1. Introduction
Due to its dependency on moderate climate conditions and its resistance to processing the
Fusarium toxin, deoxynivalenol (DON) can be often found in toxicologically-relevant concentrations in cereals in temperate climate zones [
1]. It is of special importance in pig production due to the high susceptibility of pigs, causing reduced feed intake and live weight gain, resulting in considerable economic losses [
2,
3,
4].
Several studies indicate that DON influences the systemic inflammatory response. The toxin exerts immune modulatory effects on blood leukocytes depending on the dose and frequency of exposure. Different studies have shown that a low dose exposure to
Fusarium toxins has an immune-stimulating effect due to an upregulation of transcriptional and post-transcriptional expression of cytokines, chemokines and inflammatory genes, whereas a high dose exposure has an immune-suppressive effect (reviewed in [
5]). It has further been shown that exposure to DON causes an altered immune response [
6,
7,
8] and liver cell metabolism [
9,
10] to a subsequent lipopolysaccharide (LPS) challenge
in vitro and
in vivo.
Lipopolysaccharides form the major component of the outer cell membrane of Gram-negative bacteria and are responsible for the onset of an inflammatory response in the case of systemic LPS entry [
11]. Triggering similar (immune biological) pathways, a variety of infectious pathogens, such as Gram-positive and Gram-negative bacteria, viruses and fungi, leads to identical clinical sequelae commonly described with the term sepsis [
12,
13]. Since infections with Gram-negative bacteria contribute to a substantial part of the sepsis cases worldwide, LPS-induced systemic inflammation is a well-established sepsis model in animals and humans [
11,
14,
15,
16].
On a systemic level, the recognition of LPS by the immune system causes the release of pro-inflammatory cytokines. This leads to inflammation, apoptosis, causing endothelial dysfunction, and microcirculation thrombosis, resulting in perfusion heterogeneity and microcirculatory failure [
12]. Clinically, these alterations manifest themselves in a variety of symptoms, such as hypothermia or hyperthermia, tachycardia, tachypnea, edema, central nervous dysfunction, leukocytosis and leukopenia [
11,
16,
17]. In concurrence with a pronounced inflammatory response, blood analysis reveals often lactic acidosis [
18,
19,
20], along with either dysglycemia depending on the stage of disease [
17,
18,
21].
As DON can be ubiquitous in cereals and pigs might be sub-acutely exposed on the one hand, while LPS is always present in the environment and commensal intestinal microbiota, on the other hand, pigs might be often co-exposed to both toxins at the same time. The liver possesses a central role in LPS detoxification [
22,
23] or in metabolic and immunological homeostasis [
24] of, and it has been shown before that chronic exposure to DON leads to altered liver cell metabolism [
9,
10]. We hypothesized that liver metabolism and hematological variables are altered in chronically-DON-fed animals during a subsequent LPS stimulus. An increase in systemic LPS can either be a consequence of a systemic infection [
17] or an increased passage from portal-drained viscera [
25]. In order to simulate these pathways of systemic LPS entrance and the consequences of a possible hepatic first-pass effect, we infused LPS pre- or post-hepatically. Arterial, jugular and portal blood metabolic variables were assessed to evaluate the role of the liver in this pathogenesis as a consequence of LPS infusion.
3. Discussion
In this study, LPS was used to induce an inflammatory response. All LPS-treated pigs exhibited typical clinical symptoms of an acute phase response [
16,
29,
30], such as an increased respiratory rate, fever, tremor, cyanosis, followed by hyperemic conjunctivae, injected episcleral vessels or leukopenia (Tesch
et al., 2015, submitted, [
31]). In all groups at time −30 min and within the control group over the course of the trial, no significant alterations of metabolic and hematological variables were observed, and all parameters were within their physiological range. It therefore can be stated that the performed manipulations, such as the surgery and the sampling procedure, did not constitute confounding factors.
All LPS-treated animals exhibited a lactic acidosis as a consequence of LPS infusion [
13]. Taking into consideration the observed
pO
2,
pCO
2 and lactate concentrations, we deduced that at first, acidosis was caused by a decreased
pO
2 (respiratory acidosis) and later on originated from an increase in systemic lactate concentration (metabolic acidosis) [
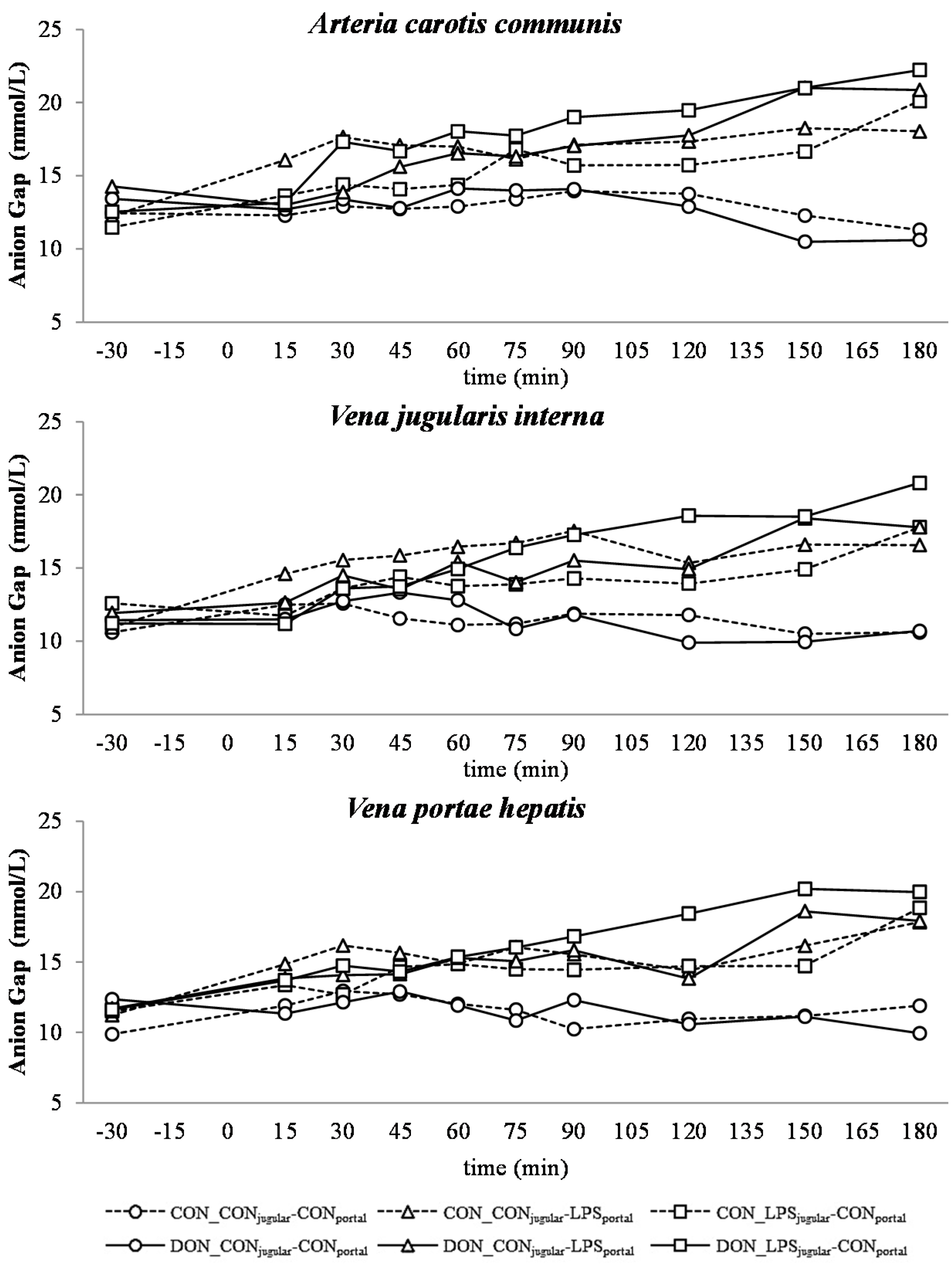
32]. This is also mirrored in the variables BE and AG, which are a reflection of the different variables that affect the acid-base balance [
33]. The alterations observed in AG can solely be ascribed to changes in HCO
3− concentrations, since other electrolytes (Na
+, K
+, Cl
−, Ca
2+) were not influenced by the LPS challenge.
Since erythrocytes occupy a central role in oxygen transport, metabolism, as well as the acid-base balance, we also assessed red hemogram variables. No biologically-relevant influence of DON or LPS was observed on red hemogram variables at any time during the trial. This is in line with a study of Grenier
et al. [
34] in which the effects of the mycotoxins DON and fumonisin, alone or in combination with a subcutaneous ovalbumin injection on different hematology variables, were investigated. These results are further confirmed by two recent studies [
35,
36] investigating the influence of low-dose (≤2 mg/kg feed) chronic oral DON exposure on red hemogram variables and electrolytes in piglets and pre-puberal gilts, respectively. However, in both studies, neither biologically-relevant alterations in red hemogram nor blood electrolytes were observed after four weeks of chronic oral DON exposure. Few studies have investigated the influence of an acute inflammatory response on sodium-potassium transport in red blood cells and skeletal muscle. Suri and colleagues [
37] and Illner
et al. [
38] observed hyponatremia and hyperpotassemia and attributed this to alteration in the transport capacity of the RBC Na
+/K
+ pump. This change in ion transport across RBC and skeletal muscles has also been observed in other studies [
39,
40], but this hypothesis was challenged later on [
41,
42]. In our study, we did not detect any changes in blood electrolytes and, based on these previous articles, might speculate that the sodium-potassium transport in red blood cells and skeletal muscle after an LPS-challenge was not changed.
A decrease in systemic
pO
2 during the initial state of an acute inflammatory response has been documented before and is probably caused by a decreased cardiac output, as well as alterations in the respiratory rate and depth [
43]. This assumption is supported by the concurrent observed increase in respiratory rate in LPS-infused animals during this trial (Tesch
et al., 2015, submitted, [
31]). After an initial decrease of arterial
pO
2, a continuous increase during the rest of the trial, accompanied by a decrease in jugular
pO
2 and no alterations in jugular pCO
2, was observed. This observation is indicative of an increase in tissue oxygen consumption, rather than a decrease in overall oxygen availability. These findings are in line with previous studies showing that the hyperlactatemia observed during sepsis is most likely caused by alterations in the glycolytic pathway, rather than hypoxia, along with increased tissue oxygen consumption [
44]. In human studies, Revelly and co-workers [
45] described an increased glucose and lactate rate of appearance in the blood of septic patients compared to healthy subjects. Furthermore, the hyperlactatemia resulted from an increased endogenous lactate production in sepsis, whereas lactate clearance was not altered compared to healthy patients, confirming the impact of altered glycolytic pathways in the development of lactic acidosis. It is further assumed that during a state of acute inflammatory response, the rate of pyruvate formation exceeds the oxidative capacity of mitochondria, causing an accumulation of pyruvate, and thereby, an increase in lactate formation [
46]. This is further potentiated by a decrease in lactate utilization [
47]. Besides an alteration in the glycolytic pathway, tissue perfusion heterogeneity is being put forward as a possible reason for hyperlactatemia accompanied by physiological systemic
pO
2 during sepsis by Gutierrez and colleagues [
18]. Under shock conditions, the blood circulation is centralized to vital organs, and non-vital tissues are characterized by a compromised peripheral vascular perfusion [
48]. In our study, we clinically observed cyanosis of the extremities and dermographism in five out of 28 LPS-treated pigs (Tesch
et al., 2015, submitted, [
31]), as well as liver hemorrhage in LPS-treated animals (Renner
et al., 2015, [
49]). In contrast to our observations, other studies with a similar setup reported dermographism in most of the LPS-treated animals, as well as macroscopic and microscopic intrahepatic hyperperfusion [
8]. These symptoms can be attributed, amongst others, to tissue perfusion heterogeneity and, thus, would fit the hypothesis voiced earlier [
18]. Further, an increased lactic acid output of leukocytes due to an increased glycolysis has been suggested as a contributing factor to the lactic acidosis observed in septic animals [
50]. In our study, leukocytes in particular were severely affected by LPS and an enhanced glycolysis, and thus, the output of lactic acid into the blood might have contributed to the present lactic acidosis (Tesch
et al., 2015, submitted, [
31]).
There was no difference between pre- or post-hepatic LPS infusion in CON-fed animals concerning the acid-base balance. An influence of DON was only observed in post-hepatic LPS-infused animals, whereas no dietary impact in pre-hepatic LPS-infused animals was observed regarding different acid-base balance variables in our trial. These results suggest a DON-related priming of post-hepatic cells involved in the exacerbation of metabolic disorders caused by LPS stimulation. Moreover, the lack of an assumed partial hepatic LPS clearance in post-hepatic LPS-infused DON-fed pigs compared to their pre-hepatic LPS-infused DON-fed counterparts might have triggered latent interactions between LPS and DON. Previously, it has been shown that an exposure to DON causes an altered immune response due to an upregulation of the transcriptional and post-transcriptional expression of cytokines, chemokines and inflammatory genes in porcine
in vitro and
in vivo studies [
6,
7,
8]. In rodent studies, LPS priming of animals prior to DON exposure resulted in a stronger cytokine response compared to vehicle-treated animals [
51], as well as simultaneous LPS and DON treatment of RAW264.7 macrophages [
52]. However, we observed a uniform increase of TNF-α after 30 min with peak values at 60 min in all LPS-infused pigs, irrespective of the site of infusion or dietary treatment. Therefore, in our study, we could not confirm a superinduction of TNF-α in LPS-treated animals fed with a DON-contaminated diet (Tesch
et al., 2015, submitted, [
31]).
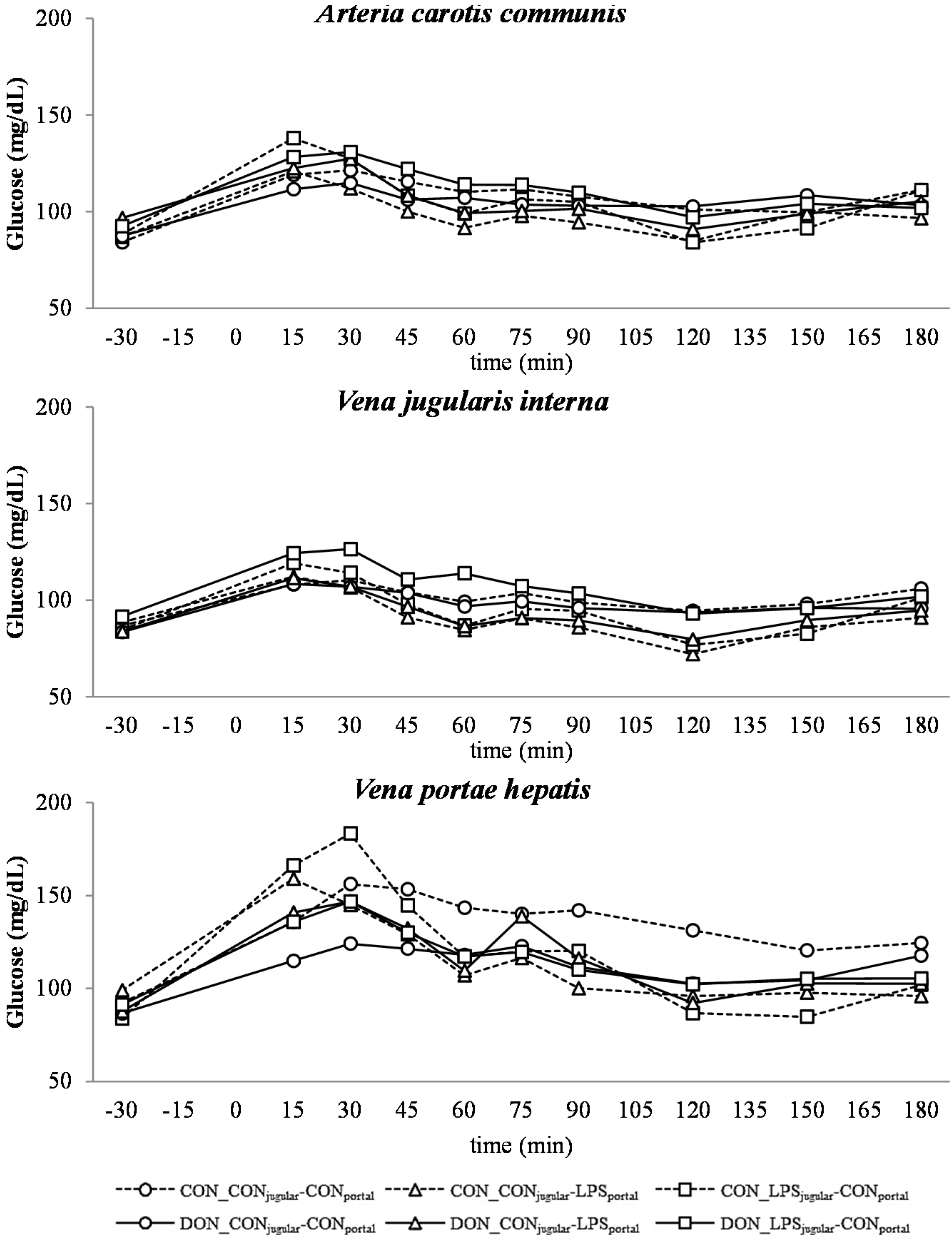
Similar to other studies that have investigated the influence of a systemic inflammatory response on blood glucose levels, we observed an initial hyperglycemia followed by eu- and hypoglycemia [
21,
53]. However, this kinetic was not significantly distinguishable from the post-prandial increase in blood glucose, which was observed in all groups as LPS infusion superimposed with the post-prandial effects.
The DON fed animals exhibited a markedly lower portal glucose level, which can most likely be attributed to the negative effects of DON on glucose transport across the intestinal barrier, as described previously by Halawa
et al. [
54]. This effect was also observed in several other studies using different animal and
in vitro models. In chickens, DON inhibited the jejunal SGLT-1 activity (sodium-linked glucose transporter 1), responsible for active glucose uptake into enterocytes from lumen [
55]. Furthermore, in human cell line HT-29, SGLT-1, GLUT-5 (glucose transporter 5,
d-fructose associated) and GLUT-1 (passive
d-glucose transporter) were inhibited by DON in a dose-dependent manner [
56]. However, other studies in swine did not confirm this effect on SGLT-1 activity in brush border vesicles derived from the jejunum [
57]. In addition to this DON effect, an additive effect of LPS infusion was detected. All LPS-infused groups had lower portal glucose concentrations 45 min after infusion start compared to the total control group. Generally under shock conditions, hypoperfusion of the intestinal tract can be observed [
58,
59], and therefore, it can be hypothesized that the transport capacity of the intestinal mucosa is impaired. Furthermore, in several LPS studies, an inhibitory effect on intestinal glucose transport was observed, for instance decreased GLUT-5 [
60] and SGLT-1 [
61] levels in LPS-treated rabbits. Furthermore, Amador and co-authors [
62] observed an inhibitory effect of TNF-α on SGLT-1
ex vivo in rabbit’s intestine. We could thus hypothesize that in our study, the impaired portal glucose uptake in LPS-treated animals (45 min–180 min) might be, at least partially, attributed to an impairment in SGLT-1 transport capacity due to the increase in TNF-α (Tesch
et al., 2015, submitted, [
31]).
Our data suggest that chronic oral exposure to DON exacerbates lactic acidosis by a post-hepatic LPS-induced systemic inflammation, while a pre-hepatic LPS stimulation did not result in such amplification. This different responsiveness between pre- and post-hepatic-infused animals was not observed within the control-fed groups.
4. Experimental Section
Animal experiments were conducted according to the EC regulations concerning the protection of experimental animals and the guidelines of the German Animal Welfare Act approved by the Lower Saxony State Office for Consumer Protection and Food Safety (Lower Saxony State Office for Consumer Protection and Food Safety; File Number 33.4-42502-04-13/1274).
4.1. Experimental Design and Procedures
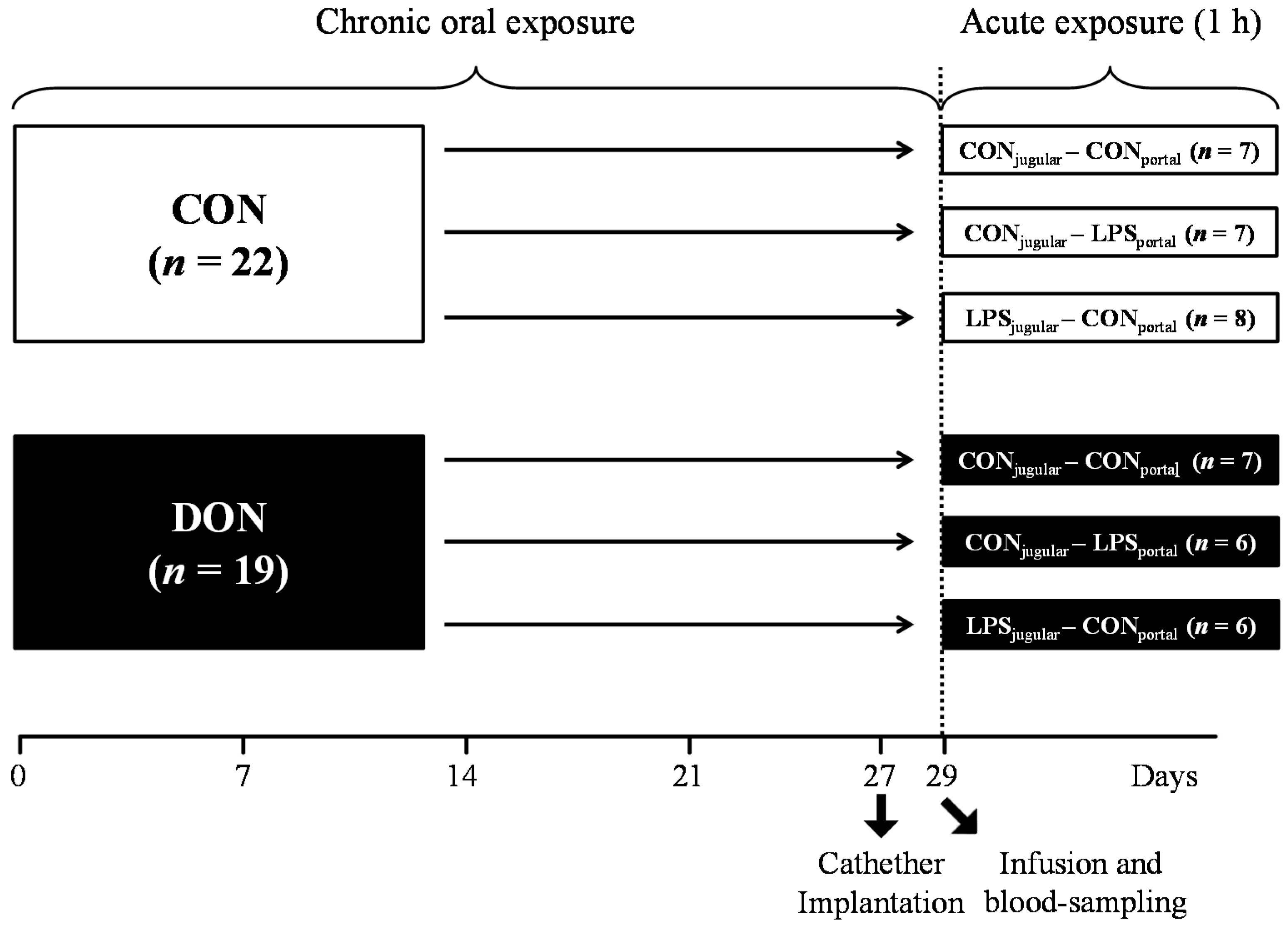
A total of 41 barrows (German landrace, Mariensee, Germany) were randomly assigned to either a group receiving natural DON-contaminated feed (DON; 4.59 mg DON/kg feed;
n = 19) or a control group (CON;
n = 22) control diet (
Table 3). Experimental groups, their treatment and the number of animals are illustrated in
Figure 9. The pigs had an average initial weight of 25.8 ± 3.7 kg (means ± SD) and were fed restrictively with 2 single portions of 700 g per day, mixed with water and provided as mash. All barrows were housed in individual floor pens during the first 21 days of the trial and subsequently transferred into individual metabolism crates (described in [
63]) until Day 29.
Table 3.
Diet composition, based on air dry matter (ADM) = 88.37%.
Table 3.
Diet composition, based on air dry matter (ADM) = 88.37%.
| Ingredients | CON % | DON % |
|---|
| barley | 53.30 | 53.30 |
| maize (non contaminated) | 15.00 | 7.50 |
| maize (contaminated) | - | 7.50 |
| soybean meal | 20.00 | 20.00 |
| rapeseed | 5.00 | 5.00 |
| soybean oil | 2.00 | 2.00 |
| Premix 1 | 3.00 | 3.00 |
| Lysine-HCl | 0.40 | 0.40 |
| l-Threonine | 0.12 | 0.12 |
| dl-Methionine | 0.15 | 0.15 |
| HCl-insoluble ash 2 | 1.00 | 1.00 |
| analysed composition | g/kg ADM | g/kg ADM |
| crude protein | 196.85 | 194.83 |
| crude fat | 47.48 | 46.51 |
| crude ash | 69.70 | 69.51 |
| crude fiber | 51.26 | 49.32 |
| deoxynivalenol mg/kg | 0.12 | 4.59 |
In order to facilitate pre- or post-hepatic blood-sampling and infusion, pigs were surgically fitted with 5 differently-located, permanent indwelling catheters under general inhalation anesthesia (Isoflurane®, CP-Pharma, Burgdorf, Germany) at Day 27 of the trial when the animals had an average body weight of 40.5 ± 3.0 kg. Permanent Silastic® catheters were manufactured from Dow Corning (Midland, TX, USA) medical-grade tubing material (1.57 mm ID and 3.18 mm OD), autoclaved and placed in the Vena jugularis interna, Vena jugularis externa, Vena lienalis, Vena portae hepatis and Arteria carotis communis. Catheters were tunneled to the neck and left flank, respectively, and fixed with catheter mounts/clamps (Arrow, Teleflex Medical GmbH, Kernen, Germany) on the skin. Catheters were fitted with three-way valves (Walther-CMP, Kiel, Germany) for sampling and flushed with heparinized physiological saline (1 mL sodium heparin (25.000 IE/5 mL) (Ratiopharm, Ulm, Germany); dissolved in 500 mL sterile 0.9% NaCl (B. Braun Melsungen AG, Melsungen, Germany) every 4 h and after each sampling to prevent blood coagulation. Two days between surgery and sampling day were allowed for recovery. Throughout this recovery period, half of the daily ration was fed (2 times 350 g/day).
At Day 29 of the trial, animals were further divided into 3 infusion groups: LPS pre-hepatic (LPS
portal-CON
jugular), LPS post-hepatic (CON
portal-LPS
jugular) or control (CON
portal-CON
jugular), illustrated in
Figure 9.
Figure 9.
Experimental design.
Figure 9.
Experimental design.
On the sampling day, the time of infusion start was set as zero, and blood samples were taken at −30, 15, 30, 45, 60, 75, 90, 120, 150 and 180 min from Vena jugularis interna, Vena portae hepatis and Arteria carotis communis. Fifteen minutes prior to infusion, pigs received 700 g of feed each. LPS was infused at 7.5 µg LPS/kg BW for 1 h (Escherichia coli-LPS, O111:B4, Sigma-Aldrich, Taufkirchen, Germany), and 0.9% NaCl was used as the control substance. Infusion was implemented using an infusion pump (IPC-N-4, ISMATEC Laboratoriumstechnik GmbH, Wertheim, Germany) and infusion tubes with a 2.06 mm inner diameter (PharMed® Ismaprene, Wertheim, Germany, ISMATEC), administered into either Vena jugularis externa or Vena lienalis. The pigs were slaughtered 195 min after infusion start.
4.2. Sample Analysis
For a red hemogram assessment, 1-mL blood samples were collected in EDTA tubes and analyzed immediately with an automated hematology analyzer (Celltac alpha MEK-6450, Nihon Kohden Corporation, Tokyo, Japan). Furthermore, blood samples (0.5 mL) for blood gases, electrolytes, pH, glucose and lactate were collected into blood sample syringes (SC-Sanguis Counting GmbH, Nümbrecht, Germany), and variables were assessed immediately using an automated blood gas and electrolyte analyzer (GEM Premier 4000, Werfen, Kirchheim, Germany). Anion gap and base excess were calculated using the equations detailed below.
4.3. Calculations
Equation used to calculate anion gap (AG) [
64]:
Na
+ = sodium; K
+ = potassium; Cl
− = chloride; HCO
3− = bicarbonate.
Equation used to calculate base excess (BE) [
65]:
cHb = total hemoglobin concentration;
pCO
2 = carbon dioxide partial pressure;
sO
2 = oxygen saturation.
4.4. Statistical Analysis
Data were evaluated by using PROC MIXED in SAS Enterprise Guide 6.1 (SAS Institute 2013, Cary, NC, USA) using a restricted maximum likelihood model (REML). Group, catheter, time and their interaction were defined as fixed factors. A “REPEATED” statement was included to account for the individual similarity at repeated measurements. The “compound symmetry” was found to be the most appropriate co-variance structure according to the corrected Akaike’s information criterion (AICC), and significant effects at different time points were further evaluated by multiple t-tests (“pairwise differences” (PDIFF)). Results are presented as least square means (LSMeans) and pooled standard error of means (PSEM).