Biosorption of B-aflatoxins Using Biomasses Obtained from Formosa Firethorn [Pyracantha koidzumii (Hayata) Rehder]
Abstract
:1. Introduction
2. Results and Discussion
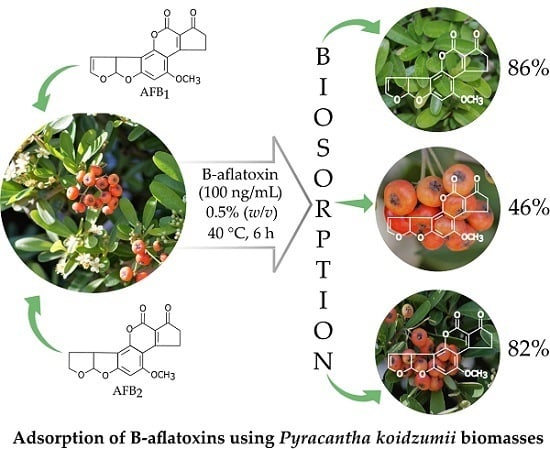
2.1. Biosorption of B-aflatoxins
2.2. Zeta Potential (ζ)
2.3. The Influence of Biomass on Aflatoxin Biosorption
2.4. FTIR Analysis
- The band at 3360 cm−1 shifted slightly to 3344 cm−1 after interaction with AF. This frequency shift may be attributed to the interaction between AF and both the hydroxyl and amine groups on the biomass surface. A shift in the spectral frequency is related to an energy change of the functional group and this is indicative that the bonding pattern of the different functional groups changed after AF biosorption [19].
- There was a markedly reduction in intensity of the carboxyl band located at 1738 cm−1 and of the –C=O chelate stretching of amide I band at 1638 cm−1, confirming the involvement of these functional groups in AF binding onto biomasses.
- There was not a strong shift in the organic phosphate group located at 1070 cm−1. However, the phosphate band intensity was significantly reduced after interaction with AF.
- Other significant changes were in the CH out-of plane deformation bands in substituted aromatic hydrocarbons located at 832 cm−1 and 765 cm−1, respectively. In addition, the absorption band at 630 cm−1 attributable to the C–CO–C bend in ketones significantly reduced the intensity after interaction with AF.
2.5. Surface Morphology of P. koidzumii Biomasses
3. Conclusions
4. Materials and Methods
4.1. Plant Material and Preparation of Biomasses
4.2. Aqueous Solution of Aflatoxins
4.3. Biosorption Assay
4.4. Aflatoxin Analysis
4.4.1. Using Immunoaffinity Columns (IAC)
4.4.2. Using Ultra Performance Liquid Chromatography (UPLC)
4.5. Characterization of the Biosorbent
4.5.1. Zeta Potential (ζ)
4.5.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
4.5.3. Scanning Electron Microscopy (SEM)
4.6. Experimental Design and Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| UPLC | Ultra performance liquid chromatography |
| FTIR | Fourier transform infrared spectroscopy |
| SEM | Scanning electron microscopy |
| AF | Aflatoxin(s) |
| AFB1 | Aflatoxin B1 |
| AFB2 | Aflatoxin B2 |
| AFG1 | Aflatoxin G1 |
| AFG2 | Aflatoxin G2 |
| AFM1 | Aflatoxin M1 |
| FAO | Food and agriculture organization |
| HSCAS | Hydrated sodium calcium aluminosilicates |
| DMSO | Dimethyl sulfoxide |
| FEEDAP | Panel on Additives and Products or Substances used in Animal Feed |
| IAC | Immunoaffinity columns |
| Rt | Retention time |
| DTGS | Deuterated triglycine sulphate |
| DR | Diffuse reflectance |
| ANOVA | Analysis of variance |
References
- Feibelman, T.P.; Cotty, P.J.; Doster, M.A.; Michailides, T.J. A morphologically distinct strain of Aspergillus nomius. Mycologia 1998, 90, 618–623. [Google Scholar] [CrossRef]
- Nesci, A.; Gsponer, N.; Etcheverry, M. Natural maize phenolic acids for control of aflatoxigenic fungi on maize. J. Food Sci. 2007, 72, M180–M185. [Google Scholar] [CrossRef] [PubMed]
- IARC. Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. Available online: http://bases.bireme.br/cgi-bin/wxislind.exe/iah/online/?IsisScript=iah/iah.xis&src=google&base=WHOLIS&lang=p&nextAction=lnk&exprSearch=9283212568&indexSearch=ID (accessed on 7 April 2016).
- Mellor, S. Mycotoxins in feed-a global challenge. Feed Mix 2001, 9, 26–28. [Google Scholar]
- Trucksess, M.W.; Richard, J.L.; Stoloff, L.; McDonald, J.S.; Brumley, W.C. Absorption and distribution patterns of aflatoxicol and aflatoxins B1 and M1 in blood and milk of cows given aflatoxin B1. Am. J. Vet. Res. 1983, 44, 1753–1756. [Google Scholar] [PubMed]
- Herzallah, S.M. Determination of aflatoxins in eggs, milk, meat and meat products using HPLC fluorescent and UV detectors. Food Chem. 2009, 114, 1141–1146. [Google Scholar] [CrossRef]
- Hussain, Z.; Khan, M.Z.; Khan, A.; Javed, I.; Saleemi, M.K.; Mahmood, S.; Asi, M.R. Residues of aflatoxin B1 in broiler meat: Effect of age and dietary aflatoxin B1 levels. Food Chem. Toxicol. 2010, 48, 3304–3307. [Google Scholar] [CrossRef] [PubMed]
- Dowling, T.S. Fumonisin and its toxic effects. Cereal Foods World 1997, 42, 13–15. [Google Scholar]
- Di Stefano, V.; Pitonzo, R.; Cicero, N.; D’Oca, M.C. Mycotoxin contamination of animal feedingstuff: Detoxification by gamma-irradiation and reduction of aflatoxins and ochratoxin A concentrations. Food Addit. Contam. A 2014, 31, 2034–2039. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.; Applebaum, R.; Brackett, R.; Marth, E. Physical, chemical and biological degradation of mycotoxins in foods and agricultural commodities. J. Food Prot. 1982, 45, 964–971. [Google Scholar]
- Bata, A.; Lasztity, R. Detoxification of mycotoxin-contaminated food and feed by microorganisms. Trends Food Sci. Technol. 1999, 10, 223–228. [Google Scholar] [CrossRef]
- Jardon-Xicotencatl, S.; Diaz-Torres, R.; Marroquin-Cardona, A.; Villarreal-Barajas, T.; Mendez-Albores, A. Detoxification of Aflatoxin-Contaminated Maize by Neutral Electrolyzed Oxidizing Water. Toxins (Basel) 2015, 7, 4294–4314. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.J.; FinkGremmels, J.; Hernandez, E. Prevention of toxic effects of mycotoxins by means of nonnutritive adsorbent compounds. J. Food Prot. 1996, 59, 631–641. [Google Scholar]
- Tunali, S.; Ozcan, A.; Kaynak, Z.; Ozcan, A.S.; Akar, T. Utilization of the Phaseolus vulgaris L. Waste biomass for decolorization of the textile dye Acid Red 57: Determination of equilibrium, kinetic and thermodynamic parameters. J. Environ. Sci. Health A Toxic/Hazard. Subst. Environ. Eng. 2007, 42, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Volesky, B. Advances in biosorption of metals: Selection of biomass types. FEMS Microbiol. Rev. 1994, 14, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Schneider, I.A.H.; Rubio, J.; Smith, R.W. Biosorption of metals onto plant biomass: Exchange adsorption or surface precipitation? Int. J. Miner. Process. 2001, 62, 111–120. [Google Scholar] [CrossRef]
- Sekhar, K.C.; Kamala, C.T.; Chary, N.S.; Anjaneyulu, Y. Removal of heavy metals using a plant biomass with reference to environmental control. Int. J. Miner. Process. 2003, 68, 37–45. [Google Scholar] [CrossRef]
- Sekhar, K.C.; Kamala, C.T.; Chary, N.S.; Sastry, A.R.K.; Rao, T.N.; Vairamani, M. Removal of lead from aqueous solutions using an immobilized biomaterial derived from a plant biomass. J. Hazard. Mater. 2004, 108, 111–117. [Google Scholar]
- Akar, T.; Anilan, B.; Gorgulu, A.; Akar, S.T. Assessment of cationic dye biosorption characteristics of untreated and non-conventional biomass: Pyracantha coccinea berries. J. Hazard. Mater. 2009, 168, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Akar, T.; Celik, S.; Akar, S.T. Biosorption performance of surface modified biomass obtained from Pyracantha coccinea for the decolorization of dye contaminated solutions. Chem. Eng. J. 2010, 160, 466–472. [Google Scholar] [CrossRef]
- Ari, A.G.; Celik, S. Biosorption potential of Orange G dye by modified Pyracantha coccinea: Batch and dynamic flow system applications. Chem. Eng. J. 2013, 226, 263–270. [Google Scholar]
- De la Federación, D.O. Norma Oficial Mexicana NOM-188-SSA1–2002, Productos y Servicios: Control de aflatoxinas en cereales para consumo humano y animal, especificaciones sanitarias. D. Of. Fed. 2002, 11, 22–41. [Google Scholar]
- Huwig, A.; Freimund, S.; Kappeli, O.; Dutler, H. Mycotoxin detoxication of animal feed by different adsorbents. Toxicol. Lett. 2001, 122, 179–188. [Google Scholar] [CrossRef]
- Diaz, D.E.; Hagler, W.M., Jr.; Blackwelder, J.T.; Eve, J.A.; Hopkins, B.A.; Anderson, K.L.; Jones, F.T.; Whitlow, L.W. Aflatoxin binders II: Reduction of aflatoxin M1 in milk by sequestering agents of cows consuming aflatoxin in feed. Mycopathologia 2004, 157, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Kutz, R.E.; Sampson, J.D.; Pompeu, L.B.; Ledoux, D.R.; Spain, J.N.; Vazquez-Anon, M.; Rottinghaus, G.E. Efficacy of Solis, NovasilPlus, and MTB-100 to reduce aflatoxin M1 levels in milk of early to mid lactation dairy cows fed aflatoxin B1. J. Dairy Sci. 2009, 92, 3959–3963. [Google Scholar] [CrossRef] [PubMed]
- Batzias, F.A.; Sidiras, D.K. Simulation of methylene blue adsorption by salts-treated beech sawdust in batch and fixed-bed systems. J. Hazard. Mater. 2007, 149, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.V.; Gonzalez-Caballero, E.; Hunter, R.J.; Koopal, L.K.; Lyklema, J. Measurement and interpretation of electrokinetic phenomena (IUPAC technical report). Pure Appl. Chem. 2005, 77, 1753–1805. [Google Scholar] [CrossRef]
- Park, D.; Yun, Y.S.; Park, J.M. Studies on hexavalent chromium biosorption by chemically-treated biomass of Ecklonia sp. Chemosphere 2005, 60, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.S.; Park, D.; Park, J.M.; Volesky, B. Biosorption of trivalent chromium on the brown seaweed biomass. Environ. Sci. Technol. 2001, 35, 4353–4358. [Google Scholar] [CrossRef] [PubMed]
- Shurvell, H. Spectra–Structure Correlations in the Mid-and Far-Infrared. In Handbook of Vibrational Spectroscopy; Wiley: Hoboken, NJ, USA, 2002. [Google Scholar]
- Ganeva, T. Leaf Epidermis Structure in Cydonia Oblonga Mill.(Rosaceae). Biotechnol. Biotechnol. Equip. 2009, 23 (Suppl. S1), 965–967. [Google Scholar] [CrossRef]
- Ganeva, T.; Uzunova, K. Leaf epidermis structure in Amelanchier ovalis Medic. (Rosaceae). Biotechnol. Biotechnol. Equip. 2010, 24 (Suppl. S1), 36–38. [Google Scholar] [CrossRef]
- Mendez-Albores, A.; Martinez-Bustos, F.; Gaytan-Martinez, M.; Moreno-Martinez, E. Effect of lactic and citric acid on the stability of B-aflatoxins in extrusion-cooked sorghum. Lett. Appl. Microbiol. 2008, 47, 1–7. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific Opinion on the Safety and Efficacy of Bentonite (dioctahedral montmorillonite) as Feed Additive for all Species. EFSA J. 2011, 9. [Google Scholar] [CrossRef]
- Horwuitz, W. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- SAS/STAT User’s Guide. Version 8. Available online: http://www.okstate.edu/sas/v8/saspdf/stat/pdfidx.htm (accessed on 7 April 2016).







| Incubation Time (h) | Leaves | Berries | Leaves/Berries |
|---|---|---|---|
| 0 | 0 | 0 | 0 |
| 3 | 0.0066 | 0.0011 | 0.0069 |
| 6 | 0.0054 | 0.0010 | 0.0047 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramales-Valderrama, R.A.; Vázquez-Durán, A.; Méndez-Albores, A. Biosorption of B-aflatoxins Using Biomasses Obtained from Formosa Firethorn [Pyracantha koidzumii (Hayata) Rehder]. Toxins 2016, 8, 218. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins8070218
Ramales-Valderrama RA, Vázquez-Durán A, Méndez-Albores A. Biosorption of B-aflatoxins Using Biomasses Obtained from Formosa Firethorn [Pyracantha koidzumii (Hayata) Rehder]. Toxins. 2016; 8(7):218. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins8070218
Chicago/Turabian StyleRamales-Valderrama, Rosa Adriana, Alma Vázquez-Durán, and Abraham Méndez-Albores. 2016. "Biosorption of B-aflatoxins Using Biomasses Obtained from Formosa Firethorn [Pyracantha koidzumii (Hayata) Rehder]" Toxins 8, no. 7: 218. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins8070218