Clostridium difficile Toxins A and B: Insights into Pathogenic Properties and Extraintestinal Effects
Abstract
:1. Introduction
2. Clostridium difficile Toxins
2.1. Regulation of Expression and Secretion of TcdA and TcdB
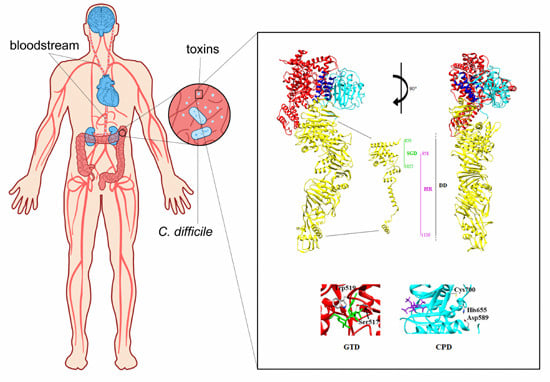
2.2. Structure of TcdA and TcdB
2.2.1. The GTD Domain (A Domain)
2.2.2. The Receptor Binding Domain (B Domain)
2.2.3. The Cysteine Protease Domain (C Domain)
2.2.4. The Hydrophobic Region (the D Domain)
3. Mechanisms of Action of TcdA and TcdB
3.1. Toxins Binding to the Cellular Surface and Endocytosis
3.2. Pore Formation and Translocation
3.3. Rho Proteins Inactivation
3.3.1. Cytopathic Effects
3.3.2. Cytotoxic Effects
Induction of the Programmed Cell Death
Activation of the Inflammasome
4. Is TcdB the Main Actor of C. difficile-Induced Cytotoxicity? Evidence for a Major Pathogenetic Role for TcdB
5. Can Toxin-Binding Agents Have a Role in Reducing Pathogenicity?
6. Are Severe Clinical Manifestations Associated to the Amount of Toxemia?
7. Extraintestinal Organ Damage and “Undervalued” Potential Clinical Implications
7.1. Heart
7.2. Kidneys
7.3. Brain
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.G.; Gerding, D.N. Clinical recognition and diagnosis of Clostridium difficile infection. Clin. Infect. Dis. 2008, 46, S12–S18. [Google Scholar] [CrossRef] [PubMed]
- Redelings, M.D.; Sorvillo, F.; Mascola, L. Increase in Clostridium difficile-related mortality rates, United States, 1999–2004. Emerg. Infect. Dis. 2007, 13, 1417–1419. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.A.; Heap, J.T.; Minton, N.P. Clostridium difficile spore germination: An update. Res. Microbiol. 2010, 161, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Anthony, D.M.; Reynolds, T.; Paton, J.; Rafter, L. Serum albumin in risk assessment for Clostridium difficile. J. Hosp. Infect. 2009, 71, 378–379. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Louie, T.; Mullane, K.; Weiss, K.; Lentnek, A.; Golan, Y.; Kean, Y.; Sears, P. Derivation and validation of a simple clinical bedside score (ATLAS) for Clostridium difficile infection which predicts response to therapy. BMC Infect. Dis. 2013, 13, 148. [Google Scholar] [CrossRef] [PubMed]
- Islam, J.; Taylor, A.L.; Rao, K.; Huffnagle, G.; Young, V.B.; Rajkumar, C.; Cohen, J.; Papatheodorou, P.; Aronoff, D.M.; Llewelyn, M.J. The role of the humoral immune response to Clostridium difficile toxins A and B in susceptibility to Clostridium difficile infection: A case-control study. Anaerobe 2014, 27, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, S.; Friedrich, A.W.; Garcia-Almodovar, E.; Gallone, M.S.; Taglietti, F.; Topino, S.; Galati, V.; Johnson, E.; D’Arezzo, S.; Petrosillo, N. Clostridium difficile infection among hospitalized HIV-infected individuals: Epidemiology and risk factors: Results from a case-control study (2002–2013). BMC Infect. Dis. 2015, 15, 194. [Google Scholar] [CrossRef] [PubMed]
- Leffler, D.A.; Lamont, J.T. Clostridium difficile infection. N. Engl. J. Med. 2015, 373, 287–288. [Google Scholar] [PubMed]
- Vardakas, K.Z.; Polyzos, K.A.; Patouni, K.; Rafailidis, P.I.; Samonis, G.; Falagas, M.E. Treatment failure and recurrence of Clostridium difficile infection following treatment with vancomycin or metronidazole: A systematic review of the evidence. Int. J. Antimicrob. Agents 2012, 40, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bakken, J.S.; Borody, T.; Brandt, L.J.; Brill, J.V.; Demarco, D.C.; Franzos, M.A.; Kelly, C.; Khoruts, A.; Louie, T.; Martinelli, L.P.; et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin. Gastroenterol. Hepatol. 2011, 9, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Warny, M.; Pepin, J.; Fang, A.; Killgore, G.; Thompson, A.; Brazier, J.; Frost, E.; McDonald, L.C. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 2005, 366, 1079–1084. [Google Scholar] [CrossRef]
- Rupnik, M.; Wilcox, M.H.; Gerding, D.N. Clostridium difficile infection: New developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 2009, 7, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Quesada-Gomez, C.; Lopez-Urena, D.; Chumbler, N.; Kroh, H.K.; Castro-Pena, C.; Rodriguez, C.; Orozco-Aguilar, J.; Gonzalez-Camacho, S.; Rucavado, A.; Guzman-Verri, C.; et al. Analysis of TcdB proteins within the hypervirulent clade 2 reveals an impact of RhoA glucosylation on Clostridium difficile proinflammatory activities. Infect. Immun. 2016, 84, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Carman, R.J.; Stevens, A.L.; Lyerly, M.W.; Hiltonsmith, M.F.; Stiles, B.G.; Wilkins, T.D. Clostridium difficile binary toxin (CDT) and diarrhea. Anaerobe 2011, 17, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Jank, T.; Belyi, Y.; Aktories, K. Bacterial glycosyltransferase toxins. Cell. Microbiol. 2015, 17, 1752–1765. [Google Scholar] [CrossRef] [PubMed]
- Jank, T.; Giesemann, T.; Aktories, K. Rho-glucosylating Clostridium difficile toxins A and B: New insights into structure and function. Glycobiology 2007, 17, 15R–22R. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, R.N.; Lacy, D.B. Toward a structural understanding of Clostridium difficile toxins A and B. Front. Cell. Infect. Microbiol. 2012, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Gerding, D.N.; Johnson, S.; Rupnik, M.; Aktories, K. Clostridium difficile binary toxin CDT: Mechanism, epidemiology, and potential clinical importance. Gut Microbes 2014, 5, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Knapp, O.; Benz, R.; Popoff, M.R. Pore-forming activity of clostridial binary toxins. Biochim. Biophys. Acta 2016, 1858, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Braun, V.; Hundsberger, T.; Leukel, P.; Sauerborn, M.; von Eichel-Streiber, C. Definition of the single integration site of the pathogenicity locus in Clostridium difficile. Gene 1996, 181, 29–38. [Google Scholar] [CrossRef]
- Hammond, G.A.; Johnson, J.L. The toxigenic element of Clostridium difficile strain CPI 10463. Microb. Pathog. 1995, 19, 203–213. [Google Scholar] [CrossRef]
- Hundsberger, T.; Braun, V.; Weidmann, M.; Leukel, P.; Sauerborn, M.; von Eichel-Streiber, C. Transcription analysis of the genes tcda-e of the pathogenicity locus of Clostridium difficile. Eur. J. Biochem. 1997, 244, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Mani, N.; Dupuy, B. Regulation of toxin synthesis in Clostridium difficile by an alternative RNA polymerase sigma factor. Proc. Natl. Acad. Sci. USA 2001, 98, 5844–5849. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.S.; Wee, B.Y.; Song, K.P. Evidence for holin function of tcde gene in the pathogenicity of Clostridium difficile. J. Med. Microbiol. 2001, 50, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Govind, R.; Dupuy, B. Secretion of Clostridium difficile toxins A and B requires the holin-like protein TcdE. PLoS Pathog. 2012, 8, e1002727. [Google Scholar] [CrossRef] [PubMed]
- Olling, A.; Seehase, S.; Minton, N.P.; Tatge, H.; Schroter, S.; Kohlscheen, S.; Pich, A.; Just, I.; Gerhard, R. Release of TcdA and TcdB from Clostridium difficile cdi 630 is not affected by functional inactivation of the tcdE gene. Microb. Pathog. 2012, 52, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Govind, R.; Fitzwater, L.; Nichols, R. Observations on the role of TcdE isoforms in Clostridium difficile toxin secretion. J. Bacteriol. 2015, 197, 2600–2609. [Google Scholar] [CrossRef] [PubMed]
- Matamouros, S.; England, P.; Dupuy, B. Clostridium difficile toxin expression is inhibited by the novel regulator TcdC. Mol. Microbiol. 2007, 64, 1274–1288. [Google Scholar] [CrossRef] [PubMed]
- Cartman, S.T.; Kelly, M.L.; Heeg, D.; Heap, J.T.; Minton, N.P. Precise manipulation of the Clostridium difficile chromosome reveals a lack of association between the TcdC genotype and toxin production. Appl. Environ. Microbiol. 2012, 78, 4683–4690. [Google Scholar] [CrossRef] [PubMed]
- Bakker, D.; Smits, W.K.; Kuijper, E.J.; Corver, J. TcdC does not significantly repress toxin expression in Clostridium difficile 630ΔErm. PLoS ONE 2012, 7, e43247. [Google Scholar] [CrossRef] [PubMed]
- Just, I.; Selzer, J.; Wilm, M.; von Eichel-Streiber, C.; Mann, M.; Aktories, K. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature 1995, 375, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, F.; Busch, C.; Prepens, U.; Just, I.; Aktories, K. Localization of the glucosyltransferase activity of Clostridium difficile toxin B to the N-terminal part of the holotoxin. J. Biol. Chem. 1997, 272, 11074–11078. [Google Scholar] [PubMed]
- Chumbler, N.M.; Rutherford, S.A.; Zhang, Z.; Farrow, M.A.; Lisher, J.P.; Farquhar, E.; Giedroc, D.P.; Spiller, B.; Melnyk, R.A.; Lacy, D.B. Crystal structure of Clostridium difficile toxin A. Nat. Microbiol. 2016, 1, 1–6. [Google Scholar] [CrossRef]
- Genisyuerek, S.; Papatheodorou, P.; Guttenberg, G.; Schubert, R.; Benz, R.; Aktories, K. Structural determinants for membrane insertion, pore formation and translocation of Clostridium difficile toxin B. Mol. Microbiol. 2011, 79, 1643–1654. [Google Scholar] [CrossRef] [PubMed]
- Von Eichel-Streiber, C.; Sauerborn, M.; Kuramitsu, H.K. Evidence for a modular structure of the homologous repetitive C-terminal carbohydrate-binding sites of Clostridium difficile toxins and Streptococcus mutans glucosyltransferases. J. Bacteriol. 1992, 174, 6707–6710. [Google Scholar] [PubMed]
- Manse, J.S.; Baldwin, M.R. Binding and entry of Clostridium difficile toxin B is mediated by multiple domains. FEBS Lett. 2015, 589, 3945–3951. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, R.N.; Chagot, B.; Cover, M.; Chazin, W.J.; Spiller, B.; Lacy, D.B. Structure-function analysis of inositol hexakisphosphate-induced autoprocessing in Clostridium difficile toxin A. J. Biol. Chem. 2009, 284, 21934–21940. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Elliott, B.; Chang, B.J.; Golledge, C.L.; Riley, T.V. Clostridium difficile-associated diarrhoea. Intern. Med. J. 2007, 37, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, M.S.; Roberts, A.P.; Hussain, H.; Williams, R.J.; Allan, E.; Mullany, P. Horizontal gene transfer converts non-toxigenic Clostridium difficile strains into toxin producers. Nat. Commun. 2013, 4, 2601. [Google Scholar] [CrossRef] [PubMed]
- Dingle, K.E.; Elliott, B.; Robinson, E.; Griffiths, D.; Eyre, D.W.; Stoesser, N.; Vaughan, A.; Golubchik, T.; Fawley, W.N.; Wilcox, M.H.; et al. Evolutionary history of the Clostridium difficile pathogenicity locus. Genome Biol. Evol. 2014, 6, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Onderdonk, A.B.; Johnston, J.; Mayhew, J.W.; Gorbach, S.L. Effect of dissolved oxygen and Eh and Bacteroides fragilis during continuous culture. Appl. Environ. Microbiol. 1976, 31, 168–172. [Google Scholar] [PubMed]
- Yamakawa, K.; Karasawa, T.; Ikoma, S.; Nakamura, S. Enhancement of Clostridium difficile toxin production in biotin-limited conditions. J. Med. Microbiol. 1996, 44, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, S.; Dupuy, B.; Mukherjee, K.; Norin, E.; Burman, L.G.; Akerlund, T. Expression of Clostridium difficile toxins A and B and their sigma factor TcdD is controlled by temperature. Infect. Immun. 2003, 71, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Bouillaut, L.; Dubois, T.; Sonenshein, A.L.; Dupuy, B. Integration of metabolism and virulence in Clostridium difficile. Res. Microbiol. 2015, 166, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, B.; Sonenshein, A.L. Regulated transcription of Clostridium difficile toxin genes. Mol. Microbiol. 1998, 27, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, S.; Lindberg, A.; Norin, E.; Burman, L.G.; Akerlund, T. Toxins, butyric acid, and other short-chain fatty acids are coordinately expressed and down-regulated by cysteine in Clostridium difficile. Infect. Immun. 2000, 68, 5881–5888. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, T.; Maegawa, T.; Nojiri, T.; Yamakawa, K.; Nakamura, S. Effect of arginine on toxin production by Clostridium difficile in defined medium. Microbiol. Immunol. 1997, 41, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, S.; Burman, L.G.; Akerlund, T. Suppression of toxin production in Clostridium difficile VPI 10463 by amino acids. Microbiology 1999, 145, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y. Carbon catabolite control of the metabolic network in Bacillus subtilis. Biosci. Biotechnol. Biochem. 2009, 73, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Sonenshein, A.L. Cody, a global regulator of stationary phase and virulence in Gram-positive bacteria. Curr. Opin. Microbiol. 2005, 8, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Brekasis, D.; Paget, M.S. A novel sensor of NADH/NAD+ redox poise in Streptomyces coelicolor A3(2). EMBO J. 2003, 22, 4856–4865. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, R.N.; Chambers, M.G.; Ng, K.K.; Ohi, M.D.; Lacy, D.B. Structural organization of the functional domains of Clostridium difficile toxins A and B. Proc. Natl. Acad. Sci. USA 2010, 107, 13467–13472. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.H.; Roberts, A.K.; Shone, C.C.; Acharya, K.R. Super toxins from a super bug: Structure and function of Clostridium difficile toxins. Biochem. J. 2011, 436, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Voth, D.E.; Ballard, J.D. Clostridium difficile toxins: Mechanism of action and role in disease. Clin. Microbiol. Rev. 2005, 18, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Jank, T.; Aktories, K. Structure and mode of action of clostridial glucosylating toxins: The ABCD model. Trends Microbiol. 2008, 16, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Rupnik, M.; Pabst, S.; Rupnik, M.; von Eichel-Streiber, C.; Urlaub, H.; Soling, H.D. Characterization of the cleavage site and function of resulting cleavage fragments after limited proteolysis of Clostridium difficile toxin B (TcdB) by host cells. Microbiology 2005, 151, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Zhang, H.; Cai, C.; Zhu, S.; Zhou, Y.; Yang, X.; He, R.; Li, C.; Guo, S.; Li, S.; et al. Chondroitin sulfate proteoglycan 4 functions as the cellular receptor for Clostridium difficile toxin B. Cell Res. 2015, 25, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Egerer, M.; Giesemann, T.; Jank, T.; Satchell, K.J.; Aktories, K. Auto-catalytic cleavage of Clostridium difficile toxins A and B depends on cysteine protease activity. J. Biol. Chem. 2007, 282, 25314–25321. [Google Scholar] [CrossRef] [PubMed]
- Olling, A.; Goy, S.; Hoffmann, F.; Tatge, H.; Just, I.; Gerhard, R. The repetitive oligopeptide sequences modulate cytopathic potency but are not crucial for cellular uptake of Clostridium difficile toxin A. PLoS ONE 2011, 6, e17623. [Google Scholar] [CrossRef] [PubMed]
- Reinert, D.J.; Jank, T.; Aktories, K.; Schulz, G.E. Structural basis for the function of Clostridium difficile toxin B. J. Mol. Biol. 2005, 351, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Mesmin, B.; Robbe, K.; Geny, B.; Luton, F.; Brandolin, G.; Popoff, M.R.; Antonny, B. A phosphatidylserine-binding site in the cytosolic fragment of Clostridium sordellii lethal toxin facilitates glucosylation of membrane-bound Rac and is required for cytotoxicity. J. Biol. Chem. 2004, 279, 49876–49882. [Google Scholar] [CrossRef] [PubMed]
- Geissler, B.; Tungekar, R.; Satchell, K.J. Identification of a conserved membrane localization domain within numerous large bacterial protein toxins. Proc. Natl. Acad. Sci. USA 2010, 107, 5581–5586. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, R.N.; Chumbler, N.M.; Rutherford, S.A.; Farrow, M.A.; Friedman, D.B.; Spiller, B.; Lacy, D.B. Structural determinants of Clostridium difficile toxin A glucosyltransferase activity. J. Biol. Chem. 2012, 287, 8013–8020. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Eugenio, L.; Schorr, M.; Hussack, G.; Tanha, J.; Kitova, E.N.; Klassen, J.S.; Ng, K.K. Structural basis for antibody recognition in the receptor-binding domains of toxins A and B from Clostridium difficile. J. Biol. Chem. 2014, 289, 2331–2343. [Google Scholar] [CrossRef] [PubMed]
- Barth, H.; Pfeifer, G.; Hofmann, F.; Maier, E.; Benz, R.; Aktories, K. Low pH-induced formation of ion channels by Clostridium difficile toxin B in target cells. J. Biol. Chem. 2001, 276, 10670–10676. [Google Scholar] [CrossRef] [PubMed]
- Spyres, L.M.; Daniel, J.; Hensley, A.; Qa’Dan, M.; Ortiz-Leduc, W.; Ballard, J.D. Mutational analysis of the enzymatic domain of Clostridium difficile toxin B reveals novel inhibitors of the wild-type toxin. Infect. Immun. 2003, 71, 3294–3301. [Google Scholar] [CrossRef] [PubMed]
- Jank, T.; Reinert, D.J.; Giesemann, T.; Schulz, G.E.; Aktories, K. Change of the donor substrate specificity of Clostridium difficile toxin B by site-directed mutagenesis. J. Biol. Chem. 2005, 280, 37833–37838. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Olarte, E.; Weidmann, M.; Eichel-Streiber, C.; Thelestam, M. Toxins A and B from Clostridium difficile differ with respect to enzymatic potencies, cellular substrate specificities, and surface binding to cultured cells. J. Clin. Investig. 1997, 100, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Kreimeyer, I.; Euler, F.; Marckscheffel, A.; Tatge, H.; Pich, A.; Olling, A.; Schwarz, J.; Just, I.; Gerhard, R. Autoproteolytic cleavage mediates cytotoxicity of Clostridium difficile toxin A. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Olarte, E.; Low, P.; Freer, E.; Norlin, T.; Weidmann, M.; von Eichel-Streiber, C.; Thelestam, M. A novel cytotoxin from Clostridium difficile serogroup F is a functional hybrid between two other large clostridial cytotoxins. J. Biol. Chem. 1999, 274, 11046–11052. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.K.; Stuart, R.L.; Mackin, K.E.; Carter, G.P.; Kotsanas, D.; Francis, M.J.; Easton, M.; Dimovski, K.; Elliott, B.; Riley, T.V.; et al. Emergence of a ribotype 244 strain of Clostridium difficile associated with severe disease and related to the epidemic ribotype 027 strain. Clin. Infect. Dis. 2014, 58, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, J.H.; Bos, J.L. Specificity in Ras and Rap signaling. J. Biol. Chem. 2009, 284, 10995–10999. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.; Dreger, S.; Gerhard, R.; Barth, H.; Just, I.; Genth, H. Difference in the cytotoxic effects of toxin B from Clostridium difficile strain VPI 10463 and toxin B from variant Clostridium difficile strain 1470. Infect. Immun. 2007, 75, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Krivan, H.C.; Clark, G.F.; Smith, D.F.; Wilkins, T.D. Cell surface binding site for Clostridium difficile enterotoxin: Evidence for a glycoconjugate containing the sequence Gala1-3Gal, B1-4GlcNAc. Infect. Immun. 1986, 53, 573–581. [Google Scholar] [PubMed]
- Von Eichel-Streiber, C.; Sauerborn, M. Clostridium difficile toxin A carries a C-terminal repetitive structure homologous to the carbohydrate binding region of streptococcal glycosyltransferases. Gene 1990, 96, 107–113. [Google Scholar] [CrossRef]
- Ho, J.G.; Greco, A.; Rupnik, M.; Ng, K.K. Crystal structure of receptor-binding C-terminal repeats from Clostridium difficile toxin A. Proc. Natl. Acad. Sci. USA 2005, 102, 18373–18378. [Google Scholar] [CrossRef] [PubMed]
- Dingle, T.; Wee, S.; Mulvey, G.L.; Greco, A.; Kitova, E.N.; Sun, J.; Lin, S.; Klassen, J.S.; Palcic, M.M.; Ng, K.K.; et al. Functional properties of the carboxy-terminal host cell-binding domains of the two toxins, TcdA and TcdB, expressed by Clostridium difficile. Glycobiology 2008, 18, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Demarest, S.J.; Salbato, J.; Elia, M.; Zhong, J.; Morrow, T.; Holland, T.; Kline, K.; Woodnutt, G.; Kimmel, B.E.; Hansen, G. Structural characterization of the cell wall binding domains of Clostridium difficile toxins A and B; evidence that Ca2+ plays a role in toxin A cell surface association. J. Mol. Biol. 2005, 346, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Ho, J.G.; Lin, S.J.; Palcic, M.M.; Rupnik, M.; Ng, K.K. Carbohydrate recognition by Clostridium difficile toxin A. Nat. Struct. Mol. Biol. 2006, 13, 460–461. [Google Scholar] [CrossRef] [PubMed]
- Reineke, J.; Tenzer, S.; Rupnik, M.; Koschinski, A.; Hasselmayer, O.; Schrattenholz, A.; Schild, H.; von Eichel-Streiber, C. Autocatalytic cleavage of Clostridium difficile toxin B. Nature 2007, 446, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Albesa-Jove, D.; Bertrand, T.; Carpenter, E.P.; Swain, G.V.; Lim, J.; Zhang, J.; Haire, L.F.; Vasisht, N.; Braun, V.; Lange, A.; et al. Four distinct structural domains in Clostridium difficile toxin B visualized using SAXS. J. Mol. Biol. 2010, 396, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Chumbler, N.M.; Farrow, M.A.; Lapierre, L.A.; Franklin, J.L.; Haslam, D.B.; Goldenring, J.R.; Lacy, D.B. Clostridium difficile Toxin B causes epithelial cell necrosis through an autoprocessing-independent mechanism. PLoS Pathog. 2012, 8, e1003072. [Google Scholar] [CrossRef]
- Lanis, J.M.; Barua, S.; Ballard, J.D. Variations in TcdB activity and the hypervirulence of emerging strains of Clostridium difficile. PLoS Pathog. 2010, 6, e1001061. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.; Lupardus, P.J.; Gersch, M.M.; Puri, A.W.; Albrow, V.E.; Garcia, K.C.; Bogyo, M. Defining an allosteric circuit in the cysteine protease domain of Clostridium difficile toxins. Nat. Struct. Mol. Biol. 2011, 18, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Florin, I.; Thelestam, M. Internalization of Clostridium difficile cytotoxin into cultured human lung fibroblasts. Biochim. Biophys. Acta 1983, 763, 383–392. [Google Scholar] [CrossRef]
- Qa’Dan, M.; Spyres, L.M.; Ballard, J.D. pH-induced conformational changes in Clostridium difficile toxin B. Infect. Immun. 2000, 68, 2470–2474. [Google Scholar] [CrossRef] [PubMed]
- Hamza, T.; Zhang, Z.; Melnyk, R.A.; Feng, H. Defective mutations within the translocation domain of Clostridium difficile toxin B impair disease pathogenesis. Pathog. Dis. 2016, 74, ftv098. [Google Scholar] [CrossRef] [PubMed]
- Papatheodorou, P.; Zamboglou, C.; Genisyuerek, S.; Guttenberg, G.; Aktories, K. Clostridial glucosylating toxins enter cells via clathrin-mediated endocytosis. PLoS ONE 2010, 5, e10673. [Google Scholar] [CrossRef] [PubMed]
- Pothoulakis, C.; Galili, U.; Castagliuolo, I.; Kelly, C.P.; Nikulasson, S.; Dudeja, P.K.; Brasitus, T.A.; LaMont, J.T. A human antibody binds to alpha-galactose receptors and mimics the effects of Clostridium difficile toxin A in rat colon. Gastroenterology 1996, 110, 1704–1712. [Google Scholar] [CrossRef] [PubMed]
- Na, X.; Kim, H.; Moyer, M.P.; Pothoulakis, C.; LaMont, J.T. Gp96 is a human colonocyte plasma membrane binding protein for Clostridium difficile toxin A. Infect. Immun. 2008, 76, 2862–2871. [Google Scholar] [CrossRef] [PubMed]
- LaFrance, M.E.; Farrow, M.A.; Chandrasekaran, R.; Sheng, J.; Rubin, D.H.; Lacy, D.B. Identification of an epithelial cell receptor responsible for Clostridium difficile TcdB-induced cytotoxicity. Proc. Natl. Acad. Sci. USA 2015, 112, 7073–7078. [Google Scholar] [CrossRef] [PubMed]
- Goy, S.D.; Olling, A.; Neumann, D.; Pich, A.; Gerhard, R. Human neutrophils are activated by a peptide fragment of Clostridium difficile toxin B presumably via formyl peptide receptor. Cell. Microbiol. 2015, 17, 893–909. [Google Scholar] [CrossRef] [PubMed]
- Schorch, B.; Song, S.; van Diemen, F.R.; Bock, H.H.; May, P.; Herz, J.; Brummelkamp, T.R.; Papatheodorou, P.; Aktories, K. Lrp1 is a receptor for Clostridium perfringens TpeL toxin indicating a two-receptor model of clostridial glycosylating toxins. Proc. Natl. Acad. Sci. USA 2014, 111, 6431–6436. [Google Scholar] [CrossRef] [PubMed]
- Egerer, M.; Giesemann, T.; Herrmann, C.; Aktories, K. Autocatalytic processing of Clostridium difficile toxin B. Binding of inositol hexakisphosphate. J. Biol. Chem. 2009, 284, 3389–3395. [Google Scholar] [CrossRef] [PubMed]
- Just, I.; Selzer, J.; von Eichel-Streiber, C.; Aktories, K. The low molecular mass GTP-binding protein Rho is affected by toxin A from Clostridium difficile. J. Clin. Investig. 1995, 95, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, Y.; Huang, T.; Feng, H. Glucosyltransferase activity of Clostridium difficile toxin B is essential for disease pathogenesis. Gut Microbes 2015, 6, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Burridge, K.; Wennerberg, K. Rho and Rac take center stage. Cell 2004, 116, 167–179. [Google Scholar] [CrossRef]
- Jaffe, A.B.; Hall, A. Rho GTPases: Biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005, 21, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Lemichez, E.; Aktories, K. Hijacking of Rho GTPases during bacterial infection. Exp. Cell Res. 2013, 319, 2329–2336. [Google Scholar] [CrossRef] [PubMed]
- Popoff, M.R. Bacterial factors exploit eukaryotic Rho GTPase signaling cascades to promote invasion and proliferation within their host. Small GTPases. 2014, 5, e983863. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sun, C.; Wang, H.; Wang, J. The role of Rho GTPases in toxicity of Clostridium difficile toxins. Toxins 2015, 7, 5254–5267. [Google Scholar] [CrossRef] [PubMed]
- Sehr, P.; Joseph, G.; Genth, H.; Just, I.; Pick, E.; Aktories, K. Glucosylation and ADP ribosylation of Rho proteins: Effects on nucleotide binding, GTPase activity, and effector coupling. Biochemistry 1998, 37, 5296–5304. [Google Scholar] [CrossRef] [PubMed]
- Genth, H.; Aktories, K.; Just, I. Monoglucosylation of RhoA at threonine 37 blocks cytosol-membrane cycling. J. Biol. Chem. 1999, 274, 29050–29056. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Olarte, E.; Freer, E.; Parra, A.; Guzman-Verri, C.; Moreno, E.; Thelestam, M. R-Ras glucosylation and transient RhoA activation determine the cytopathic effect produced by toxin B variants from toxin A-negative strains of Clostridium difficile. J. Biol. Chem. 2003, 278, 7956–7963. [Google Scholar] [CrossRef] [PubMed]
- Ottlinger, M.E.; Lin, S. Clostridium difficile toxin B induces reorganization of actin, vinculin, and talin in cultured cells. Exp. Cell Res. 1988, 174, 215–229. [Google Scholar] [CrossRef]
- Triadafilopoulos, G.; Pothoulakis, C.; O’Brien, M.J.; LaMont, J.T. Differential effects of Clostridium difficile toxins A and B on rabbit ileum. Gastroenterology 1987, 93, 273–279. [Google Scholar] [PubMed]
- Hecht, G.; Pothoulakis, C.; LaMont, J.T.; Madara, J.L. Clostridium difficile toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J. Clin. Investig. 1988, 82, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.; Pothoulakis, C.; LaMont, J.T.; Carlson, S.; Madara, J.L. C. difficile toxin A increases intestinal permeability and induces Cl− secretion. Am. J. Physiol. 1990, 259, G165–G172. [Google Scholar] [PubMed]
- Hecht, G.; Koutsouris, A.; Pothoulakis, C.; LaMont, J.T.; Madara, J.L. Clostridium difficile toxin B disrupts the barrier function of T84 monolayers. Gastroenterology 1992, 102, 416–423. [Google Scholar] [PubMed]
- Johal, S.S.; Solomon, K.; Dodson, S.; Borriello, S.P.; Mahida, Y.R. Differential effects of varying concentrations of Clostridium difficile toxin A on epithelial barrier function and expression of cytokines. J. Infect. Dis. 2004, 189, 2110–2119. [Google Scholar] [CrossRef] [PubMed]
- Just, I.; Gerhard, R. Large clostridial cytotoxins. Rev. Physiol. Biochem. Pharmacol. 2004, 152, 23–47. [Google Scholar] [PubMed]
- Yang, G.; Zhou, B.; Wang, J.; He, X.; Sun, X.; Nie, W.; Tzipori, S.; Feng, H. Expression of recombinant Clostridium difficile toxin A and B in Bacillus megaterium. BMC Microbiol. 2008, 8, 192. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, R.; Tatge, H.; Genth, H.; Thum, T.; Borlak, J.; Fritz, G.; Just, I. Clostridium difficile toxin A induces expression of the stress-induced early gene product rhob. J. Biol. Chem. 2005, 280, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Genth, H.; Huelsenbeck, J.; Hartmann, B.; Hofmann, F.; Just, I.; Gerhard, R. Cellular stability of Rho-GTPases glucosylated by Clostridium difficile toxin B. FEBS Lett. 2006, 580, 3565–3569. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.; Hirota, S.A.; Gross, O.; Li, Y.; Ulke-Lemee, A.; Potentier, M.S.; Schenck, L.P.; Vilaysane, A.; Seamone, M.E.; Feng, H.; et al. Clostridium difficile toxin-induced inflammation and intestinal injury are mediated by the inflammasome. Gastroenterology 2010, 139, 542–552.e3. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, C.; Fabbri, A.; Falzano, L.; Fattorossi, A.; Matarrese, P.; Rivabene, R.; Donelli, G. Clostridium difficile toxin B induces apoptosis in intestinal cultured cells. Infect. Immun. 1998, 66, 2660–2665. [Google Scholar] [PubMed]
- Brito, G.A.; Fujji, J.; Carneiro-Filho, B.A.; Lima, A.A.; Obrig, T.; Guerrant, R.L. Mechanism of Clostridium difficile toxin A-induced apoptosis in T84 cells. J. Infect. Dis. 2002, 186, 1438–1447. [Google Scholar] [CrossRef] [PubMed]
- Hippenstiel, S.; Schmeck, B.; N’Guessan, P.D.; Seybold, J.; Krull, M.; Preissner, K.; Eichel-Streiber, C.V.; Suttorp, N. Rho protein inactivation induced apoptosis of cultured human endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L830–L838. [Google Scholar] [CrossRef] [PubMed]
- Teichert, M.; Tatge, H.; Schoentaube, J.; Just, I.; Gerhard, R. Application of mutated Clostridium difficile toxin A for determination of glucosyltransferase-dependent effects. Infect. Immun. 2006, 74, 6006–6010. [Google Scholar] [CrossRef] [PubMed]
- Nottrott, S.; Schoentaube, J.; Genth, H.; Just, I.; Gerhard, R. Clostridium difficile toxin A-induced apoptosis is p53-independent but depends on glucosylation of Rho GTPases. Apoptosis 2007, 12, 1443–1453. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, R.; Nottrott, S.; Schoentaube, J.; Tatge, H.; Olling, A.; Just, I. Glucosylation of Rho GTPases by Clostridium difficile toxin A triggers apoptosis in intestinal epithelial cells. J. Med. Microbiol. 2008, 57, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Mahida, Y.R.; Galvin, A.; Makh, S.; Hyde, S.; Sanfilippo, L.; Borriello, S.P.; Sewell, H.F. Effect of Clostridium difficile toxin A on human colonic lamina propria cells: Early loss of macrophages followed by T-cell apoptosis. Infect. Immun. 1998, 66, 5462–5469. [Google Scholar] [PubMed]
- Qa’Dan, M.; Ramsey, M.; Daniel, J.; Spyres, L.M.; Safiejko-Mroczka, B.; Ortiz-Leduc, W.; Ballard, J.D. Clostridium difficile toxin B activates dual caspase-dependent and caspase-independent apoptosis in intoxicated cells. Cell. Microbiol. 2002, 4, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Matarrese, P.; Falzano, L.; Fabbri, A.; Gambardella, L.; Frank, C.; Geny, B.; Popoff, M.R.; Malorni, W.; Fiorentini, C. Clostridium difficile toxin B causes apoptosis in epithelial cells by thrilling mitochondria. Involvement of ATP-sensitive mitochondrial potassium channels. J. Biol. Chem. 2007, 282, 9029–9041. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, X.; Zhang, Y.; Li, S.; Chen, K.; Shi, L.; Nie, W.; Kumar, R.; Tzipori, S.; Wang, J.; et al. A chimeric toxin vaccine protects against primary and recurrent Clostridium difficile infection. Infect. Immun. 2012, 80, 2678–2688. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Hirota, S.A. The roles of host and pathogen factors and the innate immune response in the pathogenesis of Clostridium difficile infection. Mol. Immunol. 2015, 63, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Farrow, M.A.; Chumbler, N.M.; Lapierre, L.A.; Franklin, J.L.; Rutherford, S.A.; Goldenring, J.R.; Lacy, D.B. Clostridium difficile toxin B-induced necrosis is mediated by the host epithelial cell nadph oxidase complex. Proc. Natl. Acad. Sci. USA 2013, 110, 18674–18679. [Google Scholar] [CrossRef] [PubMed]
- Wohlan, K.; Goy, S.; Olling, A.; Srivaratharajan, S.; Tatge, H.; Genth, H.; Gerhard, R. Pyknotic cell death induced by Clostridium difficile TcdB: Chromatin condensation and nuclear blister are induced independently of the glucosyltransferase activity. Cell. Microbiol. 2014, 16, 1678–1692. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, J.; Gao, W.; Li, L.; Li, P.; Zhang, L.; Gong, Y.N.; Peng, X.; Xi, J.J.; Chen, S.; et al. Innate immune sensing of bacterial modifications of Rho GTPases by the pyrin inflammasome. Nature 2014, 513, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Wu, H. Structural mechanisms of inflammasome assembly. FEBS J. 2015, 282, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Linevsky, J.K.; Pothoulakis, C.; Keates, S.; Warny, M.; Keates, A.C.; Lamont, J.T.; Kelly, C.P. IL-8 release and neutrophil activation by Clostridium difficile toxin-exposed human monocytes. Am. J. Physiol. 1997, 273, G1333–G1340. [Google Scholar] [PubMed]
- Warny, M.; Keates, A.C.; Keates, S.; Castagliuolo, I.; Zacks, J.K.; Aboudola, S.; Qamar, A.; Pothoulakis, C.; LaMont, J.T.; Kelly, C.P. P38 map kinase activation by Clostridium difficile toxin A mediates monocyte necrosis, IL-8 production, and enteritis. J. Clin. Investig. 2000, 105, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Maegawa, T.; Kondo, T.; Kimura, A.; Iwakura, Y.; Nakamura, S.; Mukaida, N. Essential involvement of IFN-gamma in Clostridium difficile toxin A-induced enteritis. J. Immunol. 2004, 172, 3018–3025. [Google Scholar] [CrossRef] [PubMed]
- Jafari, N.V.; Kuehne, S.A.; Bryant, C.E.; Elawad, M.; Wren, B.W.; Minton, N.P.; Allan, E.; Bajaj-Elliott, M. Clostridium difficile modulates host innate immunity via toxin-independent and dependent mechanism(s). PLoS ONE 2013, 8, e69846. [Google Scholar] [CrossRef] [PubMed]
- Buonomo, E.L.; Madan, R.; Pramoonjago, P.; Li, L.; Okusa, M.D.; Petri, W.A., Jr. Role of interleukin 23 signaling in Clostridium difficile colitis. J. Infect. Dis. 2013, 208, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Cowardin, C.A.; Kuehne, S.A.; Buonomo, E.L.; Marie, C.S.; Minton, N.P.; Petri, W.A., Jr. Inflammasome activation contributes to interleukin-23 production in response to Clostridium difficile. MBio 2015. [Google Scholar] [CrossRef] [PubMed]
- Lyerly, D.M.; Saum, K.E.; MacDonald, D.K.; Wilkins, T.D. Effects of Clostridium difficile toxins given intragastrically to animals. Infect. Immun. 1985, 47, 349–352. [Google Scholar] [PubMed]
- Savidge, T.C.; Pan, W.H.; Newman, P.; O’Brien, M.; Anton, P.M.; Pothoulakis, C. Clostridium difficile toxin B is an inflammatory enterotoxin in human intestine. Gastroenterology 2003, 125, 413–420. [Google Scholar] [CrossRef]
- Riegler, M.; Sedivy, R.; Pothoulakis, C.; Hamilton, G.; Zacherl, J.; Bischof, G.; Cosentini, E.; Feil, W.; Schiessel, R.; LaMont, J.T.; et al. Clostridium difficile toxin B is more potent than toxin a in damaging human colonic epithelium in vitro. J. Clin. Investig. 1995, 95, 2004–2011. [Google Scholar] [CrossRef] [PubMed]
- Aktories, K. Rho proteins: Targets for bacterial toxins. Trends Microbiol. 1997, 5, 282–288. [Google Scholar] [CrossRef]
- Tucker, K.D.; Carrig, P.E.; Wilkins, T.D. Toxin A of Clostridium difficile is a potent cytotoxin. J. Clin. Microbiol. 1990, 28, 869–871. [Google Scholar] [PubMed]
- Lyras, D.; O’Connor, J.R.; Howarth, P.M.; Sambol, S.P.; Carter, G.P.; Phumoonna, T.; Poon, R.; Adams, V.; Vedantam, G.; Johnson, S.; et al. Toxin Bb is essential for virulence of Clostridium difficile. Nature 2009, 458, 1176–1179. [Google Scholar] [CrossRef] [PubMed]
- Kuehne, S.A.; Cartman, S.T.; Heap, J.T.; Kelly, M.L.; Cockayne, A.; Minton, N.P. The role of toxin A and toxin B in Clostridium difficile infection. Nature 2010, 467, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Kuehne, S.A.; Collery, M.M.; Kelly, M.L.; Cartman, S.T.; Cockayne, A.; Minton, N.P. Importance of toxin A, toxin B, and CDT in virulence of an epidemic Clostridium difficile strain. J. Infect. Dis. 2014, 209, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.; Mukherjee, J.; Parry, N.; Tzipori, S. Antibody against TcdB, but not TcdA, prevents development of gastrointestinal and systemic Clostridium difficile disease. J. Infect. Dis. 2013, 207, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.P.; Chakravorty, A.; Nguyen, T.A.P.; Mileto, S.; Schreiber, F.; Li, L.; Howarth, P.; Clare, S.; Cunningham, B.; Sambol, S.P.; et al. Defining the roles of tcdA and tcdB in localized gastrointestinal disease, systemic organ damage, and the host response during Clostridium difficile infections. MBio. 2015, 6, e00551. [Google Scholar] [CrossRef] [PubMed]
- Samra, Z.; Talmor, S.; Bahar, J. High prevalence of toxin A-negative toxin B-positive Clostridium difficile in hospitalized patients with gastrointestinal disease. Diagn. Microbiol. Infect. Dis. 2002, 43, 189–192. [Google Scholar] [CrossRef]
- Carter, G.P.; Rood, J.I.; Lyras, D. The role of toxin A and toxin B in the virulence of Clostridium difficile. Trends Microbiol. 2012, 20, 21–29. [Google Scholar] [CrossRef] [PubMed]
- McCoy, R.M.; Klick, A.; Hill, S.; Dull, R.B. Luminal toxin-binding agents for Clostridium difficile infection. J. Pharm. Pract. 2015. [Google Scholar] [CrossRef] [PubMed]
- Weiss, K. Toxin-binding treatment for Clostridium difficile: A review including reports of studies with tolevamer. Int. J. Antimicrob. Agents 2009, 33, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Louie, T.J.; Gerding, D.N.; Cornely, O.A.; Chasan-Taber, S.; Fitts, D.; Gelone, S.P.; Broom, C.; Davidson, D.M. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: Results from two multinational, randomized, controlled trials. Clin. Infect. Dis. 2014, 59, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Mogg, G.A.; George, R.H.; Youngs, D.; Johnson, M.; Thompson, H.; Burdon, D.W.; Keighley, M.R. Randomized controlled trial of colestipol in antibiotic-associated colitis. Br. J. Surg. 1982, 69, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Sturino, J.M.; Pokusaeva, K.; Carpenter, R. Effective sequestration of Clostridium difficile protein toxins by calcium aluminosilicate. Antimicrob. Agents Chemother. 2015, 59, 7178–7183. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, S.; di Masi, A.; Turla, S.; Ascenzi, P.; Gouliouris, T.; Petrosillo, N. The protective role of albumin in Clostridium difficile infection: A step toward solving the puzzle. Infect. Control. Hosp. Epidemiol. 2015, 36, 1478–1479. [Google Scholar] [CrossRef] [PubMed]
- Di Masi, A.; di Bella, S.; Turla, S.; Leboffe, L.; Arcovito, A.; Nocca, G.; Stano, P.; Ascenzi, P.; Petrosillo, N. Albumin prevents Clostridium difficile-related cytotoxicity through toxin B binding. In Proceedings of the ECCMID 2016, Amsterdam, The Netherlands, 11 April 2016.
- Taylor, N.S.; Bartlett, J.G. Binding of Clostridium difficile cytotoxin and vancomycin by anion-exchange resins. J. Infect. Dis. 1980, 141, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Giesemann, T.; Guttenberg, G.; Aktories, K. Human alpha-defensins inhibit Clostridium difficile toxin B. Gastroenterology 2008, 134, 2049–2058. [Google Scholar] [CrossRef] [PubMed]
- Bender, K.O.; Garland, M.; Ferreyra, J.A.; Hryckowian, A.J.; Child, M.A.; Puri, A.W.; Solow-Cordero, D.E.; Higginbottom, S.K.; Segal, E.; Banaei, N.; et al. A small-molecule antivirulence agent for treating Clostridium difficile infection. Sci. Transl. Med. 2015, 7, 306ra148. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.; Beilhartz, G.L.; Auger, A.; Gupta, P.; Therien, A.G.; Melnyk, R.A. Small molecule inhibitors of Clostridium difficile toxin B-induced cellular damage. Chem. Biol. 2015, 22, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Tsourous, G.I.; Raftopoulos, L.G.; Kafe, E.E.; Manoleris, E.K.; Makaritsis, K.P.; Pinis, S.G. A case of pseudomembranous colitis presenting with massive ascites. Eur. J. Intern. Med. 2007, 18, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Boaz, A.; Dan, M.; Charuzi, I.; Landau, O.; Aloni, Y.; Kyzer, S. Pseudomembranous colitis: Report of a severe case with unusual clinical signs in a young nurse. Dis. Colon Rectum. 2000, 43, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Siarakas, S.; Damas, E.; Murrell, W.G. Is cardiorespiratory failure induced by bacterial toxins the cause of sudden infant death syndrome? Studies with an animal model (the rabbit). Toxicon 1995, 33, 635–649. [Google Scholar] [CrossRef]
- Sakurai, T.; Hajiro, K.; Takakuwa, H.; Nishi, A.; Aihara, M.; Chiba, T. Liver abscess caused by Clostridium difficile. Scand. J. Infect. Dis. 2001, 33, 69–70. [Google Scholar] [PubMed]
- Shaikh, N.; Kettern, M.A.; Hanssens, Y.; Elshafie, S.S.; Louon, A. A rare and unsuspected complication of Clostridium difficile infection. Intensive Care Med. 2008, 34, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.S.; Sebastian, J.C.; Hiorns, D.; Jacob, S.; Mukerjee, P.K. Clostridium difficile and acute respiratory distress syndrome. Heart Lung 2004, 33, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Cunney, R.J.; Magee, C.; McNamara, E.; Smyth, E.G.; Walshe, J. Clostridium difficile colitis associated with chronic renal failure. Nephrol. Dial. Transplant. 1998, 13, 2842–2846. [Google Scholar] [CrossRef] [PubMed]
- Surawicz, C.M.; Brandt, L.J.; Binion, D.G.; Ananthakrishnan, A.N.; Curry, S.R.; Gilligan, P.H.; McFarland, L.V.; Mellow, M.; Zuckerbraun, B.S. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am. J. Gastroenterol. 2013, 108, 478–498. [Google Scholar] [CrossRef] [PubMed]
- Donta, S.T.; Sullivan, N.; Wilkins, T.D. Differential effects of Clostridium difficile toxins on tissue-cultured cells. J. Clin. Microbiol. 1982, 15, 1157–1158. [Google Scholar] [PubMed]
- Qualman, S.J.; Petric, M.; Karmali, M.A.; Smith, C.R.; Hamilton, S.R. Clostridium difficile invasion and toxin circulation in fatal pediatric pseudomembranous colitis. Am. J. Clin. Pathol. 1990, 94, 410–416. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, J.; Steele, J.; Sun, X.; Nie, W.; Tzipori, S.; Feng, H. An ultrasensitive rapid immunocytotoxicity assay for detecting Clostridium difficile toxins. J. Microbiol. Methods 2009, 78, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.; Chen, K.; Sun, X.; Zhang, Y.; Wang, H.; Tzipori, S.; Feng, H. Systemic dissemination of Clostridium difficile toxins A and B is associated with severe, fatal disease in animal models. J. Infect. Dis. 2012, 205, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, K.; Wu, J.; Yang, Z.; Shi, L.; Barlow, L.L.; Aronoff, D.M.; Garey, K.W.; Savidge, T.C.; von Rosenvinge, E.C.; et al. Identification of toxemia in patients with Clostridium difficile infection. PLoS ONE 2015, 10, e0124235. [Google Scholar] [CrossRef] [PubMed]
- Kyne, L.; Warny, M.; Qamar, A.; Kelly, C.P. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N. Engl. J. Med. 2000, 342, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Warny, M.; Vaerman, J.P.; Avesani, V.; Delmee, M. Human antibody response to Clostridium difficile toxin A in relation to clinical course of infection. Infect. Immun. 1994, 62, 384–389. [Google Scholar] [PubMed]
- Solomon, K.; Martin, A.J.; O’Donoghue, C.; Chen, X.; Fenelon, L.; Fanning, S.; Kelly, C.P.; Kyne, L. Mortality in patients with Clostridium difficile infection correlates with host pro-inflammatory and humoral immune responses. J. Med. Microbiol. 2013, 62, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Munoz, P.; Giannella, M.; Alcala, L.; Sarmiento, E.; Yanez, J.F.; Palomo, J.; Catalan, P.; Carbone, J.; Bouza, E. Clostridium difficile-associated diarrhea in heart transplant recipients: Is hypogammaglobulinemia the answer? J. Heart Lung Transplant. 2007, 26, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Kent, S.A.; O’Leary, K.J.; Merrigan, M.M.; Sambol, S.P.; Peterson, L.R.; Gerding, D.N. Fatal pseudomembranous colitis associated with a variant Clostridium difficile strain not detected by toxin A immunoassay. Ann. Intern. Med. 2001, 135, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Dobson, G.; Hickey, C.; Trinder, J. Clostridium difficile colitis causing toxic megacolon, severe sepsis and multiple organ dysfunction syndrome. Intensive Care Med. 2003, 29, 1030. [Google Scholar] [CrossRef] [PubMed]
- Hamm, E.E.; Voth, D.E.; Ballard, J.D. Identification of Clostridium difficile toxin B cardiotoxicity using a zebrafish embryo model of intoxication. Proc. Natl. Acad. Sci. USA 2006, 103, 14176–14181. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Camorlinga-Ponce, M.; Muñoz, O. Sensitivity in culture of epithelial cells from rhesus monkey kidney and human colon carcinoma to toxins A and B from Clostridium difficile. Toxicon 1992, 30, 419–426. [Google Scholar] [CrossRef]
- Anderson, R.J.; Ray, C.J.; Popoff, M.R. Evidence for rho protein regulation of renal tubular epithelial cell function. Kidney Int. 2000, 58, 1996–2006. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, H.S.; Santos-Neto, M.S.; Oliveira, A.V.; Lima, A.A.; Lyerly, D.M.; Fonteles, M.C. Vascular and glomerular effects of Clostridium difficile toxin A peptide on the isolated rat kidney. Braz. J. Med. Biol. Res. 1994, 27, 743–748. [Google Scholar] [PubMed]
- Linseman, D.A.; Loucks, F.A. Diverse roles of Rho family GTPases in neuronal development, survival, and death. Front. Biosci. 2008, 13, 657–676. [Google Scholar] [CrossRef] [PubMed]
- Loucks, F.A.; Le, S.S.; Zimmermann, A.K.; Ryan, K.R.; Barth, H.; Aktories, K.; Linseman, D.A. Rho family GTPase inhibition reveals opposing effects of mitogen-activated protein kinase kinase/extracellular signal-regulated kinase and janus kinase/signal transducer and activator of transcription signaling cascades on neuronal survival. J. Neurochem. 2006, 97, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz, T.R.; Loucks, F.A.; Schroeder, E.K.; Nevalainen, M.T.; Tyler, K.L.; Aktories, K.; Bouchard, R.J.; Linseman, D.A. Signal transducer and activator of transcription-5 mediates neuronal apoptosis induced by inhibition of Rac GTPase activity. J. Biol. Chem. 2012, 287, 16835–16848. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz, T.R.; Ramaswami, S.A.; Bouchard, R.J.; Aktories, K.; Linseman, D.A. Neuronal apoptosis induced by selective inhibition of Rac GTPase versus global suppression of Rho family GTPases is mediated by alterations in distinct mitogen-activated protein kinase signaling cascades. J. Biol. Chem. 2015, 290, 9363–9376. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Bella, S.; Ascenzi, P.; Siarakas, S.; Petrosillo, N.; Di Masi, A. Clostridium difficile Toxins A and B: Insights into Pathogenic Properties and Extraintestinal Effects. Toxins 2016, 8, 134. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins8050134
Di Bella S, Ascenzi P, Siarakas S, Petrosillo N, Di Masi A. Clostridium difficile Toxins A and B: Insights into Pathogenic Properties and Extraintestinal Effects. Toxins. 2016; 8(5):134. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins8050134
Chicago/Turabian StyleDi Bella, Stefano, Paolo Ascenzi, Steven Siarakas, Nicola Petrosillo, and Alessandra Di Masi. 2016. "Clostridium difficile Toxins A and B: Insights into Pathogenic Properties and Extraintestinal Effects" Toxins 8, no. 5: 134. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins8050134