; Eremin, S.; Intrasook, J.; Chung, D.-H. Monoclonal-based enzyme-linked immunosorbent assay for the detection of zearalenone in cereals. Food Addit. Contam. 2008, 25, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Zhi-hong, M. Detection on zearalenone in grains by biotin-streptavidin enzyme-linked immunosorbent assay. J. Anhui Agric. Sci. 2009, 15, 016. [Google Scholar]
- Wang, Y.-K.; Yan, Y.-X.; Li, S.-Q.; Wang, H.-A.; Ji, W.-H.; Sun, J.-H. Simultaneous quantitative determination of multiple mycotoxins in cereal and feedstuff samples by a suspension array immunoassay. J. Agric. Food Chem. 2013, 61, 10948–10953. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Quan, Y.; Lee, N.; Kennedy, I.R. Rapid determination of fumonisin B1 in food samples by enzyme-linked immunosorbent assay and colloidal gold immunoassay. J. Agric. Food Chem. 2006, 54, 2491–2495. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-K.; Shi, Y.-B.; Zou, Q.; Sun, J.-H.; Chen, Z.-F.; Wang, H.-A.; Li, S.-Q.; Yan, Y.-X. Development of a rapid and simultaneous immunochromatographic assay for the determination of zearalenone and fumonisin b1 in corn, wheat and feedstuff samples. Food Control. 2013, 31, 180–188. [Google Scholar] [CrossRef]
- Ono, E.Y.; Kawamura, O.; Ono, M.A.; Ueno, Y.; Hirooka, E.Y. A comparative study of indirect competitive ELISA and HPLC for fumonisin detection in corn of the state of paraná, brazil. Food Agric. Immunol. 2000, 12, 5–14. [Google Scholar] [CrossRef]
- Quan, Y.; Zhang, Y.; Wang, S.; Lee, N.; Kennedy, I.R. A rapid and sensitive chemiluminescence enzyme-linked immunosorbent assay for the determination of fumonisin B1 in food samples. Anal. Chim. Acta 2006, 580, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gendloff, E.; Casale, W.; Ram, B.; Tai, J.; Pestka, J.; Hart, L. Hapten-protein conjugates prepared by the mixed anhydride method: Cross-reactive antibodies in heterologous antisera. J. Immunol. Methods 1986, 92, 15–20. [Google Scholar] [CrossRef]
- Thouvenot, D.; Morfin, R.F. Radioimmunoassay for zearalenone and zearalanol in human serum: Production, properties, and use of porcine antibodies. Appl. Environ. Microbiol. 1983, 45, 16–23. [Google Scholar] [PubMed]
- Yellepeddi, V.K.; Kumar, A.; Palakurthi, S. Biotinylated poly (amido) amine (pamam) dendrimers as carriers for drug delivery to ovarian cancer cells in vitro. Anticancer Res. 2009, 29, 2933–2943. [Google Scholar] [PubMed]
- Landar, A.; Oh, J.-Y.; Giles, N.M.; Isom, A.; Kirk, M.; Barnes, S.; Darley-Usmar, V.M. A sensitive method for the quantitative measurement of protein thiol modification in response to oxidative stress. Free Radic. Biol. Med. 2006, 40, 459–468. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, X.; Sun, M.; Zhang, X.; Song, H.; Yan, Y.; Sun, J.; Li, X.; Fang, W. A Magnetic Nanoparticle Based Enzyme-Linked Immunosorbent Assay for Sensitive Quantification of Zearalenone in Cereal and Feed Samples. Toxins 2015, 7, 4216-4231. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins7104216
Zhang X, Wang X, Sun M, Zhang X, Song H, Yan Y, Sun J, Li X, Fang W. A Magnetic Nanoparticle Based Enzyme-Linked Immunosorbent Assay for Sensitive Quantification of Zearalenone in Cereal and Feed Samples. Toxins. 2015; 7(10):4216-4231. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins7104216
Chicago/Turabian StyleZhang, Xian, Xin Wang, Mengjiao Sun, Xiaofeng Zhang, Houhui Song, Yaxian Yan, Jianhe Sun, Xiaoliang Li, and Weihuan Fang. 2015. "A Magnetic Nanoparticle Based Enzyme-Linked Immunosorbent Assay for Sensitive Quantification of Zearalenone in Cereal and Feed Samples" Toxins 7, no. 10: 4216-4231. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins7104216