A Rapid and Sensitive Method to Measure the Functional Activity of Shiga Toxins in Human Serum
Abstract
:1. Introduction
2. Results and Discussion
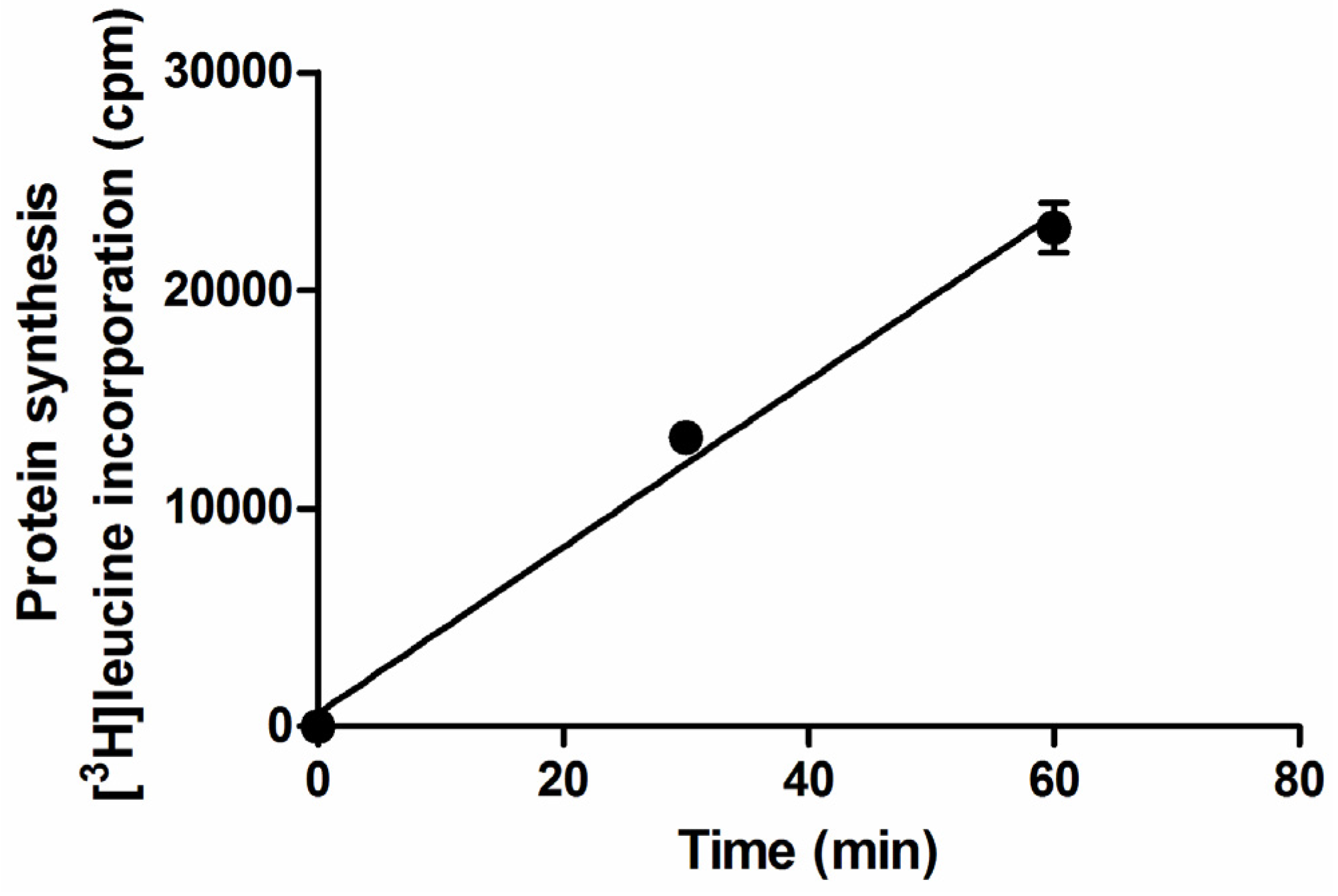
2.1. Setup of Protein Synthesis Assays with Raji Cells


2.2. Detection of Shiga Toxins in Human Serum by the Raji Cells Protein Synthesis Assay
| Treatment | IC50 (pM) | Fold-Increase | Pearson Coefficient (r) |
|---|---|---|---|
| Stx2a | 2.2 | - | −0.99 |
| Stx2a + human serum (donor 1) | 39.8 | 18.1 | −0.89 |
| Stx2a + human serum (donor 2) | 44.2 | 20.1 | −0.99 |
| Stx2a + human serum (donor 3) | 74.9 | 34.0 | −0.99 |

2.3. Detection of Shiga Toxins in Human Serum from Patients Infected by Shiga Toxin-Producing E. coli Using the Raji Cells Protein Synthesis Assay
| Patients | Age (years) | Gender | Bloody Diarrhea (days) | Detection of Shiga Toxins in Feces | |
|---|---|---|---|---|---|
| Enrollment Assay a | RT-PCR b | ||||
| 1 | 0.8 | F | 4 | Stx1+ | n.d. c |
| 2 | 2.3 | F | 5 | Stx1+ Stx2+ | Stx1+ Stx2+ eae+ |
| 3 | 14.2 | F | 4 | Stx2+ | Stx2+ eae+ |

3. Experimental Section
3.1. Toxins
3.2. Cells
3.3. Human Blood Samples
3.4. Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tarr, P.I.; Gordon, C.A.; Chandler, W.L. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 2005, 365, 1073–1086. [Google Scholar] [CrossRef]
- Trompeter, R.S.; Schwartz, R.; Chantler, C.; Dillon, M.J.; Haycock, G.B.; Kay, R.; Barratt, T.M. Haemolytic-uraemic syndrome: An analysis of prognostic features. Arch. Dis. Child. 1983, 58, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Griffin, P.M.; Tauxe, R.V. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 1991, 13, 60–98. [Google Scholar] [PubMed]
- Paton, J.C.; Paton, A.W. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 1998, 11, 450–479. [Google Scholar] [PubMed]
- Friedrich, A.W.; Bielaszewska, M.; Zhang, W.L.; Pulz, M.; Kuczius, T.; Ammon, A.; Karch, H. Escherichia coli harboring Shiga toxin 2 gene variants: Frequency and association with clinical symptoms. J. Infect. Dis. 2002, 185, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Tsurugi, K.; Yutsudo, T.; Takeda, Y.; Ogasawara, T.; Igarashi, K. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur. J. Biochem. 1988, 171, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Brigotti, M.; Carnicelli, D.; Ravanelli, E.; Vara, A.G.; Martinelli, C.; Alfieri, R.R.; Petronini, P.G.; Sestili, P. Molecular damage and induction of proinflammatory cytokines in human endothelial cells exposed to Shiga toxin 1, Shiga toxin 2, and alpha-sarcin. Infect. Immun. 2007, 75, 2201–2207. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.E.; Liu, X.H. Pathogenesis of Shiga toxin-induced hemolytic uremic syndrome. Pediatr. Nephrol. 2001, 16, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Zoja, C.; Buelli, S.; Morigi, M. Shiga toxin-associated hemolytic uremic syndrome: Pathophysiology of endothelial dysfunction. Pediatr. Nephrol. 2010, 25, 2231–2240. [Google Scholar] [CrossRef] [PubMed]
- Bauwens, A.; Betz, J.; Meisen, I.; Kemper, B.; Karch, H.; Muthing, J. Facing glycosphingolipid-Shiga toxin interaction: Dire straits for endothelial cells of the human vasculature. Cell Mol. Life Sci. 2012, 70, 425–457. [Google Scholar] [CrossRef] [PubMed]
- Cooling, L.L.; Walker, K.E.; Gille, T.; Koerner, T.A. Shiga toxin binds human platelets via globotriaosylceramide (pk antigen) and a novel platelet glycosphingolipid. Infect. Immun. 1998, 66, 4355–4366. [Google Scholar] [PubMed]
- Karpman, D.; Papadopoulou, D.; Nilsson, K.; Sjogren, A.C.; Mikaelsson, C.; Lethagen, S. Platelet activation by Shiga toxin and circulatory factors as a pathogenetic mechanism in the hemolytic uremic syndrome. Blood 2001, 97, 3100–3108. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.A.; Polanowska-Grabowska, R.K.; Fujii, J.; Obrig, T.; Gear, A.R. Shiga toxin binds to activated platelets. J. Thromb. Haemost. 2004, 2, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Geelen, J.M.; van der Velden, T.J.; van den Heuvel, L.P.; Monnens, L.A. Interactions of Shiga-like toxin with human peripheral blood monocytes. Pediatr. Nephrol. 2007, 22, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Van Setten, P.A.; van Hinsbergh, V.W.; van den Heuvel, L.P.; Preyers, F.; Dijkman, H.B.; Assmann, K.J.; van der Velden, T.J.; Monnens, L.A. Monocyte chemoattractant protein-1 and interleukin-8 levels in urine and serum of patients with hemolytic uremic syndrome. Pediatr. Res. 1998, 43, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Te Loo, D.M.; Monnens, L.A.; van der Velden, T.J.; Vermeer, M.A.; Preyers, F.; Demacker, P.N.; van Den Heuvel, L.P.; van Hinsbergh, V.W. Binding and transfer of Verocytotoxin by polymorphonuclear leukocytes in hemolytic uremic syndrome. Blood 2000, 95, 3396–3402. [Google Scholar] [PubMed]
- Brigotti, M.; Carnicelli, D.; Ravanelli, E.; Barbieri, S.; Ricci, F.; Bontadini, A.; Tozzi, A.E.; Scavia, G.; Caprioli, A.; Tazzari, P.L. Interactions between Shiga toxins and human polymorphonuclear leukocytes. J. Leukoc. Biol. 2008, 84, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Brigotti, M.; Carnicelli, D.; Arfilli, V.; Tamassia, N.; Borsetti, F.; Fabbri, E.; Tazzari, P.L.; Ricci, F.; Pagliaro, P.; Spisni, E.; et al. Identification of TLR4 as the receptor that recognizes Shiga toxins in human neutrophils. J. Immunol. 2013, 191, 4748–4758. [Google Scholar] [CrossRef] [PubMed]
- Bitzan, M.; Richardson, S.; Huang, C.; Boyd, B.; Petric, M.; Karmali, M.A. Evidence that Verotoxins (Shiga-like toxins) from Escherichia coli bind to P blood group antigens of human erythrocytes in vitro. Infect. Immun. 1994, 62, 3337–3347. [Google Scholar] [PubMed]
- Stahl, A.L.; Arvidsson, I.; Johansson, K.E.; Chromek, M.; Rebetz, J.; Loos, S.; Kristoffersson, A.C.; Bekassy, Z.D.; Morgelin, M.; Karpman, D. A novel mechanism of bacterial toxin transfer within host blood cell-derived microvesicles. PLoS Pathog. 2015, 11, e1004619. [Google Scholar] [CrossRef] [PubMed]
- Schweppe, C.H.; Hoffmann, P.; Nofer, J.R.; Pohlentz, G.; Mormann, M.; Karch, H.; Friedrich, A.W.; Muthing, J. Neutral glycosphingolipids in human blood: A precise mass spectrometry analysis with special reference to lipoprotein-associated Shiga toxin receptors. J. Lipid Res. 2010, 51, 2282–2294. [Google Scholar] [CrossRef] [PubMed]
- Lopez, E.L.; Contrini, M.M.; Glatstein, E.; Gonzalez Ayala, S.; Santoro, R.; Ezcurra, G.; Teplitz, E.; Matsumoto, Y.; Sato, H.; Sakai, K.; et al. An epidemiologic surveillance of Shiga-like toxin-producing Escherichia coli infection in Argentinean children: Risk factors and serum Shiga-like toxin 2 values. Pediatr. Infect. Dis. J. 2012, 31, 20–24. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Quinones, B.; te Loo, D.M.; Loos, S.; Scavia, G.; Brigotti, M.; Levtchenko, E.; Monnens, L.A. Serum Shiga toxin 2 values in patients during the acute phase of diarrhoea-associated haemolytic uremic syndrome. Acta Paediatr. 2015. [Google Scholar] [CrossRef] [PubMed]
- He, X.; McMahon, S.; Skinner, C.; Merrill, P.; Scotcher, M.C.; Stanker, L.H. Development and characterization of monoclonal antibodies against Shiga toxin 2 and their application for toxin detection in milk. J. Immunol. Methods 2013, 389, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Tani, S.; Matsumoto Yi, Y.; Takeda, T. Serum amyloid P component is the Shiga toxin 2-neutralizing factor in human blood. J. Biol. Chem. 2001, 276, 41576–41579. [Google Scholar] [CrossRef] [PubMed]
- Marcato, P.; Vander Helm, K.; Mulvey, G.L.; Armstrong, G.D. Serum amyloid P component binding to Shiga toxin 2 requires both A subunit and B pentamer. Infect. Immun. 2003, 71, 6075–6078. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.D.; Mulvey, G.L.; Marcato, P.; Griener, T.P.; Kahan, M.C.; Tennent, G.A.; Sabin, C.A.; Chart, H.; Pepys, M.B. Human serum amyloid P component protects against Escherichia coli O157:H7 Shiga toxin 2 in vivo: Therapeutic implications for hemolytic-uremic syndrome. J. Infect. Dis. 2006, 193, 1120–1124. [Google Scholar] [CrossRef] [PubMed]
- Karmali, M.A.; Petric, M.; Lim, C.; Fleming, P.C.; Arbus, G.S.; Lior, H. The association between idiopathic hemolytic uremic syndrome and infection by Verotoxin-producing Escherichia coli. J. Infect. Dis. 1985, 151, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, A.; Luzzi, I.; Rosmini, F.; Pasquini, P.; Cirrincione, R.; Gianviti, A.; Matteucci, M.C.; Rizzoni, G. Hemolytic-uremic syndrome and Vero cytotoxin-producing Escherichia coli infection in Italy. The HUS Italian study group. J. Infect. Dis. 1992, 166, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Brigotti, M.; Tazzari, P.L.; Ravanelli, E.; Carnicelli, D.; Rocchi, L.; Arfilli, V.; Scavia, G.; Minelli, F.; Ricci, F.; Pagliaro, P.; et al. Clinical relevance of Shiga toxin concentrations in the blood of patients with hemolytic uremic syndrome. Pediatr. Infect. Dis. J. 2011, 30, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Brigotti, M.; Arfilli, V.; Carnicelli, D.; Rocchi, L.; Calcabrini, C.; Ricci, F.; Pagliaro, P.; Tazzari, P.L.; Alfieri, R.R.; Petronini, P.G.; et al. Shiga toxin 1, as DNA repair inhibitor, synergistically potentiates the activity of the anticancer drug, mafosfamide, on Raji cells. Toxins 2013, 5, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Pulvertaft, J.V. Cytology of Burkitt’s tumour (african lymphoma). Lancet 1964, 1, 238–240. [Google Scholar] [CrossRef]
- Karpova, M.B.; Schoumans, J.; Ernberg, I.; Henter, J.I.; Nordenskjold, M.; Fadeel, B. Raji revisited: Cytogenetics of the original Burkitt’s lymphoma cell line. Leukemia 2005, 19, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Petronini, P.G.; Tramacere, M.; Mazzini, A.; Piedimonte, G.; Silvotti, L.; Borghetti, A.F. Hyperosmolarity-induced stress proteins in chick embryo fibroblasts. Exp. Cell Res. 1987, 172, 450–462. [Google Scholar] [CrossRef]
- Brigotti, M.; Alfieri, R.; Sestili, P.; Bonelli, M.; Petronini, P.G.; Guidarelli, A.; Barbieri, L.; Stirpe, F.; Sperti, S. Damage to nuclear DNA induced by Shiga toxin 1 and ricin in human endothelial cells. FASEB J. 2002, 16, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Brigotti, M.; Tazzari, P.L.; Ravanelli, E.; Carnicelli, D.; Barbieri, S.; Rocchi, L.; Arfilli, V.; Scavia, G.; Ricci, F.; Bontadini, A.; et al. Endothelial damage induced by Shiga toxins delivered by neutrophils during transmigration. J. Leukoc. Biol. 2010, 88, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Brigotti, M.; Carnicelli, D.; Arfilli, V.; Rocchi, L.; Ricci, F.; Pagliaro, P.; Tazzari, P.L.; Vara, A.G.; Amelia, M.; Manoli, F.; et al. Change in conformation with reduction of alpha-helix content causes loss of neutrophil binding activity in fully cytotoxic Shiga toxin 1. J. Biol. Chem. 2011, 286, 34514–34521. [Google Scholar] [CrossRef] [PubMed]
- Arfilli, V.; Carnicelli, D.; Rocchi, L.; Ricci, F.; Pagliaro, P.; Tazzari, P.L.; Brigotti, M. Shiga toxin 1 and ricin A chain bind to human polymorphonuclear leucocytes through a common receptor. Biochem. J. 2010, 432, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hoffmann, P.; Hulsewig, M.; Duvar, S.; Ziehr, H.; Mormann, M.; Peter-Katalinic, J.; Friedrich, A.W.; Karch, H.; Muthing, J. On the structural diversity of Shiga toxin glycosphingolipid receptors in lymphoid and myeloid cells determined by nanoelectrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 2295–2304. [Google Scholar] [CrossRef] [PubMed]
- Quinones, B.; Massey, S.; Friedman, M.; Swimley, M.S.; Teter, K. Novel cell-based method to detect Shiga toxin 2 from Escherichia coli O157:H7 and inhibitors of toxin activity. Appl. Environ. Microbiol. 2009, 75, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.; Cohen, A.S. Amyloid P component. Methods Enzymol. 1988, 163, 523–536. [Google Scholar] [PubMed]
- Ryd, M.; Alfredsson, H.; Blomberg, L.; Andersson, A.; Lindberg, A.A. Purification of Shiga toxin by alpha-d-galactose-(1→4)-beta-d-galactose-(1→4)-beta-d-glucose-(1→) receptor ligand-based chromatography. FEBS Lett. 1989, 258, 320–322. [Google Scholar] [CrossRef]
- Matussek, A.; Lauber, J.; Bergau, A.; Hansen, W.; Rohde, M.; Dittmar, K.E.; Gunzer, M.; Mengel, M.; Gatzlaff, P.; Hartmann, M.; et al. Molecular and functional analysis of Shiga toxin-induced response patterns in human vascular endothelial cells. Blood 2003, 102, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, A.; Scavia, G.; Morabito, S. Public health microbiology of Shiga toxin-producing Escherichia coli. Microbiol. Spectr. 2014, 2, 6. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arfilli, V.; Carnicelli, D.; Ardissino, G.; Torresani, E.; Scavia, G.; Brigotti, M. A Rapid and Sensitive Method to Measure the Functional Activity of Shiga Toxins in Human Serum. Toxins 2015, 7, 4564-4576. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins7114564
Arfilli V, Carnicelli D, Ardissino G, Torresani E, Scavia G, Brigotti M. A Rapid and Sensitive Method to Measure the Functional Activity of Shiga Toxins in Human Serum. Toxins. 2015; 7(11):4564-4576. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins7114564
Chicago/Turabian StyleArfilli, Valentina, Domenica Carnicelli, Gianluigi Ardissino, Erminio Torresani, Gaia Scavia, and Maurizio Brigotti. 2015. "A Rapid and Sensitive Method to Measure the Functional Activity of Shiga Toxins in Human Serum" Toxins 7, no. 11: 4564-4576. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins7114564






