A Review of Bioinsecticidal Activity of Solanaceae Alkaloids
Abstract
:1. Introduction
2. Solanaceae Secondary Metabolites
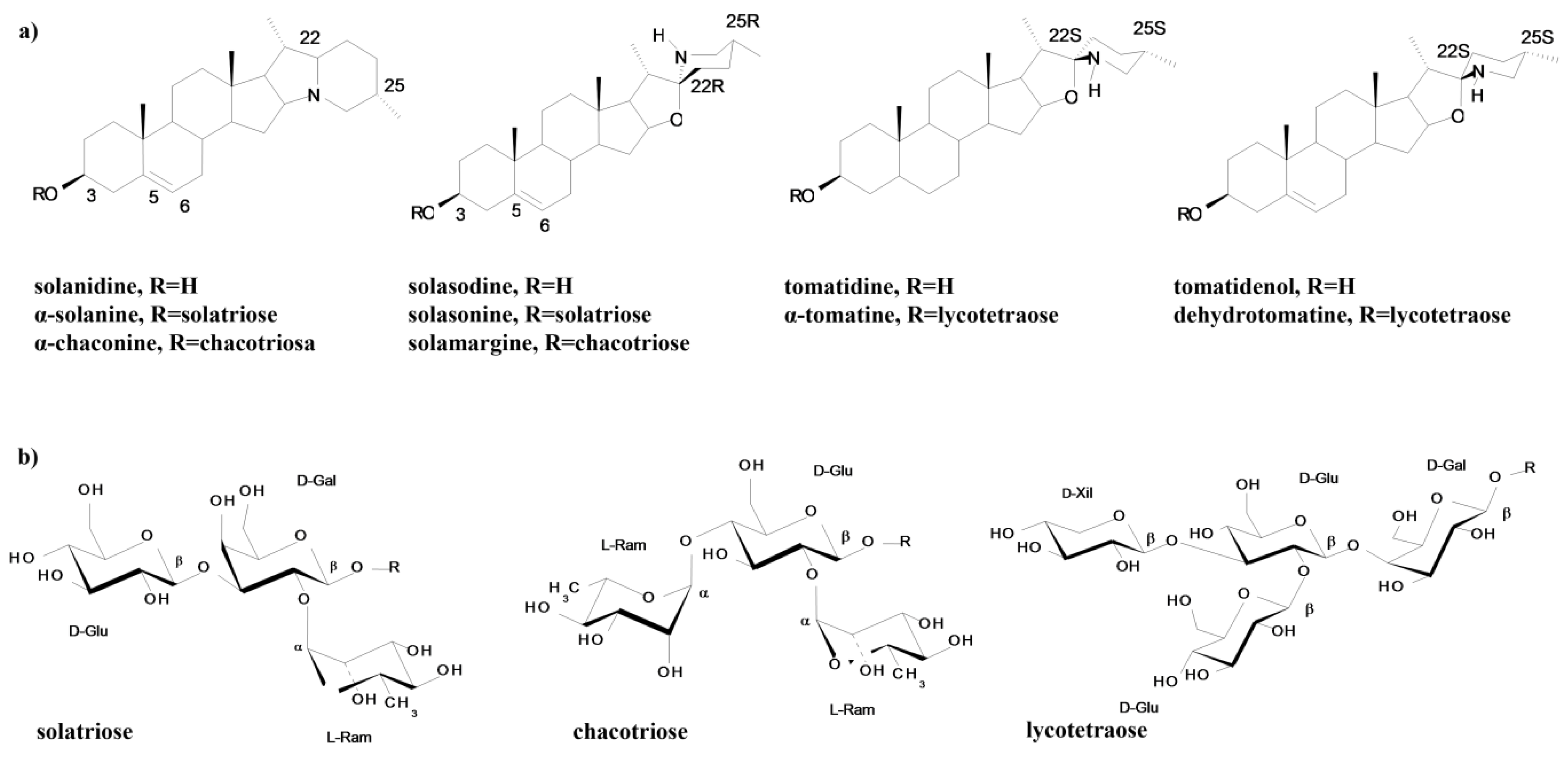
2.1. Solanaceae Alkaloids—Chemical Structure
2.2. Solanaceae Glycoalkaloids—Physiological Effects in Insects
Effects of Alkaloids on Insect Cells and Tissues
3. Solanaceae Secondary Metabolites as Bioinsecticides—Future Directions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- FAO. Save and Grow in Practice: Maize, Rice, Wheat. A Guide to Sustainable Cereal Production; FAO: Rome, Italy, 2015. [Google Scholar]
- Sallam, M.N. Insect Damage: Damage on Post-harvest; FAO: Roma, Italy, 2013; p. 38. [Google Scholar]
- Nicholson, G.M. Fighting the global pest problem: Preface to the special toxicon issue on insecticidal toxins and their potential for insect pest control. Toxicon 2007, 49, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Zalucki, M.P.; Shabbir, A.; Silva, R.; Adamson, D.; Shu-Sheng, L.; Furlong, M.J. Estimating the economic cost of one of the world's major insect pests, Plutella xylostella (lepidoptera: Plutellidae): Just how long is a piece of string? J. Econ. Entomol. 2012, 105, 1115–1129. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Malaria Report 2013; World Health Organization: Geneva, Swizerland, 2013; pp. 1–255. [Google Scholar]
- Van der Gaag, N. Pick your poison. New Int. 2000, 58, 9–11. [Google Scholar]
- Mariyono, J. Direct and indirect impacts of integrated pest management on pesticide use: A case of rice agriculture in Java, Indonesia. Pest Manage. Sci. 2008, 64, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Publ. Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Cole, D.C.; Carpio, F.; Leon, N. Economic burden of illness from pesticide poisonings in highland Ecuador. Rev. Panam. Salud Publica 2000, 8, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Soares, W.L.; Porto, M.F.D. Estimating the social cost of pesticide use: An assessment from acute poisoning in Brazil. Ecol. Econ. 2009, 68, 2721–2728. [Google Scholar] [CrossRef]
- Chowanski, S.; Kudlewska, M.; Marciniak, P.; Rosinski, G. Synthetic insecticides—is there an alternative? Pol. J. Environ. Stud. 2014, 23, 291–302. [Google Scholar]
- Ecobichon, D.J. Pesticide use in developing countries. Toxicology 2001, 160, 27–33. [Google Scholar] [CrossRef]
- Adeyemi, M.M.H. The potential of secondary metabolites in plant material as deterents against insect pests: A review. Afr. J. Pure Appl. Chem. 2010, 4, 243–246. [Google Scholar]
- Ibanez, S.; Gallet, C.; Despres, L. Plant insecticidal toxins in ecological networks. Toxins 2012, 4, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Interference of alkaloids with neuroreceptors and ion channels. In Bioactive natural products; Atta-Ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 2000; pp. 3–129. [Google Scholar]
- Pimentel, D. Environmental and economic costs of the application of pesticides primarily in the United States. Environ. Dev. Sustain. 2005, 7, 229–252. [Google Scholar] [CrossRef]
- Miresmailli, S.; Isman, M.B. Botanical insecticides inspired by plant-herbivore chemical interactions. Trends Plant Sci. 2014, 19, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, C.; Konno, K.; Wasano, N.; Nakamura, M. Differential effects of sugar-mimic alkaloids in mulberry latex on sugar metabolism and disaccharidases of eri and domesticated silkworms: Enzymatic adaptation of Bombyx mori to mulberry defense. Insect Biochem. Mol. Biol. 2007, 37, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Tangtrakulwanich, K.; Reddy, G.V.P. Development of insect resistance to plant biopesticides: An overview. In Advances in Plant Biopesticides; Singh, D., Ed.; Springer India: New Delhi, India, 2014; pp. 47–62. [Google Scholar]
- Buss, E.A.; Brown, S.G.P. Naturalproducts for managing landscape and garden pests in Florida; UF/IFAS Extension Service, University of Florida: Gainesville, FL, USA, 2002. [Google Scholar]
- Weissenberg, M.; Levy, A.; Svoboda, J.A.; Ishaaya, I. The effect of some solanum steroidal alkaloids and glycoalkaloids on larvae of the red flour beetle, Tribolium castaneum, and the tobacco hornworm, Manduca sexta. Phytochemistry 1998, 47, 203–209. [Google Scholar] [CrossRef]
- Buyukguzel, E.; Buyukguzel, K.; Snela, M.; Erdem, M.; Radtke, K.; Ziemnicki, K.; Adamski, Z. Effect of boric acid on antioxidant enzyme activity, lipid peroxidation, and ultrastructure of midgut and fat body of Galleria mellonella. Cell Biol. Toxicol. 2013, 29, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Buyukguzel, E.; Buyukguzel, K.; Erdem, M.; Adamski, Z.; Marciniak, P.; Ziemnicki, K.; Ventrella, E.; Scrano, L.; Bufo, S.A. The influence of dietary alpha-solanine on the waxmoth Galleria mellonella L. Arch. Insect Biochem. Physiol. 2013, 83, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Tomato glycoalkaloids: Role in the plant and in the diet. J. Agric. Food Chem. 2002, 50, 5751–5780. [Google Scholar] [CrossRef] [PubMed]
- Nenaah, G. Toxic and antifeedant activities of potato glycoalkaloids against Trogoderma granarium (Coleoptera: Dermestidae). J. Stored. Prod. Res. 2011, 47, 185–190. [Google Scholar] [CrossRef]
- Chopa, C.S.; Benzi, V.; Alzogaray, R.; Ferrero, A.A. Repellent activity of hexanic and ethanolic extracts from fruits of Solanum eleagnifolium (Solanaceae) against Blattella germanica (Insecta, Dictyoptera, Blattellidae) adults. Bol. Latinoam. Caribe. 2009, 8, 172–175. [Google Scholar]
- Dinesh, D.S.; Kumari, S.; Kumar, V.; Das, P. The potentiality of botanicals and their products as an alternative to chemical insecticides to sandflies (Diptera: Psychodidae): A review. J. Vector Borne Dis. 2014, 51, 1–7. [Google Scholar] [PubMed]
- Lee, M.R. Solanaceae IV: Atropa belladonna, deadly nightshade. J. R. Coll. Physicians Edinb. 2007, 37, 77–84. [Google Scholar] [PubMed]
- Lee, M.R. The Solanaceae: Foods and poisons. J. R. Coll. Physicians Edinb. 2006, 36, 162–169. [Google Scholar] [PubMed]
- Fowler, C.J. Plant sources of antimuscarinics. BJU Int. 2015, 115 Suppl. 6, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Kanteh, S.M.; Norman, J.E. Diversity of plants with pesticidal and medicinal properties in southern Sierra Leone. Biol. Agric. Hortic. 2015, 31, 18–27. [Google Scholar] [CrossRef]
- Boulogne, I.; Petit, F.; Ozier-Lafontaine, H.; Desfontaines, L.; Loranger-Merciris, G. Insecticidal and antifungal chemicals produced by plants: A review. Environ. Chem. Lett. 2012, 10, 325–347. [Google Scholar] [CrossRef]
- Jerzykiewicz, J. Alkaloids of Solanaceae (nightshade plants). Postepy Biochem. 2007, 53, 280–286. [Google Scholar] [PubMed]
- Hassine, T.; Mansour, A.; Hammami, S. Case report of fatal poisoning by Nicotina tabacum in Cattle in Tunisia. Rev. Med. Vet. (Toulouse) 2013, 164, 141–144. [Google Scholar]
- Indhumathi, T.; Mohandass, S.; Shibi, A. Acute toxicity study of ethanolic extract of Solanum incanum L. Fruit. Asian J. Pharm. Clin. Res. 2014, 7, 98–100. [Google Scholar]
- Diaz, G.J. Toxicosis by plant alkaloids in humans and animals in Colombia. Toxins 2015, 7, 5408–5416. [Google Scholar] [CrossRef] [PubMed]
- Lachman, J.; Hamouz, K.; Orsak, M.; Pivec, V. Potato glycoalkaloids and their significance in plant protection and human nutrition—Review. Rostl. Vyr. 2001, 47, 181–191. [Google Scholar]
- Sharma, R.P.; Salunkhe, D.K. Solanum glycoalkaloids. In Toxicants of plant origin; Cheeke, P.R., Ed.; CRC Press: Boca Raton, FL, USA, 1989; Volume 1, pp. 179–236. [Google Scholar]
- Hlywka, J.J.; Stephenson, G.R.; Sears, M.K.; Rickey, Y.Y. Effect of insect damage on glycoalkaloid content in potatoes (Solanum tuberosum). J. Agric. Food Chem. 1994, 42, 2545–2550. [Google Scholar] [CrossRef]
- Ji, X.H.; Rivers, L.; Zielinski, Z.; Xu, M.; MacDougall, E.; Stephen, J.; Zhang, S.C.; Wang, Y.W.; Chapman, R.G.; Keddy, P.; et al. Quantitative analysis of phenolic components and glycoalkaloids from 20 potato clones and in vitro evaluation of antioxidant, cholesterol uptake, and neuroprotective activities. Food Chem. 2012, 133, 1177–1187. [Google Scholar] [CrossRef]
- Bushway, R.J.; Ponnampalam, R. Alpha-chaconine and alpha-solanine content of potato products and their stability during several modes of cooking. J. Agric. Food Chem. 1981, 29, 814–817. [Google Scholar] [CrossRef]
- Wink, M. A short history of alkaloids. In Alkaloids: Biochemistry, Ecology and Medicinal Applications; Roberts, M.F., Wink, M., Eds.; Plenum: New York, NY, USA, 1998; pp. 11–44. [Google Scholar]
- Roddick, J.G. Steroidal glycoalkaloids: Nature and consequences of bioactivity. Adv. Exp. Med. Biol. 1996, 404, 277–295. [Google Scholar] [PubMed]
- Van Gelder, W.M.J.; Scheffer, J.J.C. Transmission of steroidal glycoalkaloids from Solanum vernei to the cultivated potato. Phytochemistry 1991, 30, 165–168. [Google Scholar] [CrossRef]
- Friedman, M.; McDonald, G.M. Potato glycoalkaloids: Chemistry, analysis, safety, and plant physiology. Crit. Rev. Plant Sci. 1997, 16, 55–132. [Google Scholar] [CrossRef]
- Dinan, L.; Harmatha, J.; Lafont, R. Chromatographic procedures for the isolation of plant steroids. J. Chromatogr. A 2001, 935, 105–123. [Google Scholar] [CrossRef]
- Friedman, M. Potato glycoalkaloids and metabolites: Roles in the plant and in the diet. J. Agric. Food Chem. 2006, 54, 8655–8681. [Google Scholar] [CrossRef] [PubMed]
- Shakya, R.; Navarre, D.A. LC-MS analysis of solanidane glycoalkaloid diversity among tubers of four wild potato species and three cultivars (Solanum tuberosum). J. Agric. Food Chem. 2008, 56, 6949–6958. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Mcdonald, G.; Haddon, W.F. Kinetics of acid-catalyzed hydrolysis of carbohydrate groups of potato glycoalkaloids alpha-chaconine and alpha-solanine. J. Agric. Food Chem. 1993, 41, 1397–1406. [Google Scholar] [CrossRef]
- Griffin, W.J.; Lin, G.D. Chemotaxonomy and geographical distribution of tropane alkaloids. Phytochemistry 2000, 53, 623–637. [Google Scholar] [CrossRef]
- Fodor, G.; Dharanipragada, R. Tropane alkaloids. Nat. Prod. Rep. 1994, 11, 443–450. [Google Scholar] [PubMed]
- Lounasmaa, M.; Tamminen, T. The tropane alkaloids: Chemistry and biology. In The Alkaloids; Cordell, G.A., Ed.; Academic Press: New York, NY, USA, 1993; Volume 44, pp. 1–114. [Google Scholar]
- EFSA. Scientific opinion of the panel on contaminants in the food chain on a request from the european commission on tropane alkaloids (from Datura sp.) as undesirable substances in animal feed. EFSA J. 2008, 691, 1–55. [Google Scholar]
- Barbosa, P.; Saunders, J.A.; Kemper, J.; Trumbule, R.; Olechno, J.; Martinat, P. Plant allelochemicals and insect parasitoids effects of nicotine on Cotesia congregata (Say) (Hymenoptera: Braconidae) and Hyposoter annulipes (Cresson) (Hymenoptera: Ichneumonidae). J. Chem. Ecol. 1986, 12, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.L. Negative aspects of interaction between host plant resistance and biological control and its implication in integrated pest management of crops. In Biocontrol Potential and Its Exploitation in Sustainable Agriculture; Upadhyay, R.K., Mukerji, K.G., Chamola, B.P., Eds.; Springer Science & Business Media New York: New York, NY, USA, 2001. [Google Scholar]
- Evangelista, W.S.; Santos, R.L.; Torres, J.B.; Zanuncio, J.C. Effect of gossypol on survival and reproduction of the zoophytophagous stinkbug Podisus nigrispinus (Dallas). Rev. Bras. Entomol. 2011, 55, 267–271. [Google Scholar] [CrossRef]
- Campbell, B.C.; Duffey, S.S. Alleviation of α-tomatine-induced toxicity to the parasitoid, Hyposoter exiguae, by phytosterols in the diet of the host, Heliothis zea. J. Chem. Ecol. 1981, 7, 927–946. [Google Scholar] [CrossRef] [PubMed]
- Hori, M.; Nakamura, H.; Fujii, Y.; Suzuki, Y.; Matsuda, K. Chemicals affecting the feeding preference of the Solanaceae-feeding lady beetle Henosepilachna vigintioctomaculata (Coleoptera: Coccinellidae). J. Appl. Entomol. 2011, 135, 121–131. [Google Scholar] [CrossRef]
- Marciniak, P.; Adamski, Z.; Bednarz, P.; Slocinska, M.; Ziemnicki, K.; Lelario, F.; Scrano, L.; Bufo, S.A. Cardioinhibitory properties of potato glycoalkaloids in beetles. Bull. Environ. Contam. Toxicol. 2010, 84, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Ventrella, E.; Marciniak, P.; Adamski, Z.; Rosinski, G.; Chowanski, S.; Falabella, P.; Scrano, L.; Bufo, S.A. Cardioactive properties of Solanaceae plant extracts and pure glycoalkaloids on Zophobas atratus. Insect Sci. 2015, 22, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Carretero, A.; Cruz, M.; Eben, A. Phenotypic plasticity of the reproductive system of female Leptinotarsa undecimlineata. Entomol. Exp. Appl. 2005, 115, 27–31. [Google Scholar] [CrossRef]
- Flanders, K.L.; Hawkes, J.G.; Radcliffe, E.B.; Lauer, F.I. Insect resistance in potatoes—Sources, evolutionary relationships, morphological and chemical defenses, and ecogeographical associations. Euphytica 1992, 61, 83–111. [Google Scholar] [CrossRef]
- Oberdorster, E.; Clay, M.A.; Cottam, D.M.; Wilmot, F.A.; McLachlan, J.A.; Milner, M.J. Common phytochemicals are ecdysteroid agonists and antagonists: A possible evolutionary link between vertebrate and invertebrate steroid hormones. J. Steroid Biochem. Mol. Biol. 2001, 77, 229–238. [Google Scholar] [CrossRef]
- Corio-Costet, M.F.; Chapuis, L.; Mouillet, J.F.; Delbecque, J.P. Sterol and ecdysteroid profiles of Serratula tinctoria (L.) - plant and cell-cultures producing steroids. Insect Biochem. Mol. Biol. 1993, 23, 175–180. [Google Scholar] [CrossRef]
- Adler, J.H.; Grebenok, R.J. Biosynthesis and distribution of insect-molting hormones in plants—A review. Lipids 1995, 30, 257–262. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, H.W.; Fang, Y.; An, S.B.; Park, D.S.; Song, H.H.; Oh, S.R.; Kim, S.Y.; Kim, S.; Kim, N.; et al. Identification of plant compounds that disrupt the insect juvenile hormone receptor complex. Proc. Natl. Acad. Sci. USA 2015, 112, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, I.M.; Martena, M.J.; Boersma, M.G.; Spiegelenberg, W.; Alink, G.M. Molecular mechanisms of toxicity of important food-borne phytotoxins. Mol. Nutr. Food. Res. 2005, 49, 131–158. [Google Scholar] [CrossRef] [PubMed]
- Wierenga, J.M.; Hollingworth, R.M. Inhibition of insect acetylcholinesterase by the potato glycoalkaloid alpha-chaconine. Nat. Toxins 1992, 1, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Adamski, Z.; Marciniak, P.; Ziemnicki, K.; Buyukguzel, E.; Erdem, M.; Buyukguzel, K.; Ventrella, E.; Falabella, P.; Cristallo, M.; Salvia, R.; et al. Potato leaf extract and its component, alpha-solanine, exert similar impacts on development and oxidative stress in Galleria mellonella L. Arch. Insect Biochem. Physiol. 2014, 87, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Barbehenn, R.; Cheek, S.; Gasperut, A.; Lister, E.; Maben, R. Phenolic compounds in red oak and sugar maple leaves have prooxidant activities in the midgut fluids of Malacosoma disstria and Orgyia leucostigma caterpillars. J. Chem. Ecol. 2005, 31, 969–988. [Google Scholar] [CrossRef] [PubMed]
- Appel, H.M. Phenolics in ecological interactions: The importance of oxidation. J. Chem. Ecol. 1993, 19, 1521–1552. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Sehnal, F. Compartmentalization of oxidative stress and antioxidant defense in the larval gut of Spodoptera littoralis. Arch. Insect Biochem. Physiol. 2006, 63, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S. Oxidative stress from environmental pollutants. Arch. Insect Biochem. Physiol. 1995, 29, 135–157. [Google Scholar] [CrossRef] [PubMed]
- Zadra, M.; Piana, M.; Brum, T.F.; Boligon, A.A.; Freitas, R.B.; Machado, M.M.; Stefanello, S.T.; Soares, F.A.; Athayde, M.L. Antioxidant activity and phytochemical composition of the leaves of Solanum guaraniticum A. St.-Hil. Molecules 2012, 17, 12560–12574. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Ali, M.; Nagella, P.; Siddiqui, N.A.; Ahmad, A. Evaluation of antioxidant activity of new constituents from the fruits of Lycium chinense. Med. Chem. Res. 2014, 23, 3852–3860. [Google Scholar] [CrossRef]
- Chung, I.M.; Yang, Y.S.; Nagella, P.; Ahn, Y.S.; Lim, J.D.; Kim, S.H.; Ahmad, A. Antioxidant activity of monoglucoside-triarabinoside from the fruits of Lycium chinense Miller. Asian J. Chem. 2014, 26, 3033–3035. [Google Scholar]
- Mohanan, P.V.; Devi, K.S. Cytotoxic potential of the preparations from Solanum trilobatum and the effect of sobatum on tumour reduction in mice. Cancer Lett. 1996, 110, 71–76. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry and anticarcinogenic mechanisms of glycoalkaloids produced by eggplants, potatoes, and tomatoes. J. Agric. Food Chem. 2015, 63, 3323–3337. [Google Scholar] [CrossRef] [PubMed]
- Keukens, E.A.; de Vrije, T.; van den Boom, C.; de Waard, P.; Plasman, H.H.; Thiel, F.; Chupin, V.; Jongen, W.M.; de Kruijff, B. Molecular basis of glycoalkaloid induced membrane disruption. Biochim. Biophys. Acta 1995, 1240, 216–228. [Google Scholar] [CrossRef]
- Mandimika, T.; Baykus, H.; Poortman, J.; Garza, C.; Kuiper, H.; Peijnenburg, A. Induction of the cholesterol biosynthesis pathway in differentiated caco-2 cells by the potato glycoalkaloid alpha-chaconine. Food Chem. Toxicol. 2007, 45, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
- Sucha, L.; Hroch, M.; Rezacova, M.; Rudolf, E.; Havelek, R.; Sispera, L.; Cmielova, J.; Kohlerova, R.; Bezrouk, A.; Tomsik, P. The cytotoxic effect of alpha-tomatine in mcf-7 human adenocarcinoma breast cancer cells depends on its interaction with cholesterol in incubation media and does not involve apoptosis induction. Oncol. Rep. 2013, 30, 2593–2602. [Google Scholar] [PubMed]
- Rosenkranz, V.; Wink, M. Alkaloids induce programmed cell death in bloodstream forms of trypanosomes (Trypanosoma b. Brucei). Molecules 2008, 13, 2462–2473. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Villegas, E.O.; Herrera-Arellano, A.; de Los Angeles Martinez-Rivera, M.; Alvarez, L.; Cano-Nepauseno, M.; Marquina, S.; Rodriguez-Tovar, A.V.; Tortoriello, J. Ultrastructural changes on clinical isolates of Trichophyton rubrum, Trichophyton mentagrophytes, and Microsporum gypseum caused by Solanum chrysotrichum saponin SC-2. Planta Med. 2009, 75, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Dekebo, A.; Kashiwagi, T.; Tebayashi, S.I.; Kim, C.S. Nitrogenous ovipositional deterrents in the leaves of sweet pepper (Capsicum annuum) at the mature stage against the leafminer, Liriomyza trifolii (Burgess). Biosci. Biotechnol. Biochem. 2007, 71, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Antonious, G.F.; Kamminga, K.; Snyder, J.C. Wild tomato leaf extracts for spider mite and cowpea aphid control. J. Environ. Sci. Health. B 2014, 49, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Singaravelan, N.; Nee’man, G.; Inbar, M.; Izhaki, I. Feeding responses of free-flying honeybees to secondary compounds mimicking floral nectars. J. Chem. Ecol. 2005, 31, 2791–2804. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Coloma, A.; Reina, M.; Medinaveitia, A.; Guadano, A.; Santana, O.; Martinez-Diaz, R.; Ruiz-Mesia, L.; Alva, A.; Grandez, M.; Diaz, R.; et al. Structural diversity and defensive properties of norditerpenoid alkaloids. J. Chem. Ecol. 2004, 30, 1393–1408. [Google Scholar] [CrossRef] [PubMed]
- Shields, V.D.; Smith, K.P.; Arnold, N.S.; Gordon, I.M.; Shaw, T.E.; Waranch, D. The effect of varying alkaloid concentrations on the feeding behavior of gypsy moth larvae, Lymantria dispar (L.) (Lepidoptera: Lymantriidae). Arthropod. Plant Interact. 2008, 2, 101–107. [Google Scholar] [CrossRef]
- Detzel, A.; Wink, M. Attraction, deterrence or intoxication of bees (Apis mellifera) by plant allelochemicals. Chemoecology 1993, 4, 8–18. [Google Scholar]
- Asano, N.; Kato, A.; Kizu, H.; Matsui, K.; Watson, A.A.; Nash, R.J. Calystegine b4, a novel trehalase inhibitor from Scopolia japonica. Carbohydr. Res. 1996, 293, 195–204. [Google Scholar] [PubMed]
- Doolittle, M.; Raina, A.; Lax, A.; Boopathy, R. Effect of natural products on gut microbes in formosan subterranean termite, Coptotermes formosanus. Int. Biodet. Biodeg. 2007, 59, 69–71. [Google Scholar] [CrossRef]
- Olszewska, J.; Tegowska, E. Opposite effect of capsaicin and capsazepine on behavioral thermoregulation in insects. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2011, 197, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Edelson, J.V.; Duthie, J.; Roberts, W. Toxicity of biorational insecticides: Activity against the green peach aphid, Myzus persicae (Sulzer). Pest Manage. Sci. 2002, 58, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska, J.; Tegowska, E. Capsaicin as an organophosphate synergist against colorado potato beetle (Leptinotarsa decemlineatasay). J. Plant Prot. Res. 2012, 52, 28–34. [Google Scholar] [CrossRef]
- Stamp, N.E.; Osier, T.L. Response of five insect herbivores to multiple allelochemicals under fluctuating temperatures. Entomol. Exp. Appl. 1998, 88, 81–96. [Google Scholar] [CrossRef]
- Weiser, L.A.; Stamp, N.E. Combined effects of allelochemicals, prey availability, and supplemental plant material on growth of a generalist insect predator. Entomol. Exp. Appl. 1998, 87, 181–189. [Google Scholar] [CrossRef]
- Soule, S.; Güntner, C.; Vázquez, A.; Argandoña, V.H.; Ferreira, F.; Moyna, P. Effect of solanum glycosides on the aphid Schizaphis graminum. J. Chem. Ecol. 1999, 25, 369–374. [Google Scholar] [CrossRef]
- Rangarajan, A.; Miller, A.R. Leptine glycoalkaloids reduce feeding by clorado potato beetle in diploid Solanum sp. Hybrids. J. Am. Soc. Hortic. Sci. 2000, 125, 689–693. [Google Scholar]
- Hollister, B.; Dickens, J.C.; Perez, F.; Deahl, K.L. Differential neurosensory responses of adult colorado potato beetle, Leptinotarsa decemlineata, to glycoalkaloids. J. Chem. Ecol. 2001, 27, 1105–1118. [Google Scholar] [CrossRef] [PubMed]
- Soule, S.; Guntner, C.; Vazquez, A.; Argandona, V.V.; Moyna, P.; Ferreira, F. An aphid repellent glycoside from Solanum laxum. Phytochemistry 2000, 55, 217–222. [Google Scholar] [CrossRef]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural products in crop protection. Bioorg. Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef] [PubMed]
- Singaravelan, N.; Inbar, M.; Ne’eman, G.; Distl, M.; Wink, M.; Izhaki, I. The effects of nectar-nicotine on colony fitness of caged honeybees. J. Chem. Ecol. 2006, 32, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Kohler, A.; Pirk, C.W.; Nicolson, S.W. Honeybees and nectar nicotine: Deterrence and reduced survival versus potential health benefits. J. Insect Physiol. 2012, 58, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Rathi, P.; Schottner, M.; Baldwin, I.T.; Pandit, S. Differences in nicotine metabolism of two Nicotiana attenuata herbivores render them differentially susceptible to a common native predator. PLoS ONE 2014, 9, e95982. [Google Scholar] [CrossRef] [PubMed]
- Mareggiani, G.; Picollo, M.I.; Zerba, E.; Burton, G.; Tettamanzi, M.C.; Benedetti-Doctorovich, M.O.; Veleiro, A.S. Antifeedant activity of withanolides from Salpichroa origanifolia on Musca domestica. J. Nat. Prod. 2000, 63, 1113–1116. [Google Scholar] [CrossRef] [PubMed]
- Mareggiani, G.; Picollo, M.I.; Veleiro, A.S.; Tettamanzi, M.C.; Benedetti-Doctorovich, M.O.; Burton, G.; Zerba, E. Response of Tribolium castaneum (Coleoptera, tenebrionidae) to Salpichroa origanifolia withanolides. J. Agric. Food Chem. 2002, 50, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Hartl, M.; Giri, A.P.; Kaur, H.; Baldwin, I.T. Serine protease inhibitors specifically defend Solanum nigrum against generalist herbivores but do not influence plant growth and development. Plant Cell 2010, 22, 4158–4175. [Google Scholar] [CrossRef] [PubMed]
- Gunter, C.; Vazquez, A.; Gonzalez, G.; Usubillaga, A.; Ferreira, F.; Moyna, P. Effect of solanum glycoalcaloids on poptato aphid, Macrosiphum euphorbiae: Part II. J. Chem. Ecol. 2000, 26, 1113–1121. [Google Scholar] [CrossRef]
- Lingampally, V.; Solanki, V.R.; Anuradha, D.L.; Sabita Raja, S. Effect of solasodine against last instar larvae of Tribolium confusum. J. Entomol. Zool. Stud. 2014, 2, 118–120. [Google Scholar]
- Mulatu, B.; Applebaum, S.W.; Kerem, Z.; Coll, M. Tomato fruit size, maturity and alpha-tomatine content influence the performance of larvae of potato tuber moth Phthorimaea operculella (Lepidoptera: Gelechiidae). Bull. Entomol. Res. 2006, 96, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Fragoyiannis, D.A.; McKinlay, R.G.; D’Mello, J.P.F. Studies of the growth, development and reproductive performance of the aphid Myzus persicae on artificial diets containing potato glycoalkaloids. Entomol. Exp. Appl. 1998, 88, 59–66. [Google Scholar] [CrossRef]
- Bloem, K.A.; Kelley, K.C.; Duffey, S.S. Differential effect of tomatine and its alleviation by cholesterol on larval growth and efficiency of food utilization in Heliothis zea and Spodoptera exigua. J. Chem. Ecol. 1989, 15, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Nenaah, G. Individual and synergistic toxicity of solanaceous glycoalkaloids against two coleopteran stored-product insects. J. Pest Sci. 2011, 84, 77–86. [Google Scholar] [CrossRef]
- Maharijaya, A.; Vosman, B.; Verstappen, F.; Steenhuis-Broers, G.; Mumm, R.; Purwito, A.; Visser, R.G.F.; Voorrips, R.E. Resistance factors in pepper inhibit larval development of thrips (Frankliniella occidentalis). Entomol. Exp. Appl. 2012, 145, 62–71. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Mikagi, E.; Mekuria, D.B.; Boru, A.D.; Tebayashi, S.; Kim, C.S. Ovipositional deterrent on mature stage of sweet pepper, Capsicum annuum, against Liriomyza trifolii (burgess). Z. Naturforsch. C 2005, 60, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Devanand, P.; Rani, P.U. Insect growth regulatory activity of the crude and purified fractions from Solanum melongena L., Lycopersicum esculentum Mill, and Capsicum annuum L. J. Biopest. 2011, 4, 118–130. [Google Scholar]
- Rani, P.U.; Devanand, P. Efficiency of different plant foliar extracts on grain protection and seed germination in maize. Res. J. Seed Scien. 2011, 4, 1–14. [Google Scholar] [CrossRef]
- Han, M.K.; Kim, S.I.; Ahn, Y.J. Insecticidal and antifeedant activities of medicinal plant extracts against Attagenus unicolor japonicus (Coleoptera: Dermestidae). J. Stored. Prod. Res. 2006, 42, 15–22. [Google Scholar] [CrossRef]
- Amoabeng, B.W.; Gurr, G.M.; Gitau, C.W.; Nicol, H.I.; Munyakazi, L.; Stevenson, P.C. Tri-trophic insecticidal effects of african plants against cabbage pests. PLoS ONE 2013, 8, e78651. [Google Scholar] [CrossRef]
- Ghosh, A.; Chandra, G. Biocontrol efficacy of Sestrum diurnum L. (Solanaceae: Solanales) against the larval forms of Anopheles stephensi. Nat. Prod. Res. 2006, 20, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Chowdhury, N.; Chandra, G. Laboratory evaluation of a phytosteroid compound of mature leaves of day jasmine (Solanaceae: Solanales) against larvae of Culex quinquefasciatus (Diptera: Culicidae) and nontarget organisms. Parasitol. Res. 2008, 103, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.D.; Patil, S.V.; Salunke, B.K.; Salunkhe, R.B. Bioefficacy of Plumbago zeylanica (Plumbaginaceae) and Cestrum nocturnum (Solanaceae) plant extracts against Aedes aegypti (Diptera: Culicide) and nontarget fish Poecilia reticulata. Parasitol. Res. 2011, 108, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Ikbal, C.; Monia, B.H.; Habib, B. Development perturbation of cotton leave noctuid with green cestrum extracts. J. Entomol. 2007, 4, 121–128. [Google Scholar]
- Zapata, N.; Budia, F.; Vinuela, E.; Medina, P. Insecticidal effects of various concentrations of selected extractions of Cestrum parqui on adult and immature Ceratitis capitata. J. Econ. Entomol. 2006, 99, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Elango, G.; Rahuman, A.A.; Kamaraj, C.; Bagavan, A.; Zahir, A.A. Efficacy of medicinal plant extracts against malarial vector, Anopheles subpictus grassi. Parasitol. Res. 2011, 108, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, A.; Ramya, S.; Gopinath, K.; Periyathambi, N.; Jayakumararaj, R.; Aruna, D. Biopesticidal effect of ethyl acetate leaf extracts of Datura metel L. (Solanaceae) on the larvae of Helicoverpa armigera (Hübner). Int. J. Pharm. Sci. Rev. Res. 2013, 18, 150–154. [Google Scholar]
- Elango, G.; Rahuman, A.A.; Kamaraj, C.; Bagavan, A.; Zahir, A.A. Screening for feeding deterrent activity of herbal extracts against the larvae of malaria vector Anopheles subpictus Grassi. Parasitol. Res. 2011, 109, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Rahuman, A.A.; Venkatesan, P.; Gopalakrishnan, G. Mosquito larvicidal activity of oleic and linoleic acids isolated from Citrullus colocynthis (Linn.) Schrad. Parasitol. Res. 2008, 103, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Abbasipour, H.; Mahmoudvand, M.; Rastegar, F.; Hosseinpour, M.H. Bioactivities of jimsonweed extract, Datura stramonium L. (Solanaceae), against Tribolium castaneum (Coleoptera: Tenebrionidae). Turk. J. Agr. Forest. 2011, 35, 623–629. [Google Scholar]
- Mehrabadi, M.; Bandani, A.R.; Saadati, F.; Mahmudvand, M. Α-amylase activity of stored products insects and its inhibition by medicinal plant extracts. J. Agric. Sci. Technol. 2011, 13, 1173–1182. [Google Scholar]
- Castillo, L.; Gonzalez-Coloma, A.; Gonzalez, A.; Diaz, M.; Santos, E.; Alonso-Paz, E.; Bassagoda, M.J.; Rossini, C. Screening of uruguayan plants for deterrent activity against insects. Ind. Crops Prod. 2009, 29, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bhattacharya, K.; Chandra, G. Efficacy of Nicotiana plumbaginifolia (Solanaceae) leaf extracts as larvicide against malarial vector Anopheles stephensi Liston 1901. Int. J. Pharm. Biol. Sci. 2015, 6, B860–B868. [Google Scholar]
- Bagalwa, J.J.; Voutquenne-Nazabadioko, L.; Sayagh, C.; Bashwira, A.S. Evaluation of the biological activity of the molluscicidal fraction of Solanum sisymbriifolium against non-target organisms. Fitoterapia 2010, 81, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, G. Insecticidal effect of plant extracts on two termite species. Arch. Phytopathology. Plant. Protect. 2011, 44, 356–361. [Google Scholar] [CrossRef]
- Schlein, Y.; Jacobson, R.L.; Muller, G.C. Sand fly feeding on noxious plants: A potential method for the control of leishmaniasis. Am. J. Trop. Med. Hyg. 2001, 65, 300–303. [Google Scholar] [PubMed]
- Rawani, A.; Ghosh, A.; Chandra, G. Mosquito larvicidal activities of Solanum nigrum L. Leaf extract against Culex quinquefasciatus Say. Parasitol. Res. 2010, 107, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, K.; Singh, S.P.; Subbarao, S.K.; Dash, A.P. Laboratory studies on mosquito larvicidal efficacy of aqueous & hexane extracts of dried fruit of Solanum nigrum Linn. Indian J. Med. Res. 2009, 130, 74–77. [Google Scholar] [PubMed]
- Ahmed, A.H.; Kamal, I.H.; Ramzy, R.M. Studies on the molluscicidal and larvicidal properties of Solanum nigrum L. Leaves ethanol extract. J. Egypt. Soc. Parasitol. 2001, 31, 843–852. [Google Scholar] [PubMed]
- Rawani, A.; Ghosh, A.; Chandra, G. Mosquito larvicidal and antimicrobial activity of synthesized nano-crystalline silver particles using leaves and green berry extract of Solanum nigrum L. (Solanaceae: Solanales). Acta Trop. 2013, 128, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Gokce, A.; Whalon, M.E.; Cam, H.; Yanar, Y.; Demirtas, I.; Goren, N. Contact and residual toxicities of 30 plant extracts to colorado potato beetle larvae. Arch. Phytopathology. Plant. Protect. 2007, 40, 441–450. [Google Scholar] [CrossRef]
- Jeyasankar, A.; Premalatha, S.; Rani, S.J. Bio-efficacy of Solanum pseudocapsicum L. (Solanaceae) against black cutworm, agrotis ipsilon hufnagel (Lepidoptera: Noctuidae). J. Biol. Sci. 2012, 12, 174–179. [Google Scholar] [CrossRef]
- Jeyasankar, A.; Premalatha, S.; Elumalai, K.; Krishnappa, K. Biological activities of Solanum pseudocapsicum (Solanaceae) against cotton bollworm, Helicoverpa armigera hubner and armyworm, Spodoptera litura Fabricius (Lepidotera: Noctuidae). Asian. Pac. J. Trop. Biomed. 2012, 2, 981–986. [Google Scholar] [CrossRef]
- Srivastava, M.; Gupta, L. Effect of formulations of Solanum surratense (family: Solanaceae) an indian desert plant on oviposition by the pulse beetle Callosobruchus chinensis Linn. Afr. J. Agric. Res. 2007, 2, 552–554. [Google Scholar]
- Muthukrishnana, J.; Pushpalathaa, E.; Kasthuribhai, A. Biological effects of four plant extracts on Culex quinquefasciatus Say larval stages. Int. J. Trop. Insect Sci. 1997, 17, 389–394. [Google Scholar] [CrossRef]
- Kamaraj, C.; Abdul Rahman, A.; Bagavan, A.; Abduz Zahir, A.; Elango, G.; Kandan, P.; Rajakumar, G.; Marimuthu, S.; Santhoshkumar, T. Larvicidal efficacy of medicinal plant extracts against Anopheles stephensi and Culex quinquefasciatus (Diptera: Culicidae). Trop. Biomed. 2010, 27, 211–219. [Google Scholar] [PubMed]
- Premalatha, S.; Elumalai, K.; Jeyasankar, A. Mosquitocidal properties of Solanum trilobatum L. (Solanaceae) leaf extracts against three important human vector mosquitoes (Diptera: Culicidae). Asian Pac. J. Trop. Med. 2013, 6, 854–858. [Google Scholar] [CrossRef]
- Zahir, A.A.; Rahuman, A.A.; Bagavan, A.; Santhoshkumar, T.; Mohamed, R.R.; Kamaraj, C.; Rajakumar, G.; Elango, G.; Jayaseelan, C.; Marimuthu, S. Evaluation of botanical extracts against Haemaphysalis bispinosa neumann and Hippobosca maculata Leach. Parasitol. Res. 2010, 107, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Singha, S.; Chandra, G. Mosquito larvicidal activity of some common spices and vegetable waste on Culex quinquefasciatus and Anopheles stephensi. Asian Pac. J. Trop. Med. 2011, 4, 288–293. [Google Scholar] [CrossRef]
- Arivoli, S.; Narendran, T.; Ignacimuthu, S. Larvicidal activity of some botanicals against Culex quinquefasciatus Say. J. Adv. Zool. 2000, 21, 19–23. [Google Scholar]
- Chowdhury, N.; Ghosh, A.; Chandra, G. Mosquito larvicidal activities of Solanum villosum berry extract against the dengue vector Stegomyia aegypti. BMC Complement. Altern. Med. 2008, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, N.; Bhattacharjee, I.; Laskar, S.; Chandra, G. Efficacy of Solanum villosum Mill. (Solanaceae: Solanales) as a biocontrol agent against fourth instar larvae of Culex quinquefasciatus Say. Turk. J. Zoo. 2007, 31, 365–370. [Google Scholar]
- Chowdhury, N.; Chatterjee, S.; Laskar, S.; Chandra, G. Larvicidal activity of Solanum villosum Mill. (Solanaceae: Solanales) leaves to Anopheles subpictus Grassi (Diptera: Culicidae) with effect on non-target Chironomus circumdatus Kieffer (Diptera: Chironomidae). J. Pest Sci. 2009, 82, 13–18. [Google Scholar] [CrossRef]
- Mahesh Kumar, P.; Murugan, K.; Kovendan, K.; Panneerselvam, C.; Prasanna Kumar, K.; Amerasan, D.; Subramaniam, J.; Kalimuthu, K.; Nataraj, T. Mosquitocidal activity of Solanum xanthocarpum fruit extract and copepod Mesocyclops thermocyclopoides for the control of dengue vector Aedes aegypti. Parasitol. Res. 2012, 111, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Mahesh Kumar, P.; Murugan, K.; Kovendan, K.; Subramaniam, J.; Amaresan, D. Mosquito larvicidal and pupicidal efficacy of Solanum xanthocarpum (family: Solanaceae) leaf extract and bacterial insecticide, Bacillus thuringiensis, against Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol. Res. 2012, 110, 2541–2550. [Google Scholar] [CrossRef] [PubMed]
- Gaur, R.; Kumar, K. Insect growth-regulating effects of Withania somnifera in a polyphagous pest, Spodoptera litura. Phytoparasitica 2010, 38, 237–241. [Google Scholar] [CrossRef]
- Abdel-Sattar, E.; El Sayed, A.M.; Zaitoon, A.; Bakhashwain, A.A. Evaluation of some medicinal plants for control of Culex pipiens mosquitoes. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 898–905. [Google Scholar]
- Smith, D.B.; Roddick, J.G.; Jones, J.L. Synergism between the potato glycoalkaloids alpha-chaconine and alpha-solanine in inhibition of snail feeding. Phytochemistry 2001, 57, 229–234. [Google Scholar] [CrossRef]
- Tak, J.H.; Jovel, E.; Isman, M.B. Comparative and synergistic activity of rosmarinus officinalis l. Essential oil constituents against the larvae and an ovarian cell line of the cabbage looper, Trichoplusia ni (Lepidoptera: Noctuidae). Pest Manage. Sci. 2015. [Google Scholar] [CrossRef]
- Akhtar, Y.; Pages, E.; Stevens, A.; Bradbury, R.; da Camara, C.A.G.; Isman, M.B. Effect of chemical complexity of essential oils on feeding deterrence in larvae of the cabbage looper. Physiol. Entomol. 2012, 37, 81–91. [Google Scholar] [CrossRef]
- Mullin, C.; Gonzalez-Coloma, A.; Gutierrez, C.; Reina, M.; Eichenseer, H.; Hollister, B.; Chyb, S. Antifeedant effects of some novel terpenoids on chrysomelidae beeltes: Comparisons with alkaloids on an alkaloid-adapted and nonadapterd species. J. Chem. Ecol. 1997, 23, 1851–1866. [Google Scholar] [CrossRef]
- Pino, O.; Sánchez, Y.; Rojas, M.M. Plant secondary metabolites as an alternative in pest management. I: Background, research approaches and trends. Revista de Protección Vegetal 2013, 28, 81–94. [Google Scholar]
- Mann, R.S.; Kaufman, P.E. Natural product pesticides: Their development, delivery and use against insect vectors. Mini-Rev. Org. Chem. 2012, 9, 185–202. [Google Scholar] [CrossRef]
- Sharma, P.P.; Pardeshi, A.B.; Vijigiri, D. Bioactivity of some medicinal plant extracts against Musca domestica L. J. Ecobiotechnology 2011, 3, 14–16. [Google Scholar]
- Delaplane, K.S. Pesticide Usage in the United States: History, Benefits, Risks, and Trends; Cooperative Extension Service, The University of Georgia College of Agriculture and Environmental Sciences: Athens, GA, USA, 1996. [Google Scholar]
- Wink, M. Evolutionary advantage and molecular modes of action of multi-component mixtures used in phytomedicine. Curr. Drug. Metab. 2008, 9, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Klonsky, K. Comparison of production costs and resource use for organic and conventional production systems. Am. J. Agr. Econ. 2012, 94, 314–321. [Google Scholar] [CrossRef]
- Amoabeng, B.W.; Gurr, G.M.; Gitau, C.W.; Stevenson, P.C. Cost:Benefit analysis of botanical insecticide use in cabbage: Implications for smallholder farmers in developing countries. Crop Prot. 2014, 57, 71–76. [Google Scholar] [CrossRef]
- Isman, M.B. A renaissance for botanical insecticides? Pest Manage. Sci. 2015, 71, 1587–1590. [Google Scholar] [CrossRef] [PubMed]

| Substance/Extract | Insect Genus/Species | Feeding * | Activity | EC50/LC50 ** | Ref. |
|---|---|---|---|---|---|
| (2S,4R)-4-hydroxy-1-methyl-2-pyrrolidine carboxylic acid | Liriomyza trifolii Burg. | C | inhibition of oviposition, deterrence | 3.7–16.0 µg/cm2 | [84] |
| 4-amin-1-β-D-ribofuranosyl-2(1H)-pirimidinone | |||||
| 4-aminobutanoic acid | |||||
| 7-O-β-D-apiofuranosyl-(1→2)-β-D-glucopyranoside | |||||
| 2-undecanone | Aphis craccivora Koch | C | increased adult mortality | 0.48 μmol/cm2 | [85] |
| 2-dodecanone | 0.32 μmol/cm2 | ||||
| 2-tridecanone | 0.22 μmol/cm2 | ||||
| 2-pentadecanone | 0.22 μmol/cm2 | ||||
| anabasine | Apis mellifera | O | antifeedance | 2-25 ppm | [86] |
| Spodoptera litura (Fabricius) | C | 60 µg/cm2 | [87] | ||
| Leptinotarsa decemlineata Say | 50 µg/cm2 | ||||
| atropine | Spodoptera litura (Fabricius) | 50 µg/cm2 | |||
| Leptinotarsa decemlineata Say | 7.38 µg/cm2 | ||||
| atropine | Lymantria dispar L. | T | antifeedance, deterrence | 4.39 nM | [88] |
| nicotine | 15.6 nM | ||||
| 28.3 nM | |||||
| scopolamine | Apis mellifera | O | deterrence | 0.03% | [89] |
| hyoscyamine | deterrence | 0.005% | |||
| lethality | 0.1% | ||||
| calystegine B4 (1α,2β,3α,4α-tetrahydroxy-nor-tropane) | Bombyx mori L., | T | midgut trehalase inhibition | 19 μM | [90] |
| Spodoptera litura | C | 40 μM | |||
| capsaicin | Coptotermes formosanus Shiraki | O | reduction of the number of microbes: Spirotrichonympha leidyi, Holomastigotoides hartmanni, Pseudotrichonympha grassii, and spirochetes present in the hindgut of a Formosan subterranean termite | 0.15–1 ppm | [91] |
| Tenebrio molitor L. | S | changes in behavioral thermoregulation | 10−7–10−4 M | [92] | |
| Myzus persicae (Sulz.) | C | increased efficiency of synthetic pesticide (neemix, pyronyl, m-pede) | 1–105 mg/L | [93] | |
| Leptinotarsa. decemlineata | C | increased metabolic rate, changes in the thermal preferences (preferring lower temperature) | 10−4–10−7 M | [94] | |
| chlorogenic acid, rutin, tomatine | Heliothis virescens F., Manduca sexta L., Pseudoplusia includes Walkler, Spodoptera frugiperda Smith | C | extended duration of molting | - | [95] |
| chlorogenic acid, rutin, tomatine | Podisus maculiventris | P | reduced development, weight and growth | 5–20 µmol/g of diet | [96] |
| laxumin A | Schizaphis graminum (Rondani) | C | decreased adult survival | 4.3 μM | [97] |
| laxumin B | 6.1 μM | ||||
| foliumin | 137 μM | ||||
| solanine | 138 μM | ||||
| chaconine | 137 μM | ||||
| tomatine | 7.3 μM | ||||
| leptine | Leptinotarsa decemlineata | C | reduced feeding | 8200 µg/g dry weight of leaf | [98] |
| leptine I | Leptinotarsa decemlineata | C | antifeedance, reduced neuronal responses to chemicals that stimulate feeding | 0.01–1 mM | [99] |
| luciamin | Schizaphis graminum | C | antifeedance, decreased adult survival | 50–500 μM | [100] |
| nicotine | aphids, whiteflies, leafhoppers, thrips and other (generally non-species specific) | - | mimicked acetylcholine and interacted with nicotinic acetylcholine receptors | - | [101] |
| Apis mellifera | O | decreased larval survival | 50 ppm | [102] | |
| deterrence, reduced survival | 3–1000 µM | [103] | |||
| increased food intake (at low concentrations), decrease food intake (at high concentrations) | 2–25 ppm | [86] | |||
| deterrence | 0.03% | [89] | |||
| lethality | 0.2% | ||||
| Cotesia congregata | P | reduced emergence, number of formed cocoons, | 0.025–0.1% | [54] | |
| Hyposoter annulipes | P | reduced emergence, number of formed cocoons, longer larval development, smaller adults | 0.025–0.1% | ||
| Manduca sexta | C | no lethal effect, decreased larval mass, | 0.1% of fresh diet | [104] | |
| Spodoptera exigua | C | lethality, decreased body mass | 0.1% of fresh diet | ||
| phytol (2E)-3,7,11,15-tetramethyl-2-hexadecen-1-ol | Liriomyza trifolii | C | oviposition deterrence | 0.1% of fresh diet | [84] |
| salpichrolide A | Musca domestica L. | O | antifeedance | 290 ppm | [105] |
| salpichrolide C | 310 ppm | ||||
| salpichrolide G | 203 ppm | ||||
| salpichrolide A, salpichrolide G | Tribolium castaneum (Herbst) | S | delay in development stage (from larva to adult) | - | [106] |
| serine protease inhibitors | Manduca sexta, Spodoptera littoralis (Fabricius) | C | inhibited digestive herbivore gut proteases | - | [107] |
| solamargine | Macrosiphum euphorbiae (Thom.) | C | deterrence, decreased reproduction rate | 50–500 μM | [108] |
| solamargine, solasonine | Manduca sexta | C | inactive | 1–3 μmol/g of diet | [21] |
| solamargine, solasonine, tomatine | Tribolium castaneum | S | inhibited larval growth | 1–3 μmol/g of diet | |
| solasodine | Macrosiphum. euphorbiae | C | deterrence, lag (delay) in appearance of new-born nymphs | 50–500 μM | [108] |
| Tribolium confusum | S | Malformations of all insect stages, decreased rate of pupations, inhibited metamorphosis, decreased adult survival | 0.1%–3.0% | [109] | |
| solasodine, tomatidine, tomatidenol | Tribolium castaneum | S | inactive | 1-3 μmol/g of diet | [21] |
| tomatidine | Macrosiphum. euphorbiae | C | deterrence and lethal to adults | 51.6 mg/L | [24] |
| solanidine | |||||
| α-tomatine | Manduca sexta | C | inhibition of larval growth | 50–500 μM | [21] |
| Hyposoter exiguae | P | prolonged larval development; disruption or prevention of pupal eclosion; morphological and anatomical malformations reduction in weight and longevity of adults | 12 μmol to 20 μmol/g of diet | [57] | |
| Phthorimaea operculella Zell. | C | negatively and significantly correlated with development rate (head capsule size) of larvae reared in the fruits | - | [110] | |
| Drosophila melanogaster | O | cytotoxic for cell line | 0.001-50 μM | [63] | |
| α-chaconine | Leptinotarsa decemlineata | C | no effects on survival, induced agitated and restless behavior | - | [24] |
| Plutella xylostella (L.) | C | ovicidal, highly toxic to deposited eggs | - | ||
| Myzus persicae | C | deterrence, mortality | - | ||
| Ceratitis capitata (Wied.) | C | decreased larval survival, lower pupal weights, extended pupation period, and increased period of adult emergence | - | ||
| Empoasca fabae Harr. | C | decreased nymph survival | - | ||
| α-chaconine | Myzus persicae | C | reduced fecundity and feeding of adults, reduced weight, increased mortality of nymphs | 0.1-1.6 mg/mL of diet | [111] |
| Pseudoplusia includes | C | lowered body weight, total weight gain, and larval survival, but not pupal weight | 18.1 μg/mg of insect | [25] | |
| α-solanine | 22.5 μg/mg of insect | ||||
| Myzus persicae | C | reduced fecundity and feeding of adults, reduced weight, increased mortality of nymphs | 0.1–1.6 mg/mL of diet | [111] | |
| α-tomatine | Heliothis zea (Boddie) | C | decreased food utilization, inhibition of larvae growth | 0.3 μmol/g of diet | [112] |
| Spodoptera exigua | C | no significant antifeedance | 1 μmol/g of diet | ||
| α-chaconine, α-solanine | Henosepilachna vigintioctomaculata Motsch. | C | stimulated feeding | - | [58] |
| Macrosiphum. euphorbiae | C | delayed the appearance and decreased the number of nymphs | - | [24] | |
| α-solanine | Spodoptera littoralis | C | no significant effects on midgut antioxidant defence system | 0.05%–0.1% in diet | [72] |
| Galleria mallonella (L.) | O | decreased survival of larvae, pupae and adults; decreased fecundity and fertility; increased malondialdehyde and protein carbonyl content in midgut and fat body of larvae; increased activity of midgut glutathione S-transferases and decreased activity of fat body glutathione S-transferases | 0.15–15 μg/g of diet | [23] | |
| increased mortality of larvae, pupae and adults; disturbance of fecundity and fertility; generation of oxidative stress; decrease in glutathione S-transferases enzymatic activity in fat body | 3.1 mg/g of diet | [69] | |||
| α-solanine | Tribolium castaneum | S | acute toxicity (high mortality) | 64.8 μg/cm2 | [113] |
| α-chaconine | 76.4 μg/cm2 | ||||
| α-tomatine | 118.0 μg/cm2 | ||||
| α-solanine | Zophobas atratus | O | decreased heart activity in pupae and adults | 10−6–10−3 M | [60] |
| α-chaconine | |||||
| α-tomatine | |||||
| solamargine | |||||
| solasonine |
| Substance/Extract | Insect Genus/Species | Feeding * | Activity | EC50/LC50 ** | Ref. |
|---|---|---|---|---|---|
| Capsicum annuum L. leaf extract | Frankliniella occidentalis (Pergande) | C, T | larvicidal, interruption of next stage development, decreased efficiency of hatched eggs | - | [114] |
| Liriomyza trifolii | C | ovipositional deterrence | 0.15–147 μg/cm2 | [115] | |
| Spodoptera litura | C | antifeedance, interfered with the molting process and caused morphological abnormalities | 0.5–5 mg/cm2 | [116] | |
| Achaea janata (L.) | C, T | ||||
| Sitophilus oryzae (L.) | S | increased adult mortality, deterrence | 1.06 mg/g of diet | [117] | |
| Tribolium castaneum | S | 1.24 mg/g of diet | |||
| C. annuum fruit extract | Attagenus unicolor japonicas Reitter | O | weak antifeedance | 1.3–5.2 mg/cm2 | [118] |
| Capsicum frutescens L. leaf extract | Plutella xylostella, | C | antifeedance, deterrence, reduced infestation | Cp 3% | [119] |
| Brevicoryne brassicae (L.) | C | ||||
| Cestrum diurnum L. leaf extract | Anopheles stephensi Sweet & Rao | O | larvicidal | 0.70% | [120] |
| Culex quinquefasciatus Say | O | 0.29% | [121] | ||
| Cestrum nocturnum L. root and leaf extract | Aedes aegypti (L.) | O | larvicidal, inhibition of pupal development and adult emergence | 15.4 ppm | [122] |
| Cestrum parqui L’Her. leaf extract | Spodoptera litoralis (Fabricius) | C | larval morality caused exuviation difficulties, molting disorders, malformations, oviposition inhibition | 2%–32% in diet | [123] |
| Ceratitis capitata | C | toxicity to neonate larvae when ingested through diet, inhibited or delayed larval development and reduced the percentages of pupae formed and adult emergence, diminished adult reproductive potential | 0.9% | [124] | |
| Datura metel L. leaf extract | Coptotermes formosanus | O | larval mortality | 298 ppm | [125] |
| Helicoverpa armigera (Hubner) | C | larval mortality, growth inhibition of the larvae, antifeedance | 5.99 μg/cm2 | [126] | |
| Anopheles subpictus Gr. | O | larvicidal | 2.11 mg/mL | [127] | |
| Culex quinquefasciatus | O | - | [128] | ||
| Datura stramonium L. leaf extract | Tribolium castaneum, | S | antifeedance, contact toxicity | 3936 mg/L | [129] |
| Corcyra cephalonica (Stainton) | S | - | |||
| Callosobruchus maculatus (F.), | S | inhibition of intestinal α-amylase activity | 0.125–2.0 mg/mL | [130] | |
| Rhyzopertha dominica (F.) | S | ||||
| Sitophilus granaries (L.) | S | ||||
| T. granarium | S, O | ||||
| Lycium cestroides Schltdl. leaf extract | Myzus persicae, | C | antifeedance, settling inhibition, contact toxicity | 0.1 mg/cm2 | [131] |
| Rhopalosiphum padi (L.), | C | ||||
| Epilachna paenulata (Germ.), | C | ||||
| Spodoptera littoralis | C | ||||
| Lycopersicum esculentum Mill. leaf extract | Zophobas atratus | O | decreased heart activity in pupae and adults | 10−6–10−3 M | [60] |
| Spodoptera litura | C | interfered with molting process and caused morphological abnormalities | 3.60 mg/cm2 | [116] | |
| Achaea janata | C, T | 3.81 mg/cm2 | |||
| Nicotiana tabacum L. leaf extract | Plutella xylostella | C | antifeedance, deterrence | - | [119] |
| Brevicoryne brassicae | C | ||||
| Nicotiana plumbaginifolia Viv. leaf extract | Anopheles stephensi Liston | O | larvicidal | 15.49 ppm | [132] |
| Solanum sisymbriifolium Lam. fruit extract | Anopheles gambiae Giles | O | larvicidal | 0.75 g/mL | [133] |
| Anopheles funestus Leeson | O | 0.75 g/mL | |||
| Solanum elaeagnifolium Cav. plant extract | Blattella germanica | O | repellence, antifeedance | 50-150 mg/mL | [26] |
| Solanum incanum L. plant extract | Amitermes messinae Full. | O | increased adult mortality | - | [134] |
| Microtermes najdensis Harris | O | - | |||
| Solanum jasminoides plant extract | Phlebotomus papatasi Harris | O | repellence, larvicidal | - | [135] |
| Solanum melongena L. fruit extract | Spodoptera litura | C | antifeedance, inhibited larval growth, interfered with molting process and caused morphological abnormalities, inhibited intestinal serine protease activity n | 0.5–5 mg/cm2 | [116] |
| Achaea janata | C, T | ||||
| S. melongena leaf extract | Sitophilus oryzae, | S | increased adult mortality, deterrence | 0.05–0.5 mg/cm2 | [117] |
| Tribolium castaneum | S | ||||
| Solanum nigrum L. leaf extract | Culex quinquefasciatus | O | increased larvae morality | 44.97 ppm | [136] |
| Anopheles culicifacies Christophers | O | larvicidal | 208.5 ppm | [137] | |
| Culex quinquefasciatus | O | 337.2 ppm | |||
| Aedes aegypti | O | 359.0 ppm | |||
| Aedes caspius (Pallas) | O | 51.29 mg/L | [138] | ||
| Culex pipiens (L.) | O | 125.89 mg/L | |||
| S. nigrum leaf and green berry extract | Culex quinquefasciatus | O | increased first instar larvae morality and also in second and third instar | 2.62 ppm | [139] |
| Anopheles stephensi | O | 2.12 ppm | |||
| S. nigrum plant extract | Leptinotarsa decemlineata | C | acute toxicity | Cp 40% | [140] |
| Solanum pseudocapsicum L. leaf and seed extracts | Agrotis ipsilon (Hufnagel) | C | antifeedance, larvicidal, deformations in next instar larvae, in pupae and adults (after larvae treatment) | 0.625%–5% | [141] |
| S. pseudocapsicum. seed extracts | Helicoverpa armigera | C | antifeedance, malformations | 0.625–5 mg/L | [142] |
| S. pseudocapsicum seed extracts | Spodoptera litura | C | |||
| Solanum suratense Burm. plant extract | Callosobruchus chinensis (L.) | S | inhibition of oviposition | 1%–10% | [143] |
| Solanum suratense Brum. leaf extract | Culex quinquefasciatus | O | larvicidal, disrupted molting and metamorphosis, induced malformation, extended larval duration and inhibited adult emergence | 23.53% | [144] |
| Solanum torvum Sw. leaf extract | Anopheles stephensi | O | larvicidal | 29.65 ppm | [145] |
| Culex quinquefasciatus | O | 20.56 ppm | |||
| Solanum trilobatum L. leaf extract | Aedes aegypti | O | larvicidal, reduced pupation ratio | 125.43 ppm | [146] |
| Anopheles stephensi | O | 127.77 ppm | |||
| Culex quinquefasciatus | O | 116.64 ppm | |||
| Hippobosca maculate (L.) | O | increased adult mortality | 432.77 ppm | [147] | |
| Solanum tuberosum L. vegetable waste extract | Culex quinquefasciatus | O | larvicidal | 1.30% | [148] |
| Anopheles stephensi | O | 1.18% | |||
| S. tuberosum leaf extract | Zophobas atratus (Fab.) | O | in vivo cardioinhibitory activity in pupae | 10−6–10−3 M | [60] |
| in vitro cardioinhibitory activity in adults | 10−7–10−1 M | [59] | |||
| T. molitor | S | non-effect on heart of adult beetle | 10−7–10−1 M | [59] | |
| Leptinotarsa decemlineata | C | increased mortality of larvae, pupae and adults; disturbance of fecundity and fertility; generation of oxidative stress; decreased GST enzymaticactivity in fat body | 1 mg/g of diet | [69] | |
| Spodoptera exigua | O | ||||
| Solanum verbasicum leaf extract | Culex quinquefasciatus | O | larvicidal | 100–1000 ppm | [149] |
| Solanum villosum Mill. fruit extract | Aedes aegypti (L.) | O | larvicidal | 0.1%–0.5% | [150] |
| Culex quinquefasciatus | O | larvicidal (inhibited larvae growth and pupation) | 321.89 ppm | [151] | |
| Anopheles subpictus | O | larvicidal | 24.20 ppm | [152] | |
| Solanum xanthocarpum Schrad. fruit extract | Aedes aegypti (L.) | O | larvicidal and pupacidal activity | 170.91 ppm | [153] |
| S. xanthocarpum shoot extract | Culex quinquefasciatus | O | contact toxicity, larvicidal and pupacidal activity | 155.29 ppm | [154] |
| Withania somnifera (L.) Dunal leaf extract | Spodoptera litoralis | C | toxicity, molt disturbances, formation of larval–pupal, pupal–adult intermediates and adultoids | 50–100 µg/insect | [155] |
| Withania somnifera plant extract | Culex pipiens | O | Reduced hatching, pupation, larvicidal activity | 132.6 ppm | [156] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowański, S.; Adamski, Z.; Marciniak, P.; Rosiński, G.; Büyükgüzel, E.; Büyükgüzel, K.; Falabella, P.; Scrano, L.; Ventrella, E.; Lelario, F.; et al. A Review of Bioinsecticidal Activity of Solanaceae Alkaloids. Toxins 2016, 8, 60. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins8030060
Chowański S, Adamski Z, Marciniak P, Rosiński G, Büyükgüzel E, Büyükgüzel K, Falabella P, Scrano L, Ventrella E, Lelario F, et al. A Review of Bioinsecticidal Activity of Solanaceae Alkaloids. Toxins. 2016; 8(3):60. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins8030060
Chicago/Turabian StyleChowański, Szymon, Zbigniew Adamski, Paweł Marciniak, Grzegorz Rosiński, Ender Büyükgüzel, Kemal Büyükgüzel, Patrizia Falabella, Laura Scrano, Emanuela Ventrella, Filomena Lelario, and et al. 2016. "A Review of Bioinsecticidal Activity of Solanaceae Alkaloids" Toxins 8, no. 3: 60. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins8030060