Ancient Venom Systems: A Review on Cnidaria Toxins
Abstract
:1. Introduction
2. Cnidarian Phylogeny
3. Cnidarian Life Cycle
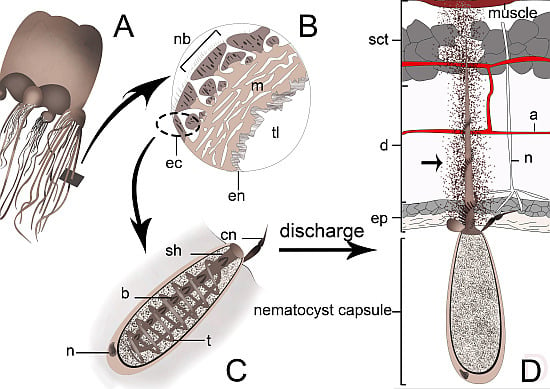
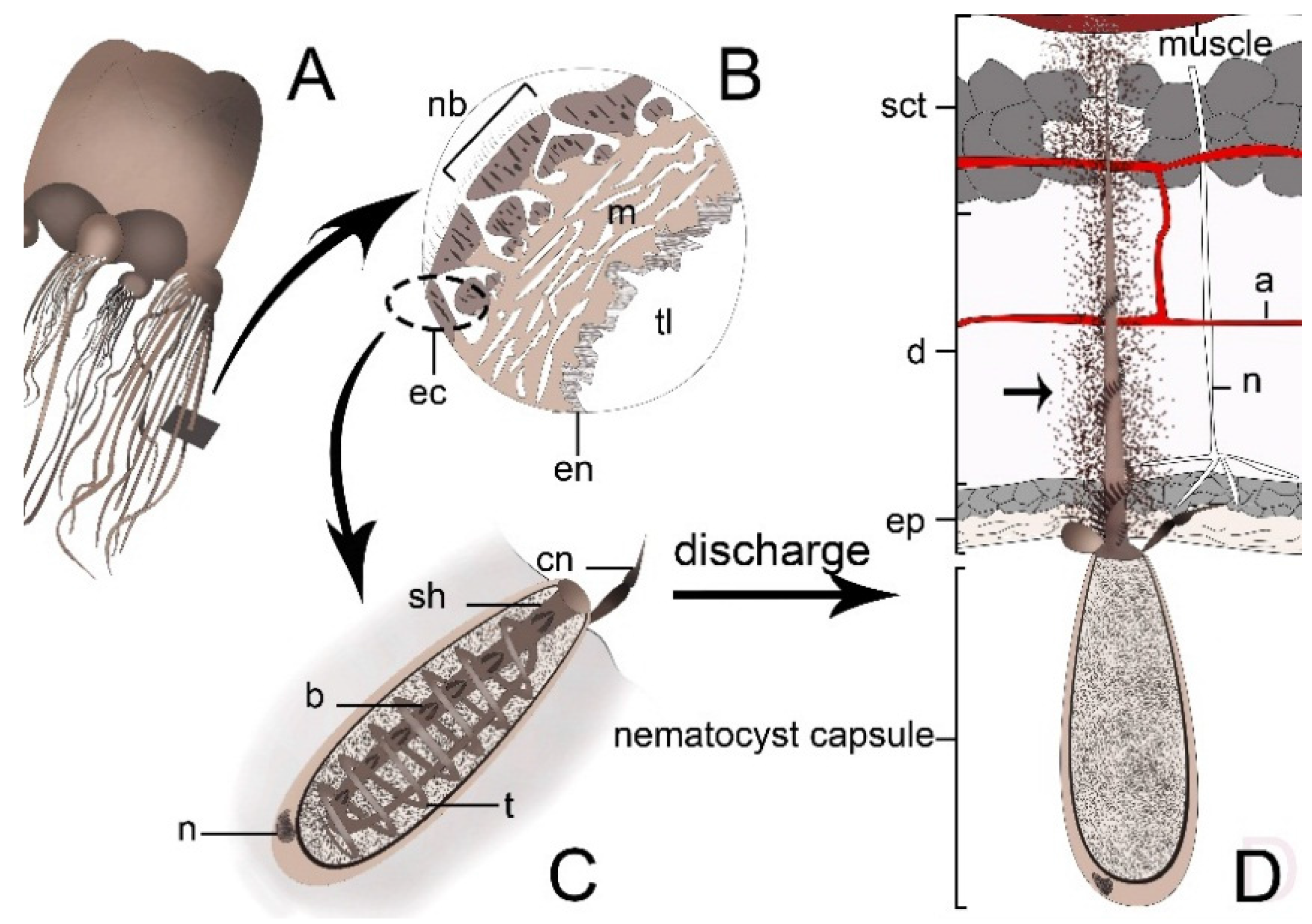
4. Cnidarian Venom Delivery System
| Toxin type | Found in class | Function | MW (kDa) | Reference |
|---|---|---|---|---|
| Enzymes | ||||
| Phospholipase A2 | Anthozoa, Cubozoa, Schyphozoa, Hydrozoa | Cytolytic, hemolytic, prey digestion | 13–45 | [44,45,46] |
| Metalloproteases | Schyphozoa, Cubozoa, Anthozoa | Cytotoxic, cytolytic, local tissue damage | 17–130 | [12,14,47,48] |
| Pore forming toxins (cytolysins) | ||||
| Actinoporins and actinoporin-like proteins | Anthozoa, Hydrozoa | Cytolytic, hemolytic, cardiovascular and respiratory arrest | 20 | [49,50,51,52,53,54] |
| Jellyfish Toxins | Cubozoa | Hemolytic, cardiotoxic, cytolytic, myotoxic, cutaneous inflammation | 42–46 | [13,55,56,57] |
| Hydralysins-related toxins | Hydrozoa, Anthozoa | Cytolytic, prey digestion | 27–31 | [43,58,59,60] |
| Membrane Attack Complex-Perforin | Anthozoa | Cytolytic, hemolytic | 60 | [61,62] |
| Neurotoxins | ||||
| NaTxs (type I-III) | Anthozoa | Neurotoxic, cardiotoxic, insecticide | 3–8 | [63,64,65,66,67] |
| KTxs (type I,III and IV,V KTxs) | Anthozoa | Neurotoxic, hypotensive, cardiotoxic, analgesic, antimicrobial, immunosuppressive, anti obesity | 3–4 | [68,69,70,71,72,73,74,75] |
| Kunitz peptides (type II KTxs) | Anthozoa | Paralytic, serine protease inhibitor | 6 | [76,77,78,79] |
| Small Cysteine-Rich Proteins (SCRiPs) and SCRiPs homologues | Anthozoa | Paralytic | 4.3–5.8 | [80] |
| ASIC Inhibitors | Anthozoa | Analgesic | 3 | [81,82] |
| TRPV1 Inhibitors | Anthozoa | Analgesic | 3 | [83,84] |
| Non-protein bioactive components | ||||
| Serotonin | Hydrozoa, Anthozoa | Vasodilation, sharp pain | - | [85] |
| Histamine | Anthozoa | Vasodilation, sharp pain | - | [86,87] |
| Bunodosine | Anthozoa | Analgesic | - | [88] |
| Caissarone | Anthozoa | Adenosine receptor antagonist | - | [89] |
5. Venom Composition
5.1. Enzymes
5.1.1. Phospholipase A2
5.1.2. Metalloproteases
5.2. Pore Forming Toxins
5.2.1. Actinoporins
5.2.2. Jellyfish Toxins (JFTs)
5.2.3. Hydralysins-Related Toxins
5.2.4. Membrane Attack Complex-Perforin
5.3. Neurotoxins
5.3.2. Voltage-gated Potasium (Kv) Channel Toxins and Kunitz peptides
5.3.3. Small Cysteine-Rich Peptides (SCRiPs)
5.3.4. ASIC Inhibitors
5.3.5. TRPV1 Inhibitors
5.4. Non-Protein Bioactive Components
6. The Role of Cnidarians Venoms in Drug Discovery
7. Envenomation
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviation
| rDNA | ribosomal DNA |
| mtDNA | mitochondrial DNA |
| PLA2 | Phospholipase A2 |
| MACP | Membrane Attack Complex-Perforin |
| SCRiPs | Small cysteine-rich peptides |
References
- Zhang, Z. Animal biodiversity: An introduction to higher-level classification and taxonomic richness. Zootaxa 2011, 3148, 7–12. [Google Scholar]
- Higgins, J.; Ford, M.; Costello, J. Transitions in morphology, nematocyst distribution, fluid motions, and prey capture during development of the scyphomedusa Cyanea capillata. Biol. Bull. 2008, 214, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, A.A.; Shohet, R.V. Cubozoan venom-induced cardiovascular collapse is caused by hyperkalemia and prevented by zinc gluconate in mice. PLoS ONE 2012, 7, e51368. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, T.; Collins, A.G.; Campbell, R. Global diversity of inland water cnidarians. Hydrobiologia 2008, 595, 35–40. [Google Scholar] [CrossRef]
- Finnerty, J.R.; Pang, K.; Burton, P.; Paulson, D.; Martindale, M.Q. Origins of bilateral symmetry: Hox and dpp expression in a sea anemone. Science 2004, 304, 1335–1337. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.W.; Wagner, G.P. The evolution of colony-level development in the Siphonophora (Cnidaria: Hydrozoa). Dev. Genes Evol. 2006, 216, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Moroz, L.L.; Kocot, K.M.; Citarella, M.R.; Dosung, S.; Norekian, T.P.; Povolotskaya, I.S.; Grigorenko, A.P.; Dailey, C.; Berezikov, E.; Buckley, K.M.; et al. The ctenophore genome and the evolutionary origins of neural systems. Nature 2014, 510, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Technau, U.; Steele, R.E. Evolutionary crossroads in developmental biology: Cnidaria. Development 2011, 138, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Iten, H.V.; Leme, J.d.M.; Marques, A.C.; Simões, M.G. Alternative interpretations of some earliest Ediacaran fossils from China. Acta Palaeontol. Pol. 2013, 58, 111–113. [Google Scholar]
- Park, E.; Hwang, D.S.; Lee, J.S.; Song, J.I.; Seo, T.K.; Won, Y.J. Estimation of divergence times in cnidarian evolution based on mitochondrial protein-coding genes and the fossil record. Mol. Phylogenet. Evol. 2012, 62, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Park, T.Y.; Woo, J.; Lee, D.J.; Lee, D.C.; Lee, S.B.; Han, Z.; Chough, S.K.; Choi, D.K. A stem-group cnidarian described from the mid-Cambrian of China and its significance for cnidarian evolution. Nat. Commun. 2011, 2, 442. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, H.; Xue, W.; Yue, Y.; Liu, S.; Xing, R.; Li, P. Jellyfish Venomics and Venom Gland Transcriptomics Analysis of Stomolophus meleagris to Reveal the Toxins Associated with Sting. J. Proteomics 2014, 106, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, D.L.; Konstantakopoulos, N.; McInerney, B.V.; Mulvenna, J.; Seymour, J.E.; Isbister, G.K.; Hodgson, W.C. Chironex fleckeri (Box Jellyfish) venom proteins: expansion of a cnidarian toxin family that elicits variable cytolytic and cardiovascular effects. J. Biol. Chem. 2014, 289, 4798–4812. [Google Scholar] [CrossRef] [PubMed]
- Jouiaei, M.; Casewell, N.R.; Yanagihara, A.A.; Nouwens, A.; Cribb, B.W.; Whitehead, D.; Jackson, T.N.; Ali, S.A.; Wagstaff, S.C.; Koludarov, I. Firing the sting: chemically induced discharge of cnidae reveals novel proteins and peptides from Box Jellyfish (Chironex fleckeri) venom. Toxins 2015, 7, 936–950. [Google Scholar] [CrossRef] [PubMed]
- Kayal, E.; Roure, B.; Philippe, H.; Collins, A.G.; Lavrov, D.V. Cnidarian phylogenetic relationships as revealed by mitogenomics. BMC. Evol. Biol. 2013, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.G. Phylogeny of Medusozoa and the evolution of cnidarian life cycles. J.Evol. Biol. 2002, 15, 418–432. [Google Scholar] [CrossRef]
- Freeman, G.; Miller, R.L. Hydrozoan eggs can only be fertilized at the site of polar body formation. Dev. Biol. 1982, 94, 142–152. [Google Scholar] [CrossRef]
- Galliot, B.; Schmid, V. Cnidarians as a model system for understanding evolution and regeneration. Int. J. Dev. Biol. 2002, 46, 39–48. [Google Scholar] [PubMed]
- Hand, C.; Uhlinger, K.R. The culture, sexual and asexual reproduction, and growth of the sea anemone Nematostella vectensis. Biol. Bull. 1992, 182, 169–176. [Google Scholar] [CrossRef]
- Loya, Y. Settlement, mortality and recruitment of a red sea scleractini an coral population. Coelenterate Ecol. Behav. 1976, 3, 89. [Google Scholar]
- Govindarajan, A.F.; Boero, F.; Halanych, K.M. Phylogenetic analysis with multiple markers indicates repeated loss of the adult medusa stage in Campanulariidae (Hydrozoa, Cnidaria). Mol. Phylogenet. Evol. 2006, 38, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Frazao, B.; Vasconcelos, V.; Antunes, A. Sea anemone (Cnidaria, Anthozoa, Actiniaria) toxins: An overview. Mar. Drugs 2012, 10, 1812–1851. [Google Scholar] [CrossRef] [PubMed]
- Orts, D.J.; Peigneur, S.; Madio, B.; Cassoli, J.S.; Montandon, G.G.; Pimenta, A.M.; Bicudo, J.E.; Freitas, J.C.; Zaharenko, A.J.; Tytgat, J. Biochemical and Electrophysiological Characterization of Two Sea Anemone Type 1 Potassium Toxins from a Geographically Distant Population of Bunodosoma caissarum. Mar. Drugs 2013, 11, 655–679. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, A.; Ozbek, S. The nematocyst: A molecular map of the cnidarian stinging organelle. Int. J. Dev. Biol. 2012, 56, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Ozbek, S. The cnidarian nematocyst: A miniature extracellular matrix within a secretory vesicle. Protoplasma 2011, 248, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Fautin, D.G. Structural diversity, systematics, and evolution of cnidae. Toxicon 2009, 54, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- David, C.N.; Ozbek, S.; Adamczyk, P.; Meier, S.; Pauly, B.; Chapman, J.; Hwang, J.S.; Gojobori, T.; Holstein, T.W. Evolution of complex structures: minicollagens shape the cnidarian nematocyst. Trends Genet. 2008, 24, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Tardent, P. The cnidarian cnidocyte, a hightech cellular weaponory. BioEssays 1995, 17, 351–362. [Google Scholar] [CrossRef]
- Gershwin, L. Nematocysts of the Cubozoa. Zootaxa 2006, 1232, 1–57. [Google Scholar]
- Peach, M.B.; Pitt, K.A. Morphology of the nematocysts of the medusae of two scyphozoans, Catostylus mosaicus and Phyllorhiza punctata (Rhizostomeae): implications for capture of prey. Invertebrate Biol. 2005, 124, 98–108. [Google Scholar] [CrossRef]
- Minagawa, S.; Sugiyama, M.; Ishida, M.; Nagashima, Y.; Shiomi, K. Kunitz-type protease inhibitors from acrorhagi of three species of sea anemones. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2008, 150, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Rifkin, J. A study of the spirocytes from the Ceriantharia and Actiniaria (Cnidaria: Anthozoa). Cell Tissue Res. 1991, 266, 365–373. [Google Scholar] [CrossRef]
- Östman, C. A guideline to nematocyst nomenclature and classification, and some notes on the systematic value of nematocysts. Sci. Mar. 2000, 64, 31–46. [Google Scholar]
- Hartwick, R.; Callanan, V.; Williamson, J. Disarming the box-jellyfish: nematocyst inhibition in Chironex fleckeri. Med. J. Aust. 1980, 1, 15–20. [Google Scholar] [PubMed]
- Rifkin, J.F.; Burnett, J.W.; Fenner, P.J. Venomous and poisonous marine animals: A medical and biological handbook; NewSouth Publishing: Sydney, Australia, 1996. [Google Scholar]
- Endean, R.; Rifkin, J.; Daddow, L. Envenomation by the box-jellyfish Chironex fleckeri: How nematocysts discharge. Hydrobiologia 1991, 216, 641–648. [Google Scholar] [CrossRef]
- Hidaka, M. Mechanism of nematocyst discharge and its cellular control. In Advances in comparative and environmental physiology; Springer-Verlag: Berlin, Germany, 1993; pp. 45–76. [Google Scholar]
- Holstein, T.; Tardent, P. An ultrahigh-speed analysis of exocytosis: Nematocyst discharge. Science 1984, 223, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Özbek, S.; Balasubramanian, P.G.; Holstein, T.W. Cnidocyst structure and the biomechanics of discharge. Toxicon 2009, 54, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Lotan, A.; Fishman, L.; Loya, Y.; Zlotkin, E. Delivery of a nematocyst toxin. Nature 1995, 375, 456. [Google Scholar] [CrossRef] [PubMed]
- Carrette, T.; Alderslade, P.; Seymour, J. Nematocyst ratio and prey in two Australian cubomedusans, Chironex fleckeri and Chiropsalmus sp. Toxicon 2002, 40, 1547–1551. [Google Scholar] [CrossRef]
- Moran, Y.; Genikhovich, G.; Gordon, D.; Wienkoop, S.; Zenkert, C.; Ozbek, S.; Technau, U.; Gurevitz, M. Neurotoxin localization to ectodermal gland cells uncovers an alternative mechanism of venom delivery in sea anemones. Proc. Biol. Sci. 2012, 279, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Fishman, Y.; Sher, D.; Zlotkin, E. Hydralysin, a novel animal group-selective paralytic and cytolytic protein from a noncnidocystic origin in hydra. Biochemistry 2003, 42, 8939–8944. [Google Scholar] [CrossRef] [PubMed]
- Nevalainen, T.J.; Peuravuori, H.J.; Quinn, R.J.; Llewellyn, L.E.; Benzie, J.A.; Fenner, P.J.; Winkel, K.D. Phospholipase A2 in cnidaria. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004, 139, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Hessinger, D.A.; Lenhoff, H.M. Mechanism of hemolysis induced by nematocyst venom: Roles of phospholipase A and direct lytic factor. Arch. Biochem. Biophys. 1976, 173, 603–613. [Google Scholar] [CrossRef]
- Mariottini, G.L.; Pane, L. Cytotoxic and cytolytic cnidarian venoms. A review on health implications and possible therapeutic applications. Toxins 2014, 6, 108–151. [Google Scholar]
- Lee, H.; Jung, E.-S.; Kang, C.; Yoon, W.D.; Kim, J.-S.; Kim, E. Scyphozoan jellyfish venom metalloproteinases and their role in the cytotoxicity. Toxicon 2011, 58, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Moran, Y.; Praher, D.; Schlesinger, A.; Ayalon, A.; Tal, Y.; Technau, U. Analysis of soluble protein contents from the nematocysts of a model sea anemone sheds light on venom evolution. Mar. Biotechnol. 2013, 15, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Uechi, G.-I.; Toma, H.; Arakawa, T.; Sato, Y. Biochemical and physiological analyses of a hemolytic toxin isolated from a sea anemone Actineria villosa. Toxicon 2005, 45, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, W.; Wang, L.H.; Wang, J.G.; Liu, X.Y.; Jiao, B.H. Purification and characterization of gigantoxin-4, a new actinoporin from the sea anemone Stichodactyla gigantea. Int. J. Biol. Sci. 2011, 7, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, V.S.; Kannan, L.; Venkateshvaran, K. Biological activity of sea anemone proteins: II. Cytolysis and cell line toxicity. Indian J. Exp. Biol. 2010, 48, 1233–1236. [Google Scholar]
- Fedorov, S.; Dyshlovoy, S.; Monastyrnaya, M.; Shubina, L.; Leychenko, E.; Kozlovskaya, E.; Jin, J.-O.; Kwak, J.-Y.; Bode, A.M.; Dong, Z. The anticancer effects of actinoporin RTX-A from the sea anemone Heteractis crispa (Radianthus macrodactylus). Toxicon 2010, 55, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Kohno, Y.; Satoh, H.; Iguchi, A.; Nagai, H. Characterization of a new hemolytic protein toxin from the sea anemone Anthopleura asiatica. Fish. Sci. 2009, 75, 1049–1054. [Google Scholar] [CrossRef]
- Glasser, E.; Rachamim, T.; Aharonovich, D.; Sher, D. Hydra actinoporin-like toxin-1, an unusual hemolysin from the nematocyst venom of Hydra magnipapillata which belongs to an extended gene family. Toxicon 2014, 91, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Takuwa, K.; Nakao, M.; Ito, E.; Miyake, M.; Noda, M.; Nakajima, T. Novel proteinaceous toxins from the box jellyfish (sea wasp) Carybdea rastoni. Biochem. Biophys. Res. Commun. 2000, 275, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Takuwa-Kuroda, K.; Nakao, M.; Oshiro, N.; Iwanaga, S.; Nakajima, T. A novel protein toxin from the deadly box jellyfish (Sea Wasp, Habu-kurage) Chiropsalmus quadrigatus. Biosci. Biotechnol. Biochem. 2002, 66, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.; Isbister, G.K.; Seymour, J.E.; Hodgson, W.C. The in vitro effects of two chirodropid (Chironex fleckeri and Chiropsalmus sp.) venoms: efficacy of box jellyfish antivenom. Toxicon 2003, 41, 703–711. [Google Scholar]
- Sher, D.; Fishman, Y.; Zhang, M.; Lebendiker, M.; Gaathon, A.; Mancheno, J.M.; Zlotkin, E. Hydralysins, a new category of β-pore-forming toxins in cnidaria. J. Biol. Chem. 2005, 280, 22847–22855. [Google Scholar] [CrossRef] [PubMed]
- Sher, D.; Fishman, Y.; Melamed-Book, N.; Zhang, M.; Zlotkin, E. Osmotically driven prey disintegration in the gastrovascular cavity of the green hydra by a pore-forming protein. FASEB J. 2008, 22, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Moran, Y.; Fredman, D.; Szczesny, P.; Grynberg, M.; Technau, U. Recurrent horizontal transfer of bacterial toxin genes to eukaryotes. Mol. Biol. Evol. 2012, 29, 2223–2230. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, N.; Kobayashi, C.; Iwanaga, S.; Nozaki, M.; Namikoshi, M.; Spring, J.; Nagai, H. A new membrane-attack complex/perforin (MACPF) domain lethal toxin from the nematocyst venom of the Okinawan sea anemone Actineria villosa. Toxicon 2004, 43, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Oshiro, N.; Takuwa-Kuroda, K.; Iwanaga, S.; Nozaki, M.; Nakajima, T. Novel proteinaceous toxins from the nematocyst venom of the Okinawan sea anemone Phyllodiscus semoni Kwietniewski. Biochem. Biophys. Res. Commun. 2002, 294, 760–763. [Google Scholar] [CrossRef]
- Norton, T.R. Cardiotonic polypeptides from Anthopleura xanthogrammica (Brandt) and A. elegantissima (Brandt). Fed. Proc. 1981, 40, 21–25. [Google Scholar] [PubMed]
- Smith, J.J.; Blumenthal, K.M. Site-3 sea anemone toxins: molecular probes of gating mechanisms in voltage-dependent sodium channels. Toxicon 2007, 49, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, T.; Schaller, C.; Schulze, C.; Sanchez-Rodriguez, J.; Dannmeier, C.; Ravens, U.; Heubach, J.F.; Eckhardt, K.; Schmidtmayer, J.; Schmidt, H.; et al. Isolation and characterisation of five neurotoxic and cardiotoxic polypeptides from the sea anemone Anthopleura elegantissima. Toxicon 2001, 39, 693–702. [Google Scholar] [CrossRef]
- Yan, F.; Cheng, X.; Ding, X.; Yao, T.; Chen, H.; Li, W.; Hu, S.; Yu, Z.; Sun, Y.; Zhang, Y.; et al. Improved insecticidal toxicity by fusing Cry1Ac of Bacillus thuringiensis with Av3 of Anemonia viridis. Curr. Microbiol. 2014, 68, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Bosmans, F.; Tytgat, J. Sea anemone venom as a source of insecticidal peptides acting on voltage-gated Na+ channels. Toxicon 2007, 49, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Diochot, S.; Schweitz, H.; Beress, L.; Lazdunski, M. Sea anemone peptides with a specific blocking activity against the fast inactivating potassium channel Kv3.4. J. Biol. Chem. 1998, 273, 6744–6749. [Google Scholar] [CrossRef]
- Diochot, S.; Loret, E.; Bruhn, T.; Beress, L.; Lazdunski, M. APETx1, a new toxin from the sea anemone Anthopleura elegantissima, blocks voltage-gated human ether-a-go-go-related gene potassium channels. Mol. Pharmacol. 2003, 64, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Diochot, S.; Baron, A.; Rash, L.D.; Deval, E.; Escoubas, P.; Scarzello, S.; Salinas, M.; Lazdunski, M. A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J. 2004, 23, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikova, T.V.; Balandin, S.V.; Aleshina, G.M.; Tagaev, A.A.; Leonova, Y.F.; Krasnodembsky, E.D.; Men’shenin, A.V.; Kokryakov, V.N. Aurelin, a novel antimicrobial peptide from jellyfish Aurelia aurita with structural features of defensins and channel-blocking toxins. Biochem. Biophys. Res. Commun. 2006, 348, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Orts, D.J.; Moran, Y.; Cologna, C.T.; Peigneur, S.; Madio, B.; Praher, D.; Quinton, L.; de Pauw, E.; Bicudo, J.E.; Tytgat, J.; et al. BcsTx3 is a founder of a novel sea anemone toxin family of potassium channel blocker. FEBS J. 2013, 280, 4839–4852. [Google Scholar] [CrossRef] [PubMed]
- Chi, V.; Pennington, M.W.; Norton, R.S.; Tarcha, E.J.; Londono, L.M.; Sims-Fahey, B.; Upadhyay, S.K.; Lakey, J.T.; Iadonato, S.; Wulff, H. Development of a sea anemone toxin as an immunomodulator for therapy of autoimmune diseases. Toxicon 2012, 59, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.K.; Eckel-Mahan, K.L.; Mirbolooki, M.R.; Tjong, I.; Griffey, S.M.; Schmunk, G.; Koehne, A.; Halbout, B.; Iadonato, S.; Pedersen, B.; et al. Selective Kv1.3 channel blocker as therapeutic for obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2013, 110, E2239–E2248. [Google Scholar]
- Jensen, J.E.; Cristofori-Armstrong, B.; Anangi, R.; Rosengren, K.J.; Lau, C.H.; Mobli, M.; Brust, A.; Alewood, P.F.; King, G.F.; Rash, L.D. Understanding the molecular basis of toxin promiscuity: The analgesic sea anemone peptide APETx2 interacts with acid-sensing ion channel 3 and hERG channels via overlapping pharmacophores. J. Med. Chem. 2014, 57, 9195–9203. [Google Scholar] [CrossRef] [PubMed]
- Peigneur, S.; Billen, B.; Derua, R.; Waelkens, E.; Debaveye, S.; Beress, L.; Tytgat, J. A bifunctional sea anemone peptide with Kunitz type protease and potassium channel inhibiting properties. Biochem. Pharmacol. 2011, 82, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Gladkikh, I.; Monastyrnaya, M.; Leychenko, E.; Zelepuga, E.; Chausova, V.; Isaeva, M.; Anastyuk, S.; Andreev, Y.; Peigneur, S.; Tytgat, J.; Kozlovkaya, E. Atypical reactive center Kunitz-type inhibitor from the sea anemone Heteractis crispa. Mar. Drugs 2012, 10, 1545–1565. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, S.; Ishida, M.; Shimakura, K.; Nagashima, Y.; Shiomi, K. Isolation and amino acid sequences of two Kunitz-type protease inhibitors from the sea anemone Anthopleura aff. xanthogrammica. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1997, 118, 381–386. [Google Scholar] [CrossRef]
- Delfin, J.; Martinez, I.; Antuch, W.; Morera, V.; Gonzalez, Y.; Rodriguez, R.; Marquez, M.; Saroyan, A.; Larionova, N.; Diaz, J. Purification, characterization and immobilization of proteinase inhibitors from Stichodactyla helianthus. Toxicon 1996, 34, 1367–1376. [Google Scholar] [CrossRef]
- Jouiaei, M.; Sunagar, K.; Federman Gross, A.; Scheib, H.; Alewood, P.F.; Moran, Y.; Fry, B.G. Evolution of an ancient venom: Recognition of a novel family of cnidarian toxins and the common evolutionary origin of sodium and potassium neurotoxins in sea anemone. Mol. Biol. Evol. 2015, 32, 1598–1610. [Google Scholar] [CrossRef] [PubMed]
- Osmakov, D.I.; Kozlov, S.A.; Andreev, Y.A.; Koshelev, S.G.; Sanamyan, N.P.; Sanamyan, K.E.; Dyachenko, I.A.; Bondarenko, D.A.; Murashev, A.N.; Mineev, K.S.; et al. Sea anemone peptide with uncommon β-hairpin structure inhibits acid-sensing ion channel 3 (ASIC3) and reveals analgesic activity. J. Biol. Chem. 2013, 288, 23116–23127. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.A.; Salceda, E.; Garateix, A.G.; Zaharenko, A.J.; Peigneur, S.; López, O.; Pons, T.; Richardson, M.; Díaz, M.; Hernández, Y. A novel sea anemone peptide that inhibits acid-sensing ion channels. Peptides 2014, 53, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Andreev, Y.A.; Kozlov, S.A.; Koshelev, S.G.; Ivanova, E.A.; Monastyrnaya, M.M.; Kozlovskaya, E.P.; Grishin, E.V. Analgesic compound from sea anemone Heteractis crispa is the first polypeptide inhibitor of vanilloid receptor 1 (TRPV1). J. Biol. Chem. 2008, 283, 23914–23921. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, S.A.; Andreev, Ya.A.; Murashev, A.N.; Skobtsov, D.I.; D’Iachenko, I.A.; Grishin, E.V. New polypeptide components from the Heteractis crispa sea anemone with analgesic activity. Russ. J. Bioorg. Chem. 2009, 35, 711–719. [Google Scholar]
- Sher, D.; Zlotkin, E. A hydra with many heads: Protein and polypeptide toxins from hydra and their biological roles. Toxicon 2009, 54, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Novak, V.; Sket, D.; Cankar, G.; Lebez, D. Partial purification of a toxin from tentacles of the sea anemone Anemonia sulcata. Toxicon 1973, 11, 411–417. [Google Scholar] [CrossRef]
- Mathias, A.; Ross, D.; Schachter, M. The distribution of 5-hydroxytryptamine, tetramethylammonium, homarine, and other substances in sea anemones. J. Physiol. 1960, 151, 296–311. [Google Scholar] [CrossRef] [PubMed]
- Zaharenko, A.J.; Picolo, G.; Ferreira, W.A., Jr.; Murakami, T.; Kazuma, K.; Hashimoto, M.; Cury, Y.; de Freitas, J.C.; Satake, M.; Konno, K. Bunodosine 391: An analgesic acylamino acid from the venom of the sea anemone Bunodosoma cangicum. J. Nat. Prod. 2011, 74, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.A.; de Freitas, J.; Porreca, F.; Eisenhour, C.M.; Lukas, R.; Huxtable, R.J. The sea anemone purine, caissarone: Adenosine receptor antagonism. Toxicon 1995, 33, 1025–1031. [Google Scholar] [CrossRef]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S.; et al. The toxicogenomic multiverse: Convergent recruitment of proteins into animal venoms. Annu. Rev. Genomics Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef] [PubMed]
- Low, D.H.; Sunagar, K.; Undheim, E.A.; Ali, S.A.; Alagon, A.C.; Ruder, T.; Jackson, T.N.; Pineda Gonzalez, S.; King, G.F.; Jones, A.; et al. Dracula’s children: Molecular evolution of vampire bat venom. J. Proteomics 2013, 89, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Gilquin, B.; Racape, J.; Wrisch, A.; Visan, V.; Lecoq, A.; Grissmer, S.; Menez, A.; Gasparini, S. Structure of the BgK-Kv1.1 complex based on distance restraints identified by double mutant cycles. Molecular basis for convergent evolution of Kv1 channel blockers. J. Biol. Chem. 2002, 277, 37406–37413. [Google Scholar]
- Talvinen, K.A.; Nevalainen, T.J. Cloning of a novel phospholipase A2 from the cnidarian Adamsia carciniopados. Comp. Biochem. Physiol..B Biochem. Mol. Biol. 2002, 132, 571–578. [Google Scholar] [CrossRef]
- Six, D.A.; Dennis, E.A. The expanding superfamily of phospholipase A2 sub enzymes: Classification and characterization. Biochim. Biophys. Acta 2000, 1488, 1–19. [Google Scholar] [CrossRef]
- Undheim, E.A.; King, G.F. On the venom system of centipedes (Chilopoda), a neglected group of venomous animals. Toxicon 2011, 57, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Vidal, N.; Norman, J.A.; Vonk, F.J.; Scheib, H.; Ramjan, S.F.; Kuruppu, S.; Fung, K.; Hedges, S.B.; Richardson, M.K.; et al. Early evolution of the venom system in lizards and snakes. Nature 2006, 439, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Wuster, W. Assembling an arsenal: Origin and evolution of the snake venom proteome inferred from phylogenetic analysis of toxin sequences. Mol. Biol. Evol. 2004, 21, 870–883. [Google Scholar] [CrossRef] [PubMed]
- Nevalainen, T.J. Phospholipases A 2 in the genome of the sea anemone Nematostella vectensis. Comp. Biochem. Physiol. D Genomics Proteomics 2008, 3, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Undheim, E.A.; Jones, A.; Clauser, K.R.; Holland, J.W.; Pineda, S.S.; King, G.F.; Fry, B.G. Clawing through evolution: Toxin diversification and convergence in the ancient lineage Chilopoda (Centipedes). J. Mol. Biol. 2014, 31, 2124–2148. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.W.; Serrano, S.M. Structural considerations of the snake venom metalloproteinases, key members of the M12 reprolysin family of metalloproteinases. Toxicon 2005, 45, 969–985. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.W.; Feil, S.C. Pore-forming protein toxins: From structure to function. Prog. Biophys. Mol. Biol. 2005, 88, 91–142. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ortega, L.; Alegre-Cebollada, J.; Garcia-Linares, S.; Bruix, M.; Martinez-Del-Pozo, A.; Gavilanes, J.G. The behavior of sea anemone actinoporins at the water-membrane interface. Biochim. Biophys. Acta 2011, 1808, 2275–2288. [Google Scholar] [CrossRef] [PubMed]
- Kristan, K.C.; Viero, G.; Dalla Serra, M.; Macek, P.; Anderluh, G. Molecular mechanism of pore formation by actinoporins. Toxicon 2009, 54, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Bakrac, B.; Gutierrez-Aguirre, I.; Podlesek, Z.; Sonnen, A.F.; Gilbert, R.J.; Macek, P.; Lakey, J.H.; Anderluh, G. Molecular determinants of sphingomyelin specificity of a eukaryotic pore-forming toxin. J. Biol. Chem. 2008, 283, 18665–18677. [Google Scholar] [CrossRef] [PubMed]
- Martínez, D.; Otero, A.; Alvarez, C.; Pazos, F.; Tejuca, M.; Eliana Lanio, M.; Gutiérrez-Aguirre, I.; Barlic, A.; Iloro, I.; Luis Arrondo, J. Effect of sphingomyelin and cholesterol on the interaction of St II with lipidic interfaces. Toxicon 2007, 49, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Mechaly, A.E.; Bellomio, A.; Gil-Cartón, D.; Morante, K.; Valle, M.; González-Mañas, J.M.; Guérin, D. Structural insights into the oligomerization and architecture of eukaryotic membrane pore-forming toxins. Structure 2011, 19, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, A.; Potrich, C.; Sabo, J.K.; Frisanco, M.; Guella, G.; Dalla Serra, M.; Anderluh, G.; Separovic, F.; Norton, R.S. Structure and activity of the N-terminal region of the eukaryotic cytolysin equinatoxin II. Biochemistry 2006, 45, 1818–1828. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Aguirre, I.; Barlic, A.; Podlesek, Z.; Macek, P.; Anderluh, G.; Gonzalez-Manas, J.M. Membrane insertion of the N-terminal α-helix of equinatoxin II, a sea anemone cytolytic toxin. Biochem. J. 2004, 384 Pt 2, 421–428. [Google Scholar] [PubMed]
- Mancheno, J.M.; Martin-Benito, J.; Martinez-Ripoll, M.; Gavilanes, J.G.; Hermoso, J.A. Crystal and electron microscopy structures of sticholysin II actinoporin reveal insights into the mechanism of membrane pore formation. Structure 2003, 11, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Tejuca, M.; Dalla Serra, M.; Potrich, C.; Alvarez, C.; Menestrina, G. Sizing the radius of the pore formed in erythrocytes and lipid vesicles by the toxin sticholysin I from the sea anemone Stichodactyla helianthus. J. Membr. Biol. 2001, 183, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.J.; Ratnapala, L.A.; Cooke, I.M.; Yanagihara, A.A. Partial purification and characterization of a hemolysin (CAH1) from Hawaiian box jellyfish Carybdea alata venom. Toxicon 2001, 39, 981–990. [Google Scholar] [CrossRef]
- Nagai, H.; Takuwa, K.; Nakao, M.; Sakamoto, B.; Crow, G.L.; Nakajima, T. Isolation and characterization of a novel protein toxin from the Hawaiian box jellyfish (sea wasp) Carybdea alata. Biochem. Biophys. Res. Commun. 2000, 275, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, D.; Burnell, J. Identification, cloning and sequencing of two major venom proteins from the box jellyfish, Chironex fleckeri. Toxicon 2007, 50, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Voskoboinik, I.; Dunstone, M.A.; Baran, K.; Whisstock, J.C.; Trapani, J.A. Perforin: structure, function, and role in human immunopathology. Immunol. Rev. 2010, 235, 35–54. [Google Scholar] [PubMed]
- Castaneda, O.; Harvey, A.L. Discovery and characterization of cnidarian peptide toxins that affect neuronal potassium ion channels. Toxicon 2009, 54, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu. Rev. Pharmacol. Toxicol. 1980, 20, 15–43. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A.; Cestèle, S.; Yarov-Yarovoy, V.; Yu, F.H.; Konoki, K.; Scheuer, T. Voltage-gated ion channels and gating modifier toxins. Toxicon 2007, 49, 124–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran, Y.; Gordon, D.; Gurevitz, M. Sea anemone toxins affecting voltage-gated sodium channels-molecular and evolutionary features. Toxicon 2009, 54, 1089–1101. [Google Scholar] [CrossRef] [PubMed]
- Loret, E.P.; del Valle, R.M.; Mansuelle, P.; Sampieri, F.; Rochat, H. Positively charged amino acid residues located similarly in sea anemone and scorpion toxins. J. Biol. Chem. 1994, 269, 16785–16788. [Google Scholar] [PubMed]
- Cariello, L.; de Santis, A.; Fiore, F.; Piccoli, R.; Spagnuolo, A.; Zanetti, L.; Parente, A. Na+ channel toxinsCalitoxin, a neurotoxic peptide from the sea anemone Calliactis parasitica: amino-acid sequence and electrophysiological properties. Biochemistry 1989, 28, 2484–2489. [Google Scholar] [CrossRef] [PubMed]
- Nir, N.; Eli, S.; Daniel, S.; Yehu, M.; Liora, T.; Ana, L.T.-M.; Michal, H.; Binyamin, H.; Eliahu, Z. AdE-1, a new inotropic Na+ channel toxin from Aiptasia diaphana, is similar to, yet distinct from, known anemone Na+ channel toxins. Biochem. J. 2013, 451, 81–90. [Google Scholar]
- Moran, Y.; Kahn, R.; Cohen, L.; Gur, M.; Karbat, I.; Gordon, D.; Gurevitz, M. Molecular analysis of the sea anemone toxin Av3 reveals selectivity to insects and demonstrates the heterogeneity of receptor site-3 on voltage-gated Na+ channels. Biochem. J. 2007, 406, 41–48. [Google Scholar] [PubMed]
- Bruhn, T. Kalicludines and Kaliseptine. J. Biol. Chem. 1995, 270, 25121–25126. [Google Scholar]
- Honma, T.; Kawahata, S.; Ishida, M.; Nagai, H.; Nagashima, Y.; Shiomi, K. Novel peptide toxins from the sea anemone Stichodactyla haddoni. Peptides 2008, 29, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.W. Treatment of Atlantic cnidarian envenomations. Toxicon 2009, 54, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Morabito, R.; La Spada, G.; Crupi, R.; Esposito, E.; Marino, A. Crude Venom from Nematocysts of the Jellyfish Pelagia noctiluca as a Tool to Study Cell Physiology. Cent. Nerv. Syst. Agents. Med. Chem. 2015, 15, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H. Palytoxin: Membrane mechanisms of action. Toxicon 2009, 54, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Habermann, E. Palytoxin acts through Na+, K+-ATPase. Toxicon 1989, 27, 1171–1187. [Google Scholar] [CrossRef]
- Gorogh, T.; Beress, L.; Quabius, E.S.; Ambrosch, P.; Hoffmann, M. Head and neck cancer cells and xenografts are very sensitive to palytoxin: Decrease of C-jun N-terminale kinase-3 expression enhances palytoxin toxicity. Mol. Cancer 2013, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Quinn, R.J.; Kashiwagi, M.; Moore, R.E.; Norton, T.R. Anticancer activity of zoanthids and the associated toxin, palytoxin, against Ehrlich ascites tumor and P-388 lymphocytic leukemia in mice. J. Pharm. Sci. 1974, 63, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Louzao, M.C.; Ares, I.R.; Cagide, E.; Espiña, B.; Vilariño, N.; Alfonso, A.; Vieytes, M.R.; Botana, L.M. Palytoxins and cytoskeleton: An overview. Toxicon 2011, 57, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Wattenberg, E.V. Modulation of protein kinase signaling cascades by palytoxin. Toxicon 2011, 57, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Giraldi, T.; Ferlan, I.; Romeo, D. Antitumour Activity of Equinatoxin. Chem.-Biol. Interact. 1976, 13, 199–203. [Google Scholar] [CrossRef]
- Batista, U.; Macek, P.; Sedmak, B. The cytotoxic and cytolytic activity of equinatoxin II from the sea anemone Actinia equina. Cell Biol. Int. Rep. 1990, 14, 1013–1024. [Google Scholar] [CrossRef]
- Lenarcic, B.; Turk, V. Thyroglobulin type-1 domains in equistatin inhibit both papain-like cysteine proteinases and cathepsin D. J. Biol. Chem. 1999, 274, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Liaudet-Coopman, E.; Beaujouin, M.; Derocq, D.; Garcia, M.; Glondu-Lassis, M.; Laurent-Matha, V.; Prébois, C.; Rochefort, H.; Vignon, F. Cathepsin D: Newly discovered functions of a long-standing aspartic protease in cancer and apoptosis. Cancer Lett. 2006, 237, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Brömme, D.; Petanceska, S. Papain-like cysteine proteases and their implications in neurodegenerative diseases. In Role of Proteases in the Pathophysiology of Neurodegenerative Diseases; Springer: New York, NY, USA, 2002; pp. 47–61. [Google Scholar]
- Ayed, Y.; Dellai, A.; Ben Mansour, H.; Bacha, H.; Abid, S. Analgesic and antibutyrylcholinestrasic activities of the venom prepared from the Mediterranean jellyfish Pelagia noctiluca. Ann. Clin. Microbiol. Antimicrob. 2012, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.J.; Angus, J.A.; Winkel, K.D.; Wright, C.E. A pharmacological investigation of the venom extract of the Australian box jellyfish, Chironex fleckeri, in cardiac and vascular tissues. Toxicol. Lett. 2012, 209, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Tibballs, J.; Yanagihara, A.A.; Turner, H.C.; Winkel, K. Immunological and toxinological responses to jellyfish stings. Inflamm. Allergy Drug Targets 2011, 10, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Bruschetta, G.; Impellizzeri, D.; Morabito, R.; Marino, A.; Ahmad, A.; Spano, N.; Spada, G.L.; Cuzzocrea, S.; Esposito, E. Pelagia noctiluca (Scyphozoa) crude venom injection elicits oxidative stress and inflammatory response in rats. Mar. Drugs 2014, 12, 2182–2204. [Google Scholar] [CrossRef] [PubMed]
- Morabito, R.; Marino, A.; Dossena, S.; La Spada, G. Nematocyst discharge in Pelagia noctiluca (Cnidaria, Scyphozoa) oral arms can be affected by lidocaine, ethanol, ammonia and acetic acid. Toxicon 2014, 83, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Birsa, L.M.; Verity, P.G.; Lee, R.F. Evaluation of the effects of various chemicals on discharge of and pain caused by jellyfish nematocysts. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 151, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Rifkin, J.F.; Fenner, P.J.; Williamson, J.A. First aid treatment of the sting from the hydroid Lytocarpus philippinus: The structure of, and in vitro discharge experiments with its nematocysts. J. Wilderness Med. 1993, 4, 252–260. [Google Scholar] [CrossRef]
- Soon, W.W.; Hariharan, M.; Snyder, M.P. High-throughput sequencing for biology and medicine. Mol. Syst. Biol. 2013, 9, 640. [Google Scholar] [CrossRef] [PubMed]
- Escoubas, P.; King, G.F. Venomics as a drug discovery platform. Expert Rev. Proteomics 2009, 6, 221–224. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jouiaei, M.; Yanagihara, A.A.; Madio, B.; Nevalainen, T.J.; Alewood, P.F.; Fry, B.G. Ancient Venom Systems: A Review on Cnidaria Toxins. Toxins 2015, 7, 2251-2271. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins7062251
Jouiaei M, Yanagihara AA, Madio B, Nevalainen TJ, Alewood PF, Fry BG. Ancient Venom Systems: A Review on Cnidaria Toxins. Toxins. 2015; 7(6):2251-2271. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins7062251
Chicago/Turabian StyleJouiaei, Mahdokht, Angel A. Yanagihara, Bruno Madio, Timo J. Nevalainen, Paul F. Alewood, and Bryan G. Fry. 2015. "Ancient Venom Systems: A Review on Cnidaria Toxins" Toxins 7, no. 6: 2251-2271. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins7062251