A Simple and Specific Noncompetitive ELISA Method for HT-2 Toxin Detection
Abstract
:1. Introduction
2. Results and Discussion
2.1. Noncompetitive ELISA and Method Optimization
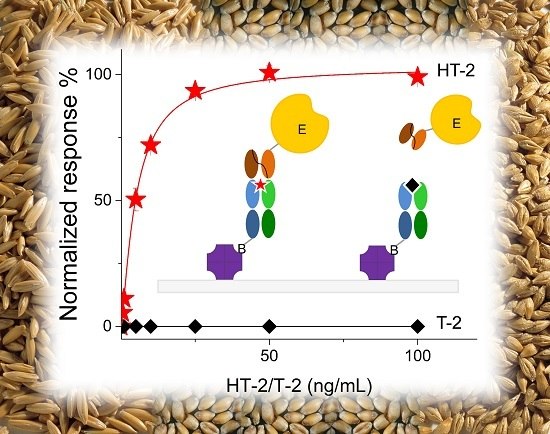
2.2. Sensitivity of the Simple Noncompetitive ELISA in Buffer
2.3. Effect of Methanol
2.4. Sample Incubation Time
2.5. Comparison of Alkaline Phosphatase Substrates
2.6. Simple Noncompetitive Immune Complex ELISA with Real Samples
2.7. Matrix Effects
3. Conclusions
4. Materials and Methods
4.1. Chemicals, Reagents and Instrumentation
4.2. Primary and Secondary Antibody Development
4.3. Construction of The ScFv-AP Plasmid
4.4. Cloning of ScFv-AP Fusion Proteins
4.5. Production and Purification of Anti-HT-2 IC ScFv-AP Fusion Proteins
4.6. Competitive HT-2 ELISA
4.7. Noncompetitive Immune Complex ELISA and Method Optimization
4.8. Sample Preparation
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Knutsen, H.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Appropriateness to set a group health based guidance value for T2 and HT2 toxin and its modified forms. EFSA J. 2017, 15, 1–53. [Google Scholar]
- Schuhmacher-Wolz, U.; Heine, K.; Schneider, K. SCIENTIFIC REPORT Submitted to EFSA Report on Toxicity Data on Trichothecene Mycotoxins HT-2 and T-2 Toxins; CT/EFSA/CONTAM/2010/03 Prepared by Klaus Schneider; FoBiG: Freiburg im Breisgau, Germany, 2010; pp. 1–57. [Google Scholar]
- Sudakin, D.L. Trichothecenes in the environment: Relevance to human health. Toxicol. Lett. 2003, 143, 97–107. [Google Scholar] [CrossRef]
- Van der Fels-Klerx, H.J. SCIENTIFIC/TECHNICAL Report Submitted to EFSA Occurrence Data of Trichothecene Mycotoxins T-2 Toxin and HT-2 Toxin in Food and Feed; RIKILT–Institute of Food Safety: Vageningen, The Netherlands, 2010; pp. 1–43. [Google Scholar]
- European Commission (EC). Recomendations on the presence of T-2 and HT-2 toxin in cereals and cereal products. Off. J. Eur. Union 2013, 9, 12–15. [Google Scholar]
- Morcia, C.; Tumino, G.; Ghizzoni, R.; Badeck, F.W.; Lattanzio, V.M.T.; Pascale, M.; Terzi, V. Occurrence of Fusarium langsethiae and T-2 and HT-2 toxins in Italian malting barley. Toxins 2016, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, Z.; Hu, X.; Zhang, Q. Advanced hyphenated chromatographic-mass spectrometry in mycotoxin determination: Current status and prospects. Mass Spectrom. Rev. 2013, 32, 420–452. [Google Scholar] [CrossRef] [PubMed]
- Porricelli, A.C.R.; Lippolis, V.; Valenzano, S.; Cortese, M.; Suman, M.; Zanardi, S.; Pascale, M. Optimization and Validation of a Fluorescence Polarization Immunoassay for Rapid Detection of T-2 and HT-2 Toxins in Cereals and Cereal-Based Products. Food Anal. Methods 2016, 9, 3310–3318. [Google Scholar] [CrossRef]
- Peters, J.; Cardall, A.; Haasnoot, W.; Nielen, M.W.F. 6-Plex microsphere immunoassay with imaging planar array detection for mycotoxins in barley. Analyst 2014, 139, 3968–3976. [Google Scholar] [CrossRef] [PubMed]
- Meneely, J.P.; Sulyok, M.; Baumgartner, S.; Krska, R.; Elliott, C.T. A rapid optical immunoassay for the screening of T-2 and HT-2 toxin in cereals and maize-based baby food. Talanta 2010, 81, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Aamot, H.U.; Hofgaard, I.S.; Brodal, G.; Elen, O.; Holen, B.; Klemsdal, S.S. Evaluation of rapid test kits for quantification of HT-2 and T-2 toxins in naturally contaminated oats. World Mycotoxin J. 2013, 6, 31–41. [Google Scholar] [CrossRef]
- Anfossi, L.; Giovannoli, C.; Baggiani, C. Mycotoxin detection. Curr. Opin. Biotechnol. 2016, 37, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Avrameas, S. Enzyme Immunoassays and Related Techniques: Development and Limitations. In New Developments in Diagnostic Virology; Bachmann, P.A., Ed.; Springer: Berlin/Heidelberg, Germany, 1983; pp. 93–99. [Google Scholar]
- Lindbladh, C.; Mosbach, K.; Bülow, L. Use of genetically prepared enzyme conjugates in enzyme immunoassay. Trends Biochem. Sci. 1993, 18, 279–283. [Google Scholar] [CrossRef]
- Suzuki, C.; Ueda, H.; Suzuki, E.; Nagamune, T. Construction, bacterial expression, and characterization of hapten-specific single-chain Fv and alkaline phosphatase fusion protein. J. Biochem. 1997, 122, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.E.; Wyckoff, H.W. Reaction mechanism of alkaline phosphatase based on crystal structures. J. Mol. Biol. 1991, 218, 449–464. [Google Scholar] [CrossRef]
- Martin, C.D.; Rojas, G.; Mitchell, J.N.; Vincent, K.J.; Wu, J.; McCafferty, J.; Schofield, D.J. A simple vector system to improve performance and utilisation of recombinant antibodies. BMC Biotechnol. 2006, 6, 46. [Google Scholar] [CrossRef]
- Shu, M.; Xu, Y.; Liu, X.; Li, Y.; He, Q.; Tu, Z.; Fu, J.; Gee, S.J.; Hammock, B.D. Anti-idiotypic nanobody-alkaline phosphatase fusion proteins: Development of a one-step competitive enzyme immunoassay for fumonisin B1 detection in cereal. Anal. Chim. Acta 2016, 924, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, Y.; Wan, D.; Xiong, Y.; He, Z.; Wang, X.; Gee, S.J.; Ryu, D.; Hammock, B.D. Development of a Nanobody-Alkaline Phosphatase Fusion Protein and Its Application in a Highly Sensitive Direct Competitive Fluorescence Enzyme Immunoassay for Detection of Ochratoxin A in Cereal. Anal. Chem. 2015, 87, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Du, X.J.; Zhou, X.N.; Li, P.; Sheng, W.; Ducancel, F.; Wang, S. Development of an Immunoassay for Chloramphenicol Based on the Preparation of a Specific Single-Chain Variable Fragment Antibody. J. Agric. Food Chem. 2016, 64, 2971–2979. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.X.; Li, Z.F.; Lei, H.T.; Sun, Y.M.; Ducancel, F.; Xu, Z.L.; Boulain, J.C.; Yang, J.Y.; Shen, Y.D.; Wang, H. Development of a single-chain variable fragment-alkaline phosphatase fusion protein and a sensitive direct competitive chemiluminescent enzyme immunoassay for detection of ractopamine in pork. Anal. Chim. Acta 2012, 736, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, H.; Liang, Y.; Yang, J.; Zhang, H.; Lei, H.; Shen, Y.; Sun, Y. Production and characterization of a single-chain Fv antibody-alkaline phosphatase fusion protein specific for clenbuterol. Mol. Biotechnol. 2010, 45, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Oyama, H.; Tanaka, E.; Kawanaka, T.; Morita, I.; Niwa, T.; Kobayashi, N. Anti-idiotype scFv-enzyme fusion proteins: A clonable analyte-mimicking probe for standardized immunoassays targeting small biomarkers. Anal. Chem. 2013, 85, 11553–11559. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.M.; Ekins, R.P. Theoretical limitations on immunoassay sensitivity. J. Immunol. Methods 1986, 87, 13–20. [Google Scholar] [CrossRef]
- Ullman, E.F.; Milburn, G.; Jelesko, J.; Radika, K.; Pirio, M.; Kempe, T.; Skold, C. Anti-immune complex antibodies enhance affinity and specificity of primary antibodies. Proc. Natl. Acad. Sci. USA 1993, 90, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- Pulli, T.; Höyhtyä, M.; Söderlund, H.; Takkinen, K. One-step homogeneous immunoassay for small analytes. Anal. Chem. 2005, 77, 2637–2642. [Google Scholar] [CrossRef] [PubMed]
- González-Techera, A.; Vanrell, L.; Last, J.A.; Hammock, B.D.; González-Sapienza, G. Phage anti-immune complex assay: General strategy for noncompetitive immunodetection of small molecules. Anal. Chem. 2007, 79, 7799–7806. [Google Scholar] [CrossRef] [PubMed]
- Omi, K.; Ando, T.; Sakyu, T.; Shirakawa, T.; Uchida, Y.; Oka, A.; Ise, N.; Aoyagi, K.; Goishi, K. Noncompetitive Immunoassay Detection System for Haptens on the Basis of Antimetatype Antibodies. Clin. Chem. 2015, 61, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Arola, H.O.; Tullila, A.; Kiljunen, H.; Campbell, K.; Siitari, H.; Nevanen, T.K. Specific Noncompetitive Immunoassay for HT-2 Mycotoxin Detection. Anal. Chem. 2016, 88, 2446–2452. [Google Scholar] [CrossRef] [PubMed]
- Meneely, J.; Ricci, F.; Vesco, S.; Abouzied, M.; Sulyok, M.; Krska, R.; Elliott, C. A comparative study of qualitative immunochemical screening assays for the combined measurement of T-2/HT-2 in cereals and cereal-based products. World Mycotoxin J. 2011, 4, 385–394. [Google Scholar] [CrossRef]
- Molinelli, A.; Grossalber, K.; Führer, M.; Baumgartner, S.; Sulyok, M.; Krska, R. Development of qualitative and semiquantitative immunoassay-based rapid strip tests for the detection of T-2 toxin in wheat and oat. J. Agric. Food Chem. 2008, 56, 2589–2594. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.M.T.; Solfrizzo, M.; Visconti, A. Enzymatic hydrolysis of T-2 toxin for the quantitative determination of total T-2 and HT-2 toxins in cereals. Anal. Bioanal. Chem. 2009, 395, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Nathanail, A.V.; Syvähuoko, J.; Malachová, A.; Jestoi, M.; Varga, E.; Michlmayr, H.; Adam, G.; Sieviläinen, E.; Berthiller, F.; Peltonen, K. Simultaneous determination of major type A and B trichothecenes, zearalenone and certain modified metabolites in Finnish cereal grains with a novel liquid chromatography-tandem mass spectrometric method. Anal. Bioanal. Chem. 2015, 407, 4745–4755. [Google Scholar] [CrossRef] [PubMed]




| Cereal Grain | Reference Samples | Noncompetitive ELISA Performance | |||
|---|---|---|---|---|---|
| T-2 ± SR 1 (µg/kg) | HT-2 ± SR 1 (µg/kg) | HT-2 ± SD (µg/kg) n = 6 | Recovery (%) | CV (%) 3 | |
| Wheat | 98 ± 20 | 25 ± 5 | 30 ± 1 | 122 | 4 |
| 195 ± 39 | 50 ± 10 | 64 ± 4 | 129 | 9 | |
| 390 ± 78 | 100 ± 20 | 131 ± 13 | 131 | 13 | |
| Barley | 100 ± 20 | 25 ± 5 | 28 ± 2 | 112 | 8 |
| 199 ± 40 | 50 ± 10 | 46 ± 2 | 92 | 4 | |
| 398 ± 80 | 100 ± 20 | 78 ± 2 | 78 | 2 | |
| Oats 2 | 8 | 25 | 33 ± 4 | 133 | 16 |
| 15 | 50 | 45 ± 8 | 90 | 16 | |
| 30 | 100 | 119 ± 18 | 119 | 18 | |
| 75 | 250 | 253 ± 7 | 101 | 3 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arola, H.O.; Tullila, A.; Nathanail, A.V.; Nevanen, T.K. A Simple and Specific Noncompetitive ELISA Method for HT-2 Toxin Detection. Toxins 2017, 9, 145. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins9040145
Arola HO, Tullila A, Nathanail AV, Nevanen TK. A Simple and Specific Noncompetitive ELISA Method for HT-2 Toxin Detection. Toxins. 2017; 9(4):145. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins9040145
Chicago/Turabian StyleArola, Henri O., Antti Tullila, Alexis V. Nathanail, and Tarja K. Nevanen. 2017. "A Simple and Specific Noncompetitive ELISA Method for HT-2 Toxin Detection" Toxins 9, no. 4: 145. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins9040145