1. Introduction
Ipomoea carnea (
I. carnea) is a shrubby plant in the Convolvulaceae family which originated in tropical America and the Caribbean, but is now found in tropical and subtropical areas worldwide including South and North America, Africa, Australia, and Asia [
1,
2].
Livestock that chronically ingest this plant suffer from extensive losses including death, abortion, and other reproductive and physiological dysfunctions, that have been reported in different countries such as Brazil [
3,
4,
5,
6], Peru [
7], and Mozambique [
8]. The intoxication typically occurs during the dry season when forage is scarce; cattle, sheep, and goats are the primary affected species [
2,
5].
The toxins in
I. carnea are the indolizidine alkaloid swainsonine and the nortropane alkaloids calystegines. Swainsonine has been demonstrated to be produced not by the plant, but by fungi living in or on the plants. Swainsonine, but not calystegines, are produced by fungi in the order Chaetothyriales that live epiphytically with
I. carnea [
9]. Swainsonine is a powerful inhibitor of lysosomal α-mannosidase and Golgi mannosidase II [
8,
10,
11,
12]. Swainsonine has also been isolated from other plants, such as
Swainsona canescens in Australia [
13],
Astragalus and
Oxytropis species, often termed “locoweeds” in North America [
14], and
Sida carpinifolia and
Turbina cordata in Brazil [
6]. Calystegines inhibit lysosomal α-galactosidase and β-glycosidase [
15], and we verified in rats, that these alkaloids potentiate the lesions produced by swansonine [
16]; however, it is unknown how the nortropane alkaloids contribute to the toxicity of
I. carnea, particularly in a ruminant model.
The inhibition of lysosomal α-mannosidase results in lysosomal accumulation of incompletely processed oligosaccharides, vacuolation, and cellular death [
17]. In goats such damage is especially severe in the CNS [
3,
8,
18,
19,
20,
21]. This vacuolar degeneration can be seen in other organs such as the thyroid, liver, pancreas, and kidneys [
22,
23].
Swainsonine’s inhibition of Golgi mannosidase II leads to changes in the synthesis, processing, and transport of glycoproteins, which causes dysfunction in hormones and membrane receptors [
24] and leads to alterations in endocrine [
25,
26,
27] and reproductive functions [
27,
28].
In goats, the signs of
I. carnea intoxication are mostly of nervous origin; clinical signs include depression, a staggering gait, muscle tremors, ataxia, nervousness, and weight loss [
5,
21,
23,
29]. Also, we verified that the administration of 7.5 g/kg BW of
I. carnea leaves to pregnant goats results in fetal death, abortion, stillbirths, cytoplasmic vacuolation in the fetal CNS, and structural and functional changes in the offspring [
21].
Many studies have shown the teratogenic effects of pesticide residues in ruminants [
30], as well as teratogenicity from toxins such as cyanide [
31,
32,
33] and some classes of alkaloids [
21,
34,
35,
36,
37,
38]. The evaluation of the teratogenic potential of toxicants in ruminants has the potential to improve animal production and thus provide numerous economic benefits to livestock producers [
38]. Protocols for developmental toxicology assessment established by regulatory agencies have received increasing importance and attention. It is known that changes in the fetus may be both structural and functional and caused at any time between fertilization and post-natal maturation [
39]. Thus, these protocols are becoming more refined and specific, detecting more subtle changes that previously went unnoticed by classical protocols, such as bone and visceral evaluations [
40]. In this sense, we have sought to refine an earlier proposed model for toxicity risk assessment in ruminants that had been developed in this laboratory [
21,
29,
32,
41]. The objective of this study was to assess the behavior of the offspring from mothers treated with
I. carnea during pregnancy in order to refine current knowledge about neonate responses.
3. Discussion
Swainsonine, but not calystegines, are produced by fungi that live epiphytically with
I. carnea.
Alternaria subs.
Undifilum produces swainsonine while living endophytically in
Swainsona canescens [
42],
Astragalus sp. [
43], and
Oxytropis sp. [
44]. Feeding rats with
Alternaria subs.
Undifilum induced clinical signs of locoism [
45]. The contribution of calystegines, if any, to the toxicity of
I. carnea has not been well established [
16]. There is no evidence that
I. carnea contains ergot-type alkaloids, nor the encoding genes for
Periglandula fungi often present in Convolvulaceous plants (D. Cook, personal communication).
Studies on the teratogenic effects of plant toxins and understanding the relationship between chemical structure and activity may serve as a model for biomedical research and accelerate the discovery of new techniques and products for treating human and animal diseases [
38]. Studies have shown the teratogenic effects of different alkaloids both for humans [
46] and for animals [
38]. Recently Welch
et al. [
47] showed that anabasine, an alkaloid from
Nicotiana glauca’ is a potent teratogenic compound for goats, causing skeletal contracture malformations and cleft palate. Similarly, our studies verified that kids whose mothers ingested
I. carnea during pregnancy may have structural changes such as arthrogryposis and retrognathia, and functional alterations such as cytoplasmic vacuolation, primarily in the CNS, but also in the liver and kidneys caused by the indolizidine alkaloid swainsonine [
21]. Therefore, to better understand the consequences of vacuolar degeneration in the CNS of neonates, we decided to evaluate maternal and neonate behavior.
A prior study conducted in our laboratory showed that pregnant goats that ingested leaves of
I. carnea at a dose of 7.5 g/kg BW had neurological signs of intoxication and also early fetal death, stillbirths, or abortion [
21]. Therefore, in this study we decided to use lower daily doses of plant (1, 3, and 5 g/kg BW leaves of
I. carnea), aiming to reduce the toxic effects of the plant on mothers and the possible indirect effect on the fetus [
48]. Clinical evaluation revealed that we succeeded in this goal since pregnant females from different groups did not show any clinical signs of poisoning by
I. carnea, and the body weight gain of pregnant females from experimental groups was similar to the control group. The correctness of the doses used was also shown by the fact that we did not observe any malformations or low birth weight in kids from any experimental groups.
Although no females showed overt clinical signs of poisoning from
I. carnea, one goat from the IC3 group and one goat from the IC5 group aborted in late pregnancy. Other causes of abortion in goats, such as leptospirosis, brucellosis, mycoplasmosis, and toxoplasmosis were negative. Swainsonine has been shown to be an abortifacient compound in previous work with
I. carnea [
49] and locoweeds [
35,
50].
Behavioral results indicate that goats fed the higher doses of
I. carnea (from IC3 and IC5 groups) spent less time paying attention to the newborn in the two hours after birth, as they sniffed and licked the kid with less frequency than did controls. Maternal behavior is a critical variable that influences the survival of the neonate [
51], particularly under extensive grazing conditions. The maternal behaviors of sniffing and licking the kid immediately after birth are crucial for clearing the head and nostrils, thus stimulating respiration [
51,
52], blood circulation [
53], and inducing the neonate to stand up and suckle [
54]. Further, such actions aid in the formation of an exclusive olfactory memory in the mother for her own neonates [
55]. Maternal inattention to the newborn after birth may result in the loss of maternal-infant bonding that is formed immediately after birth that allows mutual recognition during lactation [
56,
57]. Therefore, the decrease in maternal care observed in this study may result in a high neonatal mortality. Similarly, Pfister
et al. [
36] showed that fetal maternal-infant bonding in ewes can be impaired by ingestion of locoweed during pregnancy such that subsequent lamb survival depended on human intervention.
An important result in this study was the observation of low vigor in kids from mothers of the different groups treated with
I. carnea. Offspring vigor influences the survival of the neonate [
51] and may be responsible for the lack of bonding [
58]. After birth, lambs pass through behavioral events directed towards standing, udder seeking, and sucking [
55]. We verified that in the two hours after birth, kids from intoxicated mothers took longer to attempt to stand up and to stand, consequently they were much less mobile than were control kids. Standing and subsequent movement while teat-seeking reduces heat loss to the ground, so helping the lamb to maintain body temperature, and provides the easiest way to find the udder [
55]. In fact, we observed that the number of kids able to suckle in two hours after birth decreased in a dose-dependent manner (control, 9/9; IC1, 5/6; IC3, 4/6; and IC5, 3/8).
Suckling quickly provides essential nutrients, particularly to sustain thermoregulation, and immunoglobulins to provide passive immunity [
55,
59]. In this sense, the faster the lamb accomplishes neonatal behaviors like standing and suckling, the higher the probability of survival [
60]. These observations indicate that the alterations in behavior are potentially life-threatening to kids, as kids from treated dams were generally not able to stand, or were very slow to stand and suckle normally.
The high frequency with which kids from control group walked toward their dams in the first two hours postpartum was an excellent indicator of the kids’ ability to stand, walk, and suckle their dams. The higher vigor and desire to suckle in control kids during the first hour of life were confirmed by the increased frequency with which they nuzzled their dams front and rear halves and then suckled effectively. In the present study, we verified that control kids spent much of their time resting after suckling, during the final hour of observation; therefore major differences in behavior among control kids and kids from treated dams were not observed in this period.
Gotardo
et al. [
21] showed that the lesions in the CNS of kids whose mothers ate
I. carnea during gestation were characterized by vacuolization and loss of Purkinje cells. Therefore, it can be inferred that the changes observed in kids from treated dams during the first two hours of life are directly related to impaired CNS functions from maternal exposure and fetotoxicity [
61,
62].
Kids from treated dams that were not able to suckle were given human assistance to suckle at the conclusion of the first portion of the study. For this, their dams were restrained by hand, and the kid was attached to the teat, and all suckled at that point. Thus, the treated kids, although typically low in vigor, all survived and were available to participate in the next tests(i.e., Dam-alien goat discrimination test, kids tests of movements, and Hebb-Williams mazes).
The results of the dam-alien goat discrimination test and kid tests of movement confirmed the hypothesis of disrupted maternal-infant bonding in treated animals. Thus, these tests demonstrated the low vigor of newborns exposed in utero to I. carnea. Hence, kids from treated mothers were slower to traverse a maze to reach their dams, slower to reach their mothers around a barrier and were also unable to discriminate their dam from an alien goat at 12 h postpartum.
Goats have determinant behaviors for the survival of the newborn, and are an example of “hider” behavior at parturition [
63]. Mothers and their young spent most of their time apart from one another during the infant’s first 6–8 weeks following birth [
64]. So while the mothers graze, their kid(s) are hidden waiting for their return. This strategy is used to avoid predators; however, the success of this procedure depends on the kid’s ability to discriminate their mother, not confusing, for example, the approach of their dam with a predator. Furthermore, if a kid approaches the wrong dam, the alien dam often butts the young goat with considerable force [
65] which could cause serious injury in weak kids and further complicate their ability to survive.
There are many procedures that can be used to evaluate learning in different animal species [
66], but few studies have examined the influence of plant toxins on learning in livestock. However, such work has been done in horses [
67], sheep [
68], and cattle [
69]. Lee
et al. [
68] showed that sheep under the effect of scopolamine had learning deficits in maze tests. Other studies with mazes showed cognition changes in sheep after exposure to toxicants [
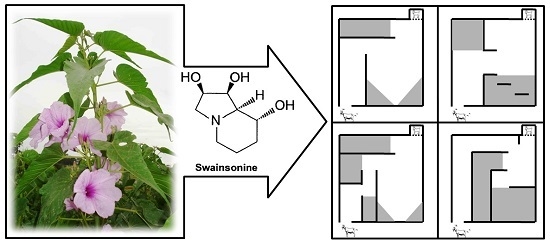
70]. Therefore, we proposed in this study to perform the assessment of learning using basically four mazes or closed fields (“Hebb-Williams A, B, C and D”), as proposed by Hebb-Williams [
71] in which kids were tested at two, four, and six weeks of age.
Data from evaluations with Hebb-Williams mazes showed that kids from experimental groups showed a higher latency time to complete the different mazes until the sixth week of life, and goats from the IC5 group committed a greater number of errors in two mazes. Therefore, kids exposed to the
I. carnea during pregnancy appeared to have reduced spatial awareness when in the maze and were less able to learn how to complete the maze. Sheep tested in mazes can retain spatial memories for many days [
68], and control kids in our study also appeared to retain memories of the maze since their performance improved every week in all mazes; however, we did not observe this response so clearly in the performances of kids from groups of
I. carnea-treated mothers. This result is similar to a study with sheep and swainsonine which showed that lambs exposed in utero did not improve their maze performance over the course of several days postpartum [
36]. Under field conditions these results may have serious implications for growth and development. Kids from treated dams may be handicapped in their ability to learn from their dam and cohorts about foraging [
72], location of food and water, and other aspects important for survival [
73].
Little work has been done to characterize the effect of other swainsonine-containing plants such as locoweeds in goats. Furlan
et al. [
74] used a high swainsonine dose of 8 mg/kg BW from
Astragalus lentiginosus, and pregnant goats became severely intoxicated in 10 days. Fetal death was observed in all locoweed-treated goats between 10 and 20 days after the beginning of locoweed treatment. Stegelmeier (unpublished personal communication) observed clinical signs of locoweed poisoning using a swainsonine dose of 1.5 mg/kg BW from
Astragalus lentiginosus dosed for 45 days to Spanish goats. A dose of 1.5 mg/kg BW from
Astragalus lentiginosus is higher than the highest swainsonine dose (1.35 mg/kg BW) used in this
I. carnea study. Further studies are needed to characterize the relative contribution, if any, of calystegines to intoxication in goats from
I. carnea compared to swainsonine alone [
75]. In a rat model, calystegines alone added little to the neurotoxic syndrome [
16], but rats are not a good model for glycosidase inhibitors. Studies are ongoing to compare the clinical signs and histopathology of swainsonine-containing locoweeds (e.g.,
Astragalus lentiginosus that contain no calystegines) and
I. carnea at equal doses of swainsonine in a goat model.